Polymorphism in the MethylenetetrahydrofolateReductase and Thymidylate Synthase GenePredicts for Response to Fluorouracil-basedChemotherapy in Advanced Gastric Cancer Patients
Lu Jian-wei, Gao Chang-ming, Wu Jian-zhong, Cao Hai-xia, Kazuo Tajima, Chen Huan-qiu, Chen Jia, Feng Ji-feng
1 Introduction
Gastric cancer is a significant health problem worldwide, with approximately 930 000 new cases diagnosed and 700 000 deaths attributed to the disease each year[1]. About 60% of cases occur in developing countries, and about 38% of cases are in China[2]. Most gastric cancer patients are diagnosed at an advanced stage, with poor prognosis and a 5-year survival rate between 5 and 15%[3]. However, chemotherapy is the standard treatment for advanced gastric cancer. A meta-analysis of randomized, controlled trials in patients with advanced gastric cancer demonstrated that chemotherapy significantly improved the median overall survival versus best supportive care[4]. In addition, combination chemotherapy was associated with a survival benefit versus single-agent chemotherapy and combination chemotherapy regimens containing 5-fluorouracil (5-FU), anthracyclines, and cisplatin were associated with a significant survival benefit versus 5-FU and anthracycline regimens without cisplatin. The V-325 study demonstrated that adding docetaxel (D) to a frequently used regimen of cisplatin and 5-fluorouracil (CF) provided benefits with regard to overall survival, response rate, time-to-disease progression, clinical benefit, and health-related quality of life[5-6].
Despite the success rates mentioned above, chemotherapeutic programs for gastric cancer have been remain unsatisfactory. A regimen may be completely ineffective or achieve complete remission (CR) in the treatment of a given patient, depending on individual differences in drug sensitivity. Thus, it is important to find a reliable marker with which to forecast drug sensitivity, and to use this marker to direct individualized treatment. Such a marker would also be significant in determining the efficacy and safety of clinical medication.
In cancer therapy, methylenetetrahydrofolate reductase (MTHFR) and thymidylate synthase (TS) are important targets of many antimetabolites, including 5-FU. Several polymorphisms exist for both genes, and MTHFR gene is subject to several polymorphisms. Of these, the 677C>T (Ala to Val at codon 222, exon 4) and 1298A>C (Glu to Ala at codon 428, exon 7) SNPs are the two most commonly linked with altered enzyme activity[7-8]. Preliminary data from in vitro studies with human cancer cells and nude mice xenograft models suggested that both C677T and A1298C may enhance the chemosensitivity to 5-FU[9-10]. The critical point for the 5-FU activity is the formation of an inhibitory ternary complex, consisting of its active metabolite 5-fluoro-2-deoxyuridine-5-monophospate (5-FdUMP), TS and 5,10-methylentetrahydrofolate, thereby inhibiting TS activity. It can be hypothesized that MTHFR polymorphisms, by increasing intracellular concentrations of 5,10-methylentetrahydrofolate, may augment the cytotoxic activity (tumor response and/or toxic side-effects) of 5-FU by enhancing the formation and stability of the ternary inhibitory complex. The TS gene has a two or three 28-bp tandem repeat sequence in 5’-untranslated region (5’-UTR), or a 6-bp deletion polymorphism at bp 1494 in the 3’-untranslated region (3’-UTR). These kinds of TS gene polymorphisms can influence TS gene expression by influencing the stability of the TS mRNA[11-12], and thus affect the drug sensitivity of cancer patients[13]. In the present work, we studied the relationship between polymorphisms of the MTHFR, the TS (3’-UTR) gene and drug sensitivity of gastric cancer to 5-FU based chemotherapy.
