乳酸菌细菌素纯化技术的检测及研究进展
刘文丽,张兰威,*,John shi,易华西
(1.哈尔滨工业大学食品科学与工程学院,黑龙江哈尔滨 150090;2.加拿大农业部圭尔夫食品研究中心,安大略圭尔夫N1G5C9)
乳酸菌细菌素纯化技术的检测及研究进展
刘文丽1,张兰威1,*,John shi2,易华西1
(1.哈尔滨工业大学食品科学与工程学院,黑龙江哈尔滨 150090;2.加拿大农业部圭尔夫食品研究中心,安大略圭尔夫N1G5C9)
细菌素可以抑制大量的食品源腐败菌和致病菌,因此,近年来受到国内外广大学者的关注,其中乳酸菌细菌素因其安全性尤为受到关注。然而要使细菌素更好地应用到生物技术领域,揭示乳酸菌细菌素的生物化学结构和作用位点就显得尤为重要,而这又是建立在乳酸菌细菌素的纯化基础上。文章结合乳酸菌细菌素纯化技术及检测的最新研究进展,讨论乳酸菌细菌素在食品和医疗领域的发展潜力。
乳酸菌,细菌素,分离,纯化,检测
近年来,随着人们生活水平和保健意识的提高,天然生物防腐剂代替化学防腐剂成为未来的发展方向。乳酸菌细菌素由于能够有效地抑制食品中的腐败菌和致病菌,受到了越来越多学者的关注,并在体内、体外实验得到了一定的验证[1-3]。但目前仅有Nisin被允许合法地以部分纯化的方式应用到食品中。
1 乳酸菌细菌素的分离纯化
乳酸菌细菌素应用到工业化生产的障碍主要归因于两个方面:a.细菌素在培养基中的产量很少;b.乳酸菌细菌素提纯费用高。复合培养基中本身含有的疏水性肽杂质的干扰,这就使乳酸菌细菌素的分离纯化需要一个复杂而又高耗费的过程,因此乳酸菌细菌素的分离纯化是目前急需解决的问题。关于乳酸菌细菌素的分离纯化,研究者们也相继进行了大量的研究[4-10],总结其方法大概包括以下两个步骤:a.浓缩培养基上清液中的肽;b.利用色谱技术分离纯化。
1.1 细菌素的浓缩
细菌素是细菌在生长过程中分泌到培养基中的产物,培养基上清液中细菌素的产量很低,所以分离乳酸菌细菌素的第一个步骤就是浓缩培养基上清液中的肽,主要方法包括盐析、有机溶剂、酸提取、细胞吸附-解析法和膜分离技术等。
1.1.1 盐析 目前比较常用的盐析方法主要是硫酸铵沉淀法,其原理是利用硫酸铵饱和溶液使培养基中的蛋白质凝聚从而从培养基中析出,其存在的缺点是在高盐浓度下,乳酸菌细菌素的活性降低。具体操作方法一般是将培养18h的产细菌素乳酸菌离心(9000×g,10m in,4℃),用70%饱和硫酸铵沉淀细胞悬浮液的蛋白[11](一些细菌素能够以较低浓度的硫酸铵沉淀,或者以小范围的饱和态就能将上清液中的蛋白沉淀下来)。细胞悬浮液在4℃过夜,并且用磁力搅拌器搅拌,盐析出的蛋白1000×g离心20m in,溶解在10mmol/L pH 7.0磷酸缓冲液或蒸馏水中,悬浮液用分子量1200透析膜或者是2500~3500的透析盒在4℃的磷酸缓冲液中透析12h。Soumaya Messaoudi等[12]利 用 盐 析 的 方 法 分 离 Lactobacillus salivarius SMXD51产生的细菌素粗蛋白,取得了很好的效果,蛋白回收率为1.36%,细菌素活性为160AU/m L,总活性1.6×104AU/m L,比活性117.6AU/mg。Ramakrishnan Srinivasan等[13]也利用35%~65%的硫酸铵回收Lactobacillus rhamnosus L34产生的细菌素,产率达到了12.32%,纯化倍数是1.79,比活性是39.77AU/mg。N Ravi Sankar等[14]利用70%的硫酸铵回收从牛奶中分离出的Lactobacillus plantarum产生的细菌素,效果显著,回收率达到了63.1%,纯化倍数是5.3倍,比活性155.7AU/mg。E Vera Pingitore等[15]将此方法应用到了salivaricin CRL 1328的分离纯化中,取得了的效果如表1所示。
