Adverse events associated with the gold probe and the injection gold probe devices used for endoscopic hemostasis: A MAUDE database analysis
Vishnu Charan Suresh Kumar,Mark Aloysius,Ganesh Aswath
Abstract BACKGROUND Gastrointestinal (GI) bleeding accounts for over half a million admissions annually and is the most common GI diagnosis requiring hospitalization in the United States. Bipolar electrocoagulation devices are used for the management of gastrointestinal bleeding. There is no data on device-related adverse events for gold probe (GP) and injection gold probe (IGP).AIM To analyze this using the Food and Drug Administration (FDA’s) Manufacturer and User Facility Device Experience (MAUDE) database from 2013 to 2023.METHODS We examined post-marketing surveillance data on GP and IGP from the FDA MAUDE database to report devicerelated and patient-related adverse events between 2013-2023. The MAUDE database is a publicly available resource providing over 4 million records relating to medical device safety. Statistical analyses were performed using IBM SPSS Statistics V.27.0 (IBM Corp.,Armonk,NY,United States).RESULTS Our search elicited 140 reports for GP and 202 reports for IGP,respec-tively,during the study period from January 2013 to August 2023. Malfunctions reportedly occurred in 130 cases for GP,and actual patient injury or event occurred in 10 patients. A total of 149 patients (74%) reported with Injection GP events suffered no significant consequences due to the device failure,but 53 patients (26%) were affected by an event.CONCLUSION GP and IGP are critical in managing gastrointestinal bleeding. This study of the FDA MAUDE database revealed the type,number,and trends of reported device-related adverse events. The endoscopist and support staff must be aware of these device-related events and be equipped to manage them if they occur.
Key Words: Hemostasis; Gastrointestinal bleeding; Endoscopy; Device failure; Bipolar coagulation; Cautery; Risks
INTRODUCTION
Gastrointestinal (GI) bleeding accounts for over half a million admissions annually and is the most common GI diagnosis requiring hospitalization in the United States[1]. Lesions with high-risk stigmata,which are associated with high rates of recurrent bleeding (50% to 80%) and result in significant morbidity if treated with medical therapy alone. Thus,the latest American College of Gastroenterology (ACG) guidelines recommend endoscopic therapy for ulcers with active spurting or oozing and nonbleeding visible vessels. The management of nonvariceal upper GI bleed (UGIB) has evolved tremendously with the advent of therapeutic endoscopic hemostasis devices and techniques. Studies have shown that thermal contact devices such as bipolar electrocoagulation and heater probes decrease the incidence of re-bleeding compared with no endoscopic therapy[2].
Overall,devices used to achieve hemostasis using thermal therapy were safe. The serious adverse events associated with these devices include uncontrollable bleeding and perforation[3]. Pooled data showed that the rate of bleeding that required urgent surgery was 0.3%,and perforation was 0.5%[4].
The gold probe (GP) and injection gold probe (IGP) (Boston Scientific Corp.,Natick,Mass.) are two commonly used devices to achieve endoscopic hemostasis. IGP can deliver an injection as well as thermal therapy. No data on devicerelated adverse events for these devices used routinely to achieve endoscopic hemostasis is available.
Thus,we aimed to evaluate the events associated with using Gold Probe and Injection Gold Probe using the Food and Drug Administration (FDA’s) Manufacturer and User Facility Device Experience (MAUDE) database from 2013 to 2023.
MATERlALS AND METHODS
We examined post-marketing surveillance data on GP and IGP from the FDA MAUDE database to report devicerelated and patient-related adverse events. The MAUDE database is a publicly available resource providing over 4 million records relating to medical device safety. The MAUDE database has medical device reports (MDRs) submitted to the FDA by mandatory reporters (manufacturers,importers,and device user facilities) and voluntary reporters such as healthcare professionals,patients and consumers (https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/search.cfm#fn1).