2 Materials and Methods
2.1Patients
We collected 173 patients of advanced gastric cancer defined by pathology in the Department of Medical Oncology, Jiangsu Province Institute of Cancer Research from May 2001 to June 2008 (Table 1). Patients were eligible for entry into the study if they had locally advanced or metastatic gastric cancer with measurable or evaluable disease. Patients had not previously received chemotherapy regimens except adjuvant chemotherapy administered more than 6 months. Histologic or cytologic confirmation of gastric adenocarcinoma was required. Patients had to be at least 18 years old and have a life expectancy >12 weeks. Eastern Cooperative Oncology Group performance status was required to be between 0 and 2. Patients were not included if they had active or extensive brain metastases, active systemic infection, inflammatory bowel disease, unstable cardiac disease, or untreated vitamin B12 deficiency. Patients who were pregnant or actively lactating were excluded. All patients gave written informed consent, and the study was approved by the Institutional Ethics Committee.

Table 1 Patient characteristics
The three different chemotherapy regimens administered in this study of patients were: FOLFOX regimen: A 2 hours infusion of oxaliplatin (100 mg/m2) and folinic acid (200 mg/m2), followed by a 46 hour continuous infusion of 5-FU (2,400 mg/m2), every 2 weeks. FP regimen: A 4 hours infusion of cisplatin (80 mg/m2), a 2 hours infusion of folinic acid (200 mg/m2/day×5), and a 24 hour continuous infusion of 5-FU (600 mg/m2/day×5), every 3 weeks. DCF regimen: A 1 hour infusion of Docetaxel (80 mg/m2), 5-FU, folinic acid and cisplatin at the same dose in FP regimen, every 4 weeks. Patients underwent chemotherapy cycles until disease progression, patient refusal or unacceptable adverse reactions.
2.2Genotypeanalysis
Samples of venous blood (2 mL) were taken before chemotherapy and placed in EDTA-containing anticoagulation tubes. Leukocytes were then separated and DNA extracted from white cells using a QIAamp DNA Extraction Kit (QIAGEN Inc., Valencia, CA). Each polymorphism was detected after PCR amplification with the corresponding primers according to our previous studies[15-16]. The restriction enzyme Hinf I was used to distinguish the 677 (C/T) polymorphism. The primers were 5’-TGAAGGAGAAGGTGTCTGCGGGA-3’ and 5’-AGGACGGTGCGGTGAGAGTG-3’. The PCR product was subjected to Hinf I restriction enzyme digestion and samples were then analyzed by electrophoresis in 3% agarose gels stained with ethidium bromide. There were three genotypes of MTHFR C677T: C/C (198 bp); C/T (198 bp/175 bp); T/T (175 bp). The restriction enzyme Mbo II was used to distinguish the 1298 (A/C) polymorphism. The primers were 5’- CTTTGGGGAGCTGAAGGACTACTAC-3’ and 5’-CACTTTGTGACCATTCCGGTTTG-3’. The PCR product was subjected to Mbo II restriction enzyme digestion and samples were then analyzed by electrophoresis in 4% agarose gels stained with ethidium bromide. There were three genotypes of MTHFR A1298C: A/A (56 bp); A/C (56 bp/84 bp); C/C (84 bp). The TS 3’-UTR sequences of the primers were 5’-CAAATCTGAGGGAGCTGAGT-3’ and 5’-CAGATAAGTGGCAGTACAGA-3’. PCR products were resolved by agarose gel (3%) electrophoresis; products with two bands of 158 bp (+6 bp) and 152 bp (-6 bp) were classified as the heterozygote genotype +6/-6 bp. A single band was obtained for the two homozygous genotypes (+6/+ 6bp and -6/-6 bp). Digestion of this single band with the restriction enzyme DraI yielded two bands of 88 and 70 bp in the case of the +6/+6 bp homozygous genotype.
2.3Statistics
We carried out statistical analyses using SPSS software package, version 11.5. Chi-square tests and Fisher’s exact test (if N≤5) were applied to analyze the relationships among genotype, therapeutic effect and toxicity.
3 Results
3.1Patientcharacteristicsandtreatmentoutcomes
The clinical characteristics of patients were summarized in Table 1. The overall response rate (RR) was 35.8%. No patient had complete response, 62 patients had partial response and 55 patients had stable disease. Fifty-six patients had progressive disease at their first evaluation. The RR of the DCF regimen group was significantly higher than that of the FP and FOLFOX regimen groups (55.8% vs 27.1%, 31.1%;P=0.006) (Table 2).

Table 2 Response rate of chemotherapy regimens and the genotypes
RR: response rate.
3.2Correlationbetweengenotypesandresponserates
We analyzed 9 germ-line polymorphisms within 2 genes. The genotypes of MTHFR C677T, A1298C and TS 3’-TUR were analyzed in 173 patients. The genotype distributions and therapeutic effect were summarized in Table 2.55 (31.8%) of the patients had the MTHFR C677T C/C genotype, 88 (50.9%) the C/T genotype, and 30 (17.3%) the T/T genotype. For A1298C, 122, 49, and 2 patients had the A/A (70.5%), A/C (28.2%), C/C (1.2%) genotypes, respectively. The RR of the MTHFR C677T T/T genotype was significantly higher than that of the C/C and C/T genotypes (73.3% vs 28.0%;P=0.000). In MTHFR A1298C, a higher RR was observed in A/A genotype compared with the C/C and A/C genotypes (41.8% vs 21.6%,P=0.011).
For the TS 3’-UTR indel, 65, 74, and 34 patients had -6 bp/-6 bp (37.6%), +6 bp/-6 bp (42.8%), and +6 bp/+6 bp (19.7%) genotypes, respectively. The RR of the -6/-6 bp and -6/+6 bp genotypes in TS 3’-UTR were significantly higher than that of the +6/+6 bp genotype (40.3% vs 17.6%,P=0.014).
The relationship between MTHFR, TS genotype and chemotherapeutic effect of different treatment regimens was summarized in Table 3. The RR of the MTHFR C677T T/T genotypes in the FOLFOX and FP regimens were significantly higher than that of the C/C and C/T genotypes (P=0.008,P=0.000), but no difference in the DCF regimen. Although the response rate of other genotypes (A1298C A/A, TS 3’UTR -6/-6 bp and -6/+6 bp) was the highest, there was no statistical difference when compared with the three regimens. The RR of different three chemotherapy regimens had no difference in the C677T T/T genotype (P=0.563), but the RR of DCF regimen was significantly higher than that of the FOLFOX and FP regimens in the C/T and C/C genotypes (P=0.000).
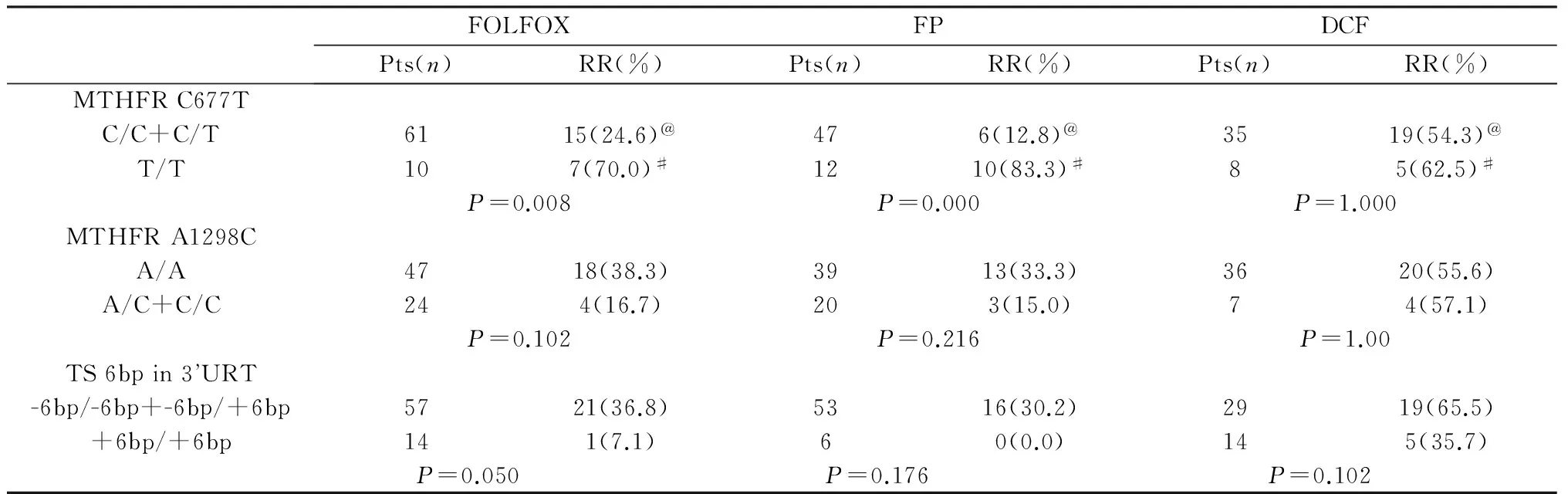
Table 3 Response rate of the genotypes in different chemotherapy regimens
RR: response rate.