It consists of four primary (Master Event,Device,Patient,Text) and two supplemental (Device Problems and Problem Code Descriptions) file types,which,when combined,provide a detailed account of an adverse event or product problem report. Healthcare professionals have used MAUDE to review events associated with specific products or procedures. Several articles referencing MAUDE have been published analyzing adverse events specific to a particular outcome,product,or body system. It is publicly available online and de-identified. Therefore,no institutional review board approval was required for this study.
Outcomes and statistical analysis
We queried the MAUDE database from January 2013 to August 2023. The MAUDE web search feature is limited to adverse event reports within the past ten years. The data was analyzed for device issues and patient adverse events. The primary outcome measure of this study was the failure modes of the endoscopic diathermyGold ProbeTM(Ò Boston Scientific) and injection diathermyInjection Gold ProbeTM(Ò Boston Scientific). Secondary outcomes included significant complications associated with device failure. The MAUDE database cannot capture the utilization of IGP in the United States; therefore,the actual incidence rate of each failure or complication type cannot be assessed. Categorical variables were presented as numbers; all statistical analyses were performed using IBM SPSS Statistics V.27.0 (IBM Corp.,Armonk,NY,United States).
RESULTS
Our search elicited 140 reports for GP and 202 reports for IGP,respectively,during the study period from January 2013 to August 2023. The procedure type for GP use was esophagogastroduodenoscopy (47) followed by colonoscopy (25),bronchoscopy (7),endoscopic retrograde cholangiopancreatography (ERCP) (6),enteroscopy (3),missing procedure information (52),Table 1. The procedure types for IGP were esophagogastroduodenoscopy (174) followed by colonoscopy (16),ERCP (11),and enteroscopy (1),Table 2.

Table 1 Reported procedure type in which gold probe was used
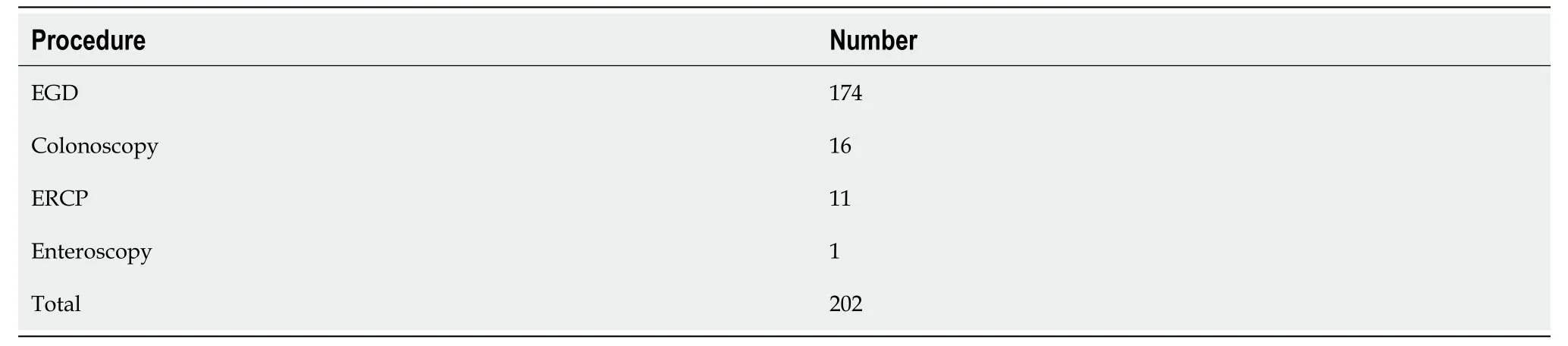
Table 2 Reported procedure type in which injection gold probe was used
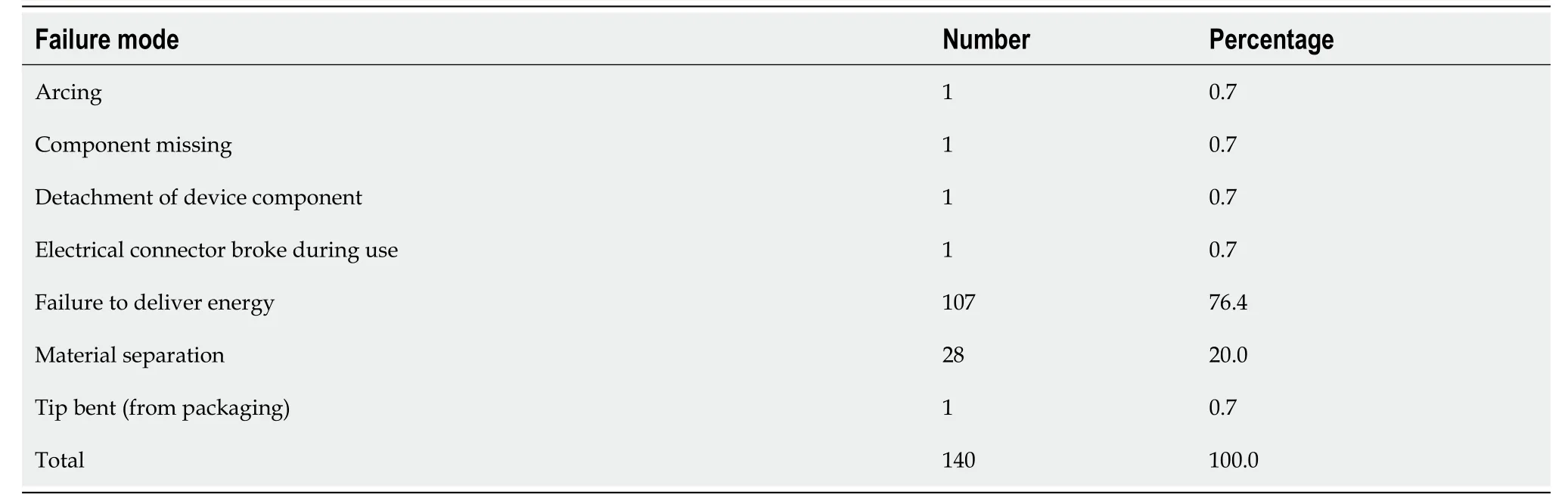
Table 3 Failure Modes of gold probe (Primary Outcomes)
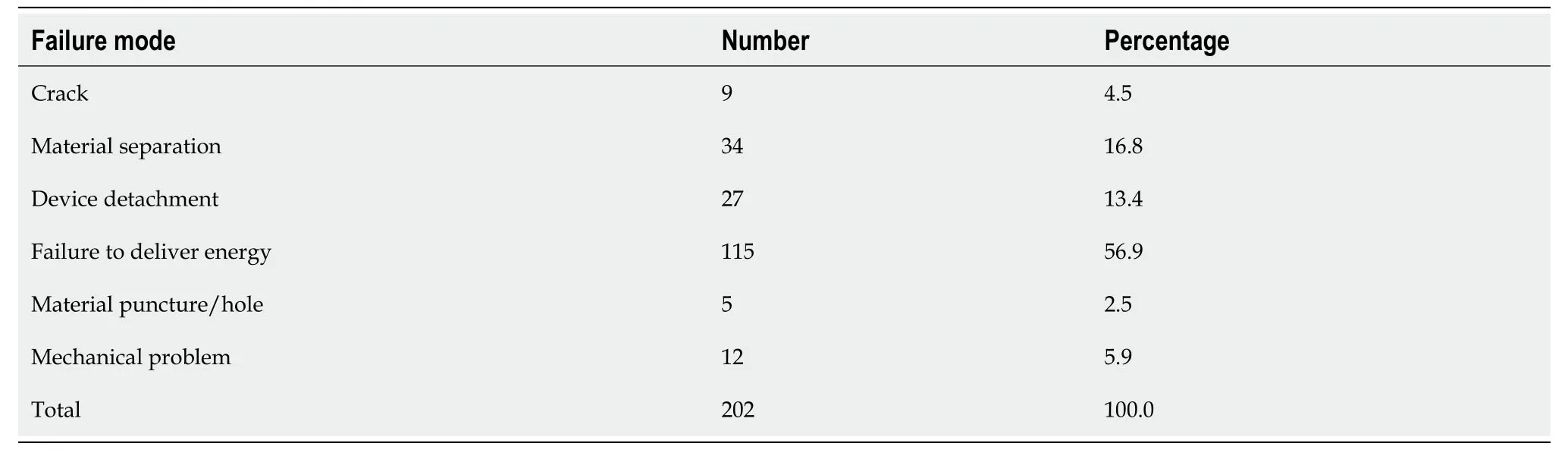
Table 4 Failure modes of injection gold probe (Primary Outcomes)
Primary outcomes outlining failure modes for the GP and IGP is outlined in Tables 3 and 4. GP failure modes were failure to deliver energy (107),followed by material separation or fracture of the probe tip (28),arcing (1),missing component (1),bent tip (1),and detachment of device (2). IGP failure modes were failure to deliver energy (115),followed by material separation or fracture of the probe tip (34),crack (9),device detachment (27),material puncture (5),and mechanical problems (12).
Malfunctions reportedly occurred in 130 cases for GP,and actual patient injury or event occurred in 10 patients. In assessing secondary outcomes,no deaths were reported,although two patients experienced prolonged hemorrhage and two fiberoptic endoscopes were damaged by the device; 7 patients required a secondary procedure to retrieve the detached probe. Most patients with a reported GP event suffered no significant consequences due to the device failure (93%),but 7% required a second procedure or experienced prolonged stay or discomfort,Table 5. Most patients reported with IGP events (74%) suffered no significant consequences due to the device failure,but 26% of patients were affected by an event (prolonged hemorrhage,need for a secondary procedure due to a detached probe),Table 6. Reports by year decreased significantly after 2017 for both GP and IGP,Table 7.
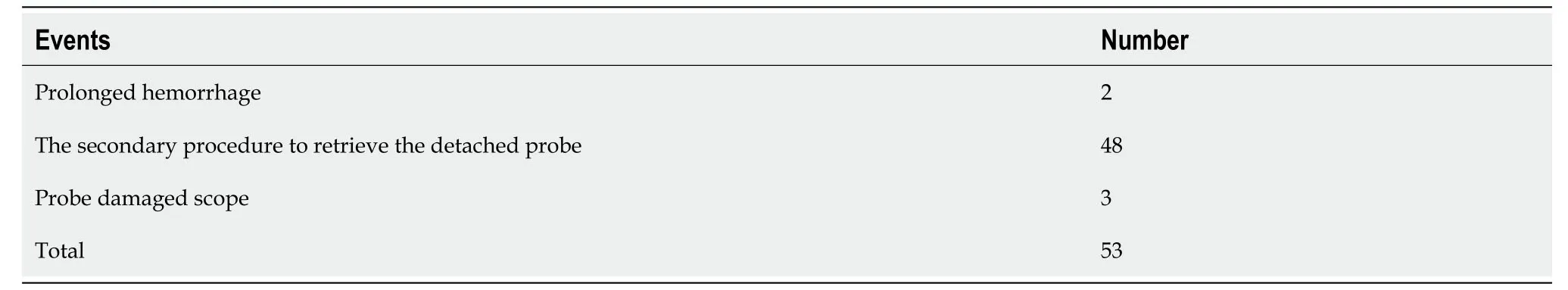
Table 6 Events affecting patients or equipment with injection gold probe failure (Secondary Outcomes)

Table 7 Reports by year (2013-2023)
DlSCUSSlON
Our study comprehensively analyzes events reported with GP and IGP from 2013 to 2023. For both GP and IGP,the most reported problem is the "failure to deliver energy." Investigating the root cause of this recurrent issue with these devices is imperative. If user error is identified as a significant factor,offering additional training to the healthcare professionals using these devices and refining the user guidelines would be beneficial.