@: The RR of DCF regimen was significantly higher than that of the FOLFOX and FP regimens in the C/T and C/C genotypes,P=0.000.
#: The RR of three different chemotherapy regimens were no difference in the C677T T/T genotype,P=0.563.
3.3Correlationbetweengenotypesandchemotherapeutictoxicity
The Grade 3/4 toxicity of chemotherapy regimens and the genotypes were reported in Table 4. Overall, myelosuppression, emesis, oral stomatitis, diarrhea were the predominant toxicity. There was no difference in toxicity according to comparisons among the three regimens.

Table 4 Grade 3 and 4 toxicity of chemotherapy regimens and the genotypes
Patients with the MTHFR C677T T/T genotypes had a significantly higher incidence of grade 3/4 emesis (66.7%) and stomatitis (30.0%) than patients with the C/T or C/C genotypes (41.3%, 9.8%;P=0.011, 0.003). On analysis, patients with the MTHFR A1298C A/A genotype had a higher incidence of grade 3/4 stomatitis (17.2%) and diarrhea (13.9%) than patients with A/C and C/C genotypes (3.9%, 2.0%;P=0.025, 0.026). There was no difference in toxicity for patients with the TS 3’-UTR genotypes.
On further analysis, in FP and DCF regimens, MTHFR C677T T/T genotypes had a significantly higher incidence of grade 3/4 stomatitis (33.3%, 4 of 12 patients;37.5%,3 of 8 patients) than patients with the C/T or C/C genotypes (4.3%, 2 of 47 patients,P=0.013; 5.7%,2 of 35 patients,P=0.037). MTHFR C677T T/T genotypes in DCF had a significantly higher incidence of grade 3/4 diarrhea than patients with the C/T or C/C genotypes (37.5%, 3 of 8 patients vs 5.7%, 2 of 35 patients;P=0.037). MTHFR A1298C A/A genotypes in FOLFOX regimen had a significantly higher incidence of grade 3/4 stomatitis than patients with the A/C or C/C genotypes (23.4%, 11 of 47 patients vs 4.2%, 1 of 24 patients;P=0.049).
4 Discussion
The goal of many pharmacogenetic investigations is to identify genetic markers that predict either drug toxicity or efficacy. In recent years, several pharmacogenetic analyses have been performed to examine the association between MTHFR C677T and A1298C and the outcome of patients treated with fluoropyrimidine-based chemotherapy. A majority of investigations have been conducted in advanced colorectal cancer patients receiving 5-FU-based therapy mainly as first-line treatment. Some of these studies, considering both tumor material and normal tissue, reported that MTHFR C677T genetic variant, but not A1298C, is significantly associated with increased tumor response rate and time to progression to 5-FU-based therapy[17-18].
At present, only a few studies analyzed the role of MTHFR polymorphisms on efficacy after fluoropyrimidine therapy in gastric cancer. Ruzzo A et al. evaluated the MTHFR polymorphisms in 175 patients with advanced gastric cancer treated with fluorouracil/cisplatin palliative chemotherapy. The response rate was not related to MTHFR C677T genotype (response rate: 38%, 42% and 37% in C/C, C/T and T/T, respectively;P=0.20)[19]. In the clinical study (Lee et al.), the relationship between the C677T polymorphism and survival to fluoropyrimidine-based chemotherapy in 40 patients with gastric cancer was determined. MTHFR C677T polymorphisms were not associated with better survival of gastric cancer patients with 5-FU chemotherapy[20]. On the contrary, Huang and colleagues analyzed the MTHFR and TS 3’-UTR polymorphisms in 116 patients with gastric cancer treated with 5-FU-based adjuvant chemotherapy. It was found that the overall survival (OS) of the TS +6 bp/+6 bp genotype was significantly shorter than those in patients with the -6 bp/-6 bp (P= 0.017) and +6 bp/-6 bp (P= 0.022) genotype. The relapse-free survival and OS of the MTHFR C677T C/C genotype were significantly worse than those in patients with the T/T or C/T genotype (P= 0.043 and 0.040, respectively)[21].