The significantly higher number of reported events with IGP devices than with GP devices is noteworthy. While a higher usage frequency might contribute to the increased reporting,the pronounced rate of patient-related adverse events stemming from IGP failures cannot be dismissed lightly. Especially concerning are instances requiring repeat procedures,as they amplify the risk profile for patients and accentuate the resource burdens on healthcare institutions.
The manufacturer for the GP and IGP reports patient-related adverse events,including perforation,bleeding,aspiration pneumonia,and septicemia/infection,and reports a potential electrical hazard to the patient and operator with possible adverse including fulguration,burns,stimulation,and cardiac arrhythmia[5]. However,there have been no studies so far that have looked at the device-related events that could occur with GP and IGP. Our study is the first to analyze the device-related events reported. It sheds light on device-related complications,thus enhancing the existing knowledge pool crucial for daily clinical applications. Data regarding other bipolar devices was sparse and thus a comparative analysis could not be done.
The 2021 ACG guidelines for managing UGIB strongly recommend endoscopic hemostatic therapy with bipolar electrocoagulation,heater probe,or injection of absolute ethanol for patients with UGIB due to ulcers. Several studies have proven the efficacy and overall safety of GP and IGP to manage gastrointestinal hemorrhage[2,6,7]. The safety and efficacy of bipolar devices have been also established while managing lower GI bleeding[8,9]. GP and IGP are Bipolar devices used to manage GI bleeding during endoscopy. Given the ubiquity of these bipolar devices in clinical scenarios,endoscopists,and auxiliary staff must be apprised of potential device-related pitfalls.
Interestingly,the findings of this study also suggest that there was a decline in the events for both IGP and GP from 2017. Endoscopists familiarity with the device and adequate training in its usage,and manufacturer’s improvement of the quality of the device could have led to fewer events. Usage of other hemostatic devices could have also contributed to this. Over-the-Scope Clips (OTSC) has been shown to be as effective as standard therapy in non-variceal upper gastrointestinal bleeding since 2017[10]. OTSC has also proven effective in large ulcers up to 5 cm[11],with a high success rate of hemostasis (80%) even in recurrent bleeding and has also competed with GP and IGP as first line hemostatic method since 2017[12].
At around the same time,hemostatic aerosolized powders such as TC 325 (Hemospray) have become part of the hemostatic armamentarium available to the endoscopist,especially effective in the setting of diffuse mucosal bleeding[13,15].
These newer hemostatic technologies may have contributed to a decline in use of IGP and GP since 2017. It’s also conceivable that the manufacturing process may have effectively addressed the prior device failure reports to redesign and improve quality control hence leading to a decline in the device malfunction/failure reports since 2017.
Guidelines for non-variceal upper gastrointestinal bleeding have emphasized that epinephrine injection needs to be combined with a secondary hemostatic modality and hence IGP use may have increased over GP use. IGP conveniently uses both injection and thermocoagulation sequentially without interruption to introduce another hemostatic method endoscopically. This may have contributed to increase in IGP use over GP and consequently higher device malfunction reports[15].
This study has limitations. The MAUDE web search feature is limited to adverse event reports within the past ten years. This passive surveillance system has its limitations. There is a potential for submission of incomplete,inaccurate,untimely,unverified,or biased data. In addition,the incidence or prevalence of an event cannot be determined from this reporting system alone due to under-reporting of events,inaccuracies in reports,lack of verification that the device caused the reported event,and lack of information about the frequency of the device use.
CONCLUSION
GP and IGP are critical in managing gastrointestinal bleeding. This study of the FDA MAUDE database revealed the type,number,and trends of reported device-related adverse events. The endoscopist and support staff must be aware of these device-related events and be equipped to manage them if they occur.
ARTlCLE HlGHLlGHTS
Research motivation
Gold probe (GP) and gold probe (GP) are vital in managing gastrointestinal bleeding,yet they present notable risks. Awareness of these risks is essential for endoscopists and support staff. The study highlights the need for improved device safety and better management strategies in case of device failure.
Research objectives
The analysis revealed 140 reports for GP and 202 reports for IGP,with the majority of device failures being attributed to the failure to deliver energy. While most events did not lead to significant patient consequences,a notable proportion (26% for IGP) resulted in adverse outcomes like prolonged hemorrhage or the need for secondary procedures.
Research methods
The study utilized post-marketing surveillance data from the Food and Drug Administration (FDA’s) Manufacturer and User Facility Device Experience (MAUDE) database,analyzing reports for GP and IGP from January 2013 to August 2023. Statistical analyses were performed using IBM SPSS Statistics V.27.0 to identify primary and secondary outcome measures.
Research results
The primary objective is to evaluate the events associated with the use of GP and IGP,specifically focusing on the types and frequencies of device failures and their impact on patient outcomes.
Research conclusions
The motivation for this research stems from the lack of comprehensive data on device-related adverse events for GP and IGP,devices commonly used in managing gastrointestinal bleeding,despite their widespread clinical use.
Research perspectives
This study investigates the device-related adverse events associated with the use of GP and IGP in endoscopic hemostasis,leveraging data from the FDA's MAUDE database over a decade (2013-2023).
Research background
The findings underscore the need for ongoing surveillance,device improvement,and consideration of emerging hemostatic technologies. Further research into device design and usage guidelines could enhance safety and efficacy in clinical practice.
FOOTNOTES
Author contributions:Suresh Kumar VC contributed to conceptualization,design,manuscript writing,and editing; Aloysius M contributed to design,statistical analysis,manuscript writing,and editing; Aswath G contributed to manuscript review and editing.
lnstitutional review board statement:This is a de-identified database-based study thus it was determined that no ethical approval/IRB is required.
lnformed consent statement:For this study,we utilized a de-identified database,specifically the FDA’s Manufacturer and User Facility Device Experience (MAUDE) database,which contains anonymized and publicly available data. Given the retrospective and deidentified nature of the data analyzed,this study did not involve direct interaction with patients or access to identifiable patient information. Consequently,in accordance with ethical guidelines and research standards,informed consent was not required for this database-based study.
Conflict-of-interest statement:There are no conflicts of interest to report.
Data sharing statement:No additional data are available.
STROBE statement:The authors have read the STROBE Statement-checklist of items,and the manuscript was prepared and revised according to the STROBE Statement-checklist of items.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:United States
ORClD number:Vishnu Charan Suresh Kumar 0000-0002-9472-2869; Mark Aloysius 0000-0001-6191-0524; Ganesh Aswath 0000-0002-1354-9225.
Corresponding Author's Membership in Professional Societies:American College of Gastroenterology; American Society for Gastrointestinal Endoscopy; American Gastroenterological Association.
S-Editor:Liu JH
L-Editor:A
P-Editor:Cai YX
 World Journal of Gastrointestinal Endoscopy2024年1期
World Journal of Gastrointestinal Endoscopy2024年1期
- World Journal of Gastrointestinal Endoscopy的其它文章
- Propofol sedation in routine endoscopy: A case series comparing target controlled infusion vs manually controlled bolus concept
- Bowel preparation protocol for hospitalized patients ages 50 years or older: A randomized controlled trial
- Safety and efficacy of modified endoscopic ultrasound-guided selective N-butyl-2-cyanoacrylate injections for gastric variceal hemorrhage in left-sided portal hypertension
- Upper gastrointestinal bleeding in Bangladeshi children: Analysis of 100 cases