In a preliminary report we performed before, the response rate for C677T T/T genotype was 83.3% compared with 8.3% and 15.2% for C677T C/C and C/T genotype, respectively (P<0.001)[22]. In the present work, we found the correlation between the MTHFR genotype and the outcome of chemotherapy. The RR of the MTHFR C677T T/T genotype was significantly higher than that of the C/C and C/T genotypes (73.3% vs 28.0%;P=0.000). A higher RR was observed in MTHFR A1298C A/A genotype compared with the C/C and A/C genotypes (41.8% vs 21.6%,P=0.011). In analyses of the different treatment regimens, the RR of the MTHFR C677T T/T genotypes in the FOLFOX or FP regimens was significantly higher than that of the C/C and C/T genotypes, but no difference in the DCF regimen. Also, we found no correlation between the MTHFR A1298C genotype and the outcome of chemotherapy when compared with the three regimens.
Of interest is the finding that the RR of DCF regimen in the MTHFR C677T C/C and C/T genotype was significantly higher than that of the FOLFOX and FP regimens in this cohort, but no difference in the T/T genotype. Paz et al. showed that MTHFR T allele was associated with global genomic hypomethylation[23]. Friso et al. indicated that the MTHFR C677T polymorphism influences peripheral blood mononuclear cell DNA methylation status through an interaction with folate status[24]. Hypomethylation of DNA has mechanistic implications and can lead to gene activation. Several studies demonstrated that methylation of CHFR correlates specifically with sensitivity to microtubule inhibitors[25-26]. The incidence of cell death after exposure to docetaxel was markedly higher among the methylated gastric cell lines than that among the unmethylated ones[25]. Therefore, methylation of the drug sensitivity gene correlates with enhanced sensitivity to anticancer drug (eg. docetaxel and paclitaxel). Our research showed that adding docetaxle to a regimen of cisplatin and 5-fluorouracil in the patients of MTHFR C allele provided benefits with regard to response rate.
The TS gene polymorphism attracts considerable attention in recent years. Several studies indicated that the number of repeats is predictive of the effect of FU. Kawakami et al. found the presence of at least one high TS expression genotype, TS 5’UTR 3G/3G, 3G/3C, 2R/3G or 3’UTR +6 bp/+6 bp, predicted for worse overall survival in patients with gastric cancer receiving adjuvant fluoropyrimidine therapies compared with patients without any high expression genotypes[27]. Sharma and colleagues showed that TS genotypes did not influence clinical response or overall survival in patients with metastatic colorectal cancer to capecitabine therapy[28]. One reason for the contradictory results may be that these studies used normal tissue, which is different from the most previous investigations applying tumor tissue. In our study we used normal tissue, the RR of the -6/-6 bp and -6/+6 bp genotypes in TS 3’UTR was significantly higher than that of the +6/+6 bp genotype (40.3% vs 17.6%,P=0.014). For Chinese people, therefore, it is clinically more valuable to analyze TS 3’-UTR polymorphism.
Many studies also observed a relationship between gene polymorphism and chemotherapeutic side effects. In a study of advanced breast cancer, MTHFR C677T and A1298C genotypes were not related to toxicity from capecitabine therapy[29]. In another study of advanced colorectal cancer patients receiving fluoropyrimidine-based therapies, similar incidences of neutropenia, diarrhea, nausea, vomiting, and mucositis were observed for patients with differing C677T genotypes[30]. In a recent study of metastatic colorectal cancer to capecitabine therapy, Sharma et al. showed that patients with the MTHFR C677 T/T genotype had a lower incidence of grade 2/3 toxicity than patients with C/T and C/C genotypes (P<0.05). Patients with the A/A genotype suffered less grade 2/3 toxicity than patients with A/C and C/C genotypes (P<0.01), whereas the TS genotypes had no influence[28]. Our work showed that SNPs in MTHFR are predictive of grade 3/4 toxicity to fluoropyrimidine-based therapies. Patients with the MTHFR C677T T/T genotypes had a significantly higher incidence of grade 3/4 emesis and stomatitis than patients with the C/T or C/C genotypes. Patients with the MTHFR A1298C A/A genotype had a higher incidence of grade 3/4 stomatitis and diarrhea than patients with A/C and C/C genotypes. The value of TS polymorphisms in predicting outcome to 5-FU-based therapy was not clearly established and requires further investigation.
Our studies indicated that detection of MTHFR and TS 3’-UTR polymorphism can be used to guide the choice of 5-FU based chemotherapy on advanced gastric cancer. It is also hoped that the present pilot study will stimulate such studies, particularly as part of large clinical trials, which could eventually result in a strategy for selecting patients with advanced gastric cancer based on pharmacogenetic analysis.
[1] Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world[J]. J Clin Oncol, 2006,24:2137-2150.
[2] Parkin DM, Pisani P, Ferlay J. Estimates of the worldwide incidence of 25 major cancers in 1990[J]. Int J Cancer, 1999,80(6):827-841.
[3] Berardi R, Scartozzi M, Romagnoli E, et al. Gastric cancer treatment: a systematic review[J]. Oncol Rep, 2004,11:911-916.
[4] Wagner AD, Grothe W, Haerting J, et al. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data[J]. J Clin Oncol, 2006,24(18):2903-2909.
[5] Van Cutsem E, Moiseyenko VM, Tjulandin S, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group[J]. J Clin Oncol, 2006,24:4991-4997.
[6] Ajani JA, Moiseyenko VM, Tjulandin S, et al. Clinical benefit with docetaxel plus fluorouracil and cisplatin compared with cisplatin and fluorouracil in a phase III trial of advanced gastric or gastroesophageal cancer adenocarcinoma: the V-325 Study Group[J]. J Clin Oncol, 2007,25:3205-3209.
[7] Frosst P, Blom HJ, Milos R, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase[J]. Nat Genet, 1995,10:111-113.
[8] Weisberg I, Tran P, Christensen B, et al. A second genetic polymorphism in methylenetetrahydrofolate reductase (MTHFR) associated with decreased enzyme activity[J]. Mol Genet Metab, 1998,64:169-172.
[9] Sohn KJ, Croxford R, Yates Z, et al. Effect of the methylenetetrahydrofolate reductase C677T polymorphism on chemosensitivity of colon and breast cancer cells to 5-fluorouracil and methotrexate[J]. J Natl Cancer Inst, 2004,96:134-144.
[10] Etienne MC, Ilc K, Formento JL, et al. Thymidylate synthase and methylenetetrahydrofolate reductase gene polymorphisms: relationships with 5-fluorouracil sensitivity[J]. Br J Cancer, 2004,90:526-534.
[11] Kaneda S, Takeishi K, Ayusawa D, et al. Role in translation of a triple tandemly repeated sequence in the 5’-untranslated region of human thymidylate synthase mRNA[J]. Nucleic Acids Res, 1987,15:1259-1270.
[12] Trinh BN, Ong CN, Coetzee GA, et al. Thymidylate synthase: a novel genetic determinant of plasma homocysteine and folate levels[J]. Hum Genet, 2002,111:299-302.
[13] Lu JW, Gao CM, Wu JZ, et al. Polymorphism in the 3’-untranslated region of the thymidylate synthase gene and sensitivity of stomach cancer to fluoropyrimidine-based chemotherapy[J]. J Hum Genet, 2006,51:155-160.
[14] Miller AB, Hoogstraten B, Staquet M, et al. Reporting results of cancer treatment[J]. Cancer, 1981,47:207-214.
[15] Cao HX, Gao CM, Takezaki T, et al. Genetic polymorphisms of methylenetetrahydrofolate reductase and susceptibility to colorectal cancer[J]. Asian Pac J Cancer Prev, 2008,9:203-208.
[16] Gao CM, Takezaki T, Wu JZ, et al. Polymorphisms in thymidylate synthase and methylenetetrahydrofolate reductase genes and the susceptibility to esophageal and stomach cancer with smoking[J]. Asian Pac J Cancer Prev, 2004,5:133-138.
[17] Jakobsen A, Nielsen JN, Gyldenkerne N, et al. Thymidylate synthase and methylenetetrahydrofolate reductase gene polymorphism in normal tissue as predictors of fluorouracil sensitivity[J]. J Clin Oncol, 2005,23:1365-1369.
[18] Etienne MC, Formento JL, Chazal M, et al. Methylenetetrahydrofolate reductase gene polymorphisms and response to fluorouracil-based treatment in advanced colorectal cancer patients[J]. Pharmacogenetics, 2004,14:785-792.
[19] Ruzzo A, Graziano F, Kawakami K, et al. Pharmacogenetic profiling and clinical outcome of patients with advanced gastric cancer treated with palliative chemotherapy[J]. J Clin Oncol, 2006,24:1883-1891.
[20] Lee J, Jeong CK, Hong SP, et al.Clinical significance of thymidylate synthase and methylenetetrahydrofolate reductase gene polymorphism in Korean patients with gastric cancer[J]. Korean J Gastroenterol, 2005,46:32-38.
[21] Huang ZH, Hua D, Li LH. The polymorphisms of TS and MTHFR predict survival of gastric cancer patients treated with fluorouracil-based adjuvant chemotherapy in Chinese population[J]. Cancer Chemother Pharmacol, 2009,63:911-918.
[22] Lu JW, Gao CM, Wu JZ, et al. Relationship of methylenetetrahydrofolate reductase C677T polymorphism and chemosensitivity to 5-fluorouracil in gastric carcinoma [J]. Ai Zheng, 2004,23:958-62.
[23] Paz MF, Avila S, Fraga MF, et al. Germ-line variants in methyl-group metabolism genes and susceptibility to DNA methylation in normal tissues and human primary tumors[J]. Cancer Res, 2002,62:4519-4524.
[24] Friso S, Choi SW, Girelli D, et al. A common mutation in the 5,10-methylenetetrahydrofolate reductase gene affects genomic DNA methylation through an interaction with folate status[J]. Proc Natl Acad Sci, 2002,99:5606-5611.
[25] Satoh A, Toyota M, Itoh F, et al. Epigenetic inactivation of CHFR and sensitivity to microtubule inhibitors in gastric cancer[J]. Cancer Res, 2003,63:8606-8613.
[26] Yanokura M, Banno K, Kawaguchi M, et al. Relationship of aberrant DNA hypermethylation of CHFR with sensitivity to taxanes in endometrial cancer[J]. Oncol Rep, 2007,17:41-48.
[27] Kawakami K, Graziano F, Watanabe G, et al. Prognostic role of thymidylate synthase polymorphisms in gastric cancer patients treated with surgery and adjuvant chemotherapy[J]. Clin Cancer Res, 2005,11:3778-3783.
[28] Sharma R, Hoskins JM, Rivory LP, et al. Thymidylate synthase and methylenetetrahydrofolate reductase gene polymorphisms and toxicity to capecitabine in advanced colorectal cancer patients[J]. Clin Cancer Res, 2008,14:817-825.
[29] Largillier R, Etienne-Grimaldi MC, Formento JL, et al. Pharmacogenetics of capecitabine in advanced breast cancer patients[J]. Clin Cancer Res, 2006,12:5496-5502.
[30] Cohen V, Panet-Raymond V, Sabbaghian N, et al. Methylenetetrahydrofolate reductase polymorphism in advanced colorectal cancer: a novel genomic predictor of clinical response to fluoropyrimidine-based chemotherapy[J]. Clin Cancer Res, 2003,9:1611-1615.