1.1.2 有机溶剂和真空浓缩[16-17]主要是利用正丙醇和氯化钠的混合物抽提,丙酮沉淀,但收率并不理想。据报道,这种方法已经成功地应用到Lactococcin B、Pediocin PA1和Lacticin Q的浓缩中,一般具体操作是用3倍体积冷丙酮处理无细胞上清液,在-30℃过夜,然后离心回收蛋白。Dong M in Chung等[18]利用3倍体积冷丙酮在-20℃处理Pediococcus acidilactici WRL-1产生的细菌素2h,在液相中得到95.2%±2.5%片球菌素活性,片球菌素通过蒸发有机溶剂的方法获得。
1.1.3 细胞吸附-解析法 Yang等[19]提出利用细胞吸附-解析法来回收乳酸菌细菌素,主要是根据大多数细菌素在一个特定的pH范围下能够完全吸附在细胞表面,而在较低pH下自动从细胞表面脱落下来的原理,虽然这种方法具有高效、简便、无污染等特点,但进行大规模工业化生产,仍需进一步研究。具体操作方法为菌株在相应培养基上培养过夜,70℃加热25m in使微生物失去活性。培养基的pH用5mol/L的NaOH调节pH 7.0,使细菌素吸附到细胞上,离心收集细胞,用原始培养基40倍体积的5mmol/L磷酸钠缓冲溶液(pH 7.0)洗两次,用0.1mol/L NaCl(pH 2.0)重悬,在4℃下搅拌1h从细胞表面释放细菌素分子,释放的细菌素在4℃14000×g离心30min,上清液用于下一步研究。运用这种方法来回收salivaricin CRL 1328,收率很低,原因可能是salivaricin CRL 1328是两组分细菌素,此方法的优点主要是减少了杂蛋白的干扰。用细胞膜吸附提取salivaricin CRL 1328[15]的实例见表2。
1.1.4 酸提取和吸附剂吸附 Daba等[20]和Coventry等[21]又提出了利用酸提取和吸附剂吸附乳酸菌细菌素,但效果并不理想,虽然得到相对较高的纯品,但必须通过透析的步骤,这将大大降低肽的回收率。
1.1.5 膜分离技术 Daoudi L等[22]提出利用膜分离技术浓缩细菌素,此方法比较适合大规模工业化生产,但其弊端是容易造成膜污染,而膜不易清洗,从而降低膜性能,使成本提高。陈琳等[23]利用采用截留分子质量1ku和3ku的超滤膜分离植物乳杆菌KLDS1.0391发酵液中的细菌素,研究主要超滤操作参数对膜通量和细菌素效价的影响,确定超滤法分离细菌素的条件。结果表明,超滤法分离物乳杆菌KLDS 1.0391发酵液中细菌素的最适条件为:采用截留分子质量为3ku的超滤膜进行分离,超滤温度30℃,操作压力0.140MPa,超滤时间120m in,细菌素的效价由574.99IU/m L提高到1849.40IU/m L,比活力为185.96IU/mg,纯化倍数为8.0,浓缩倍数为3.0。
1.2 色谱分离
由于乳酸菌细菌素的浓缩步骤仅仅能减少工作量,并不能得到高纯品,所以,后续的色谱分离步骤还是必不可少的,主要包括离子交换色谱、疏水层析、凝胶过滤和反相色谱。由于乳酸菌细菌素带正电荷并具有疏水性残基,所以常用的分离方法是利用阳离子交换色谱将乳酸菌细菌素吸附到柱子上,然后再用NaCl梯度洗脱,最后再利用反相色谱将活性片段浓缩,来提高样品的纯度和减少培养基中杂质肽的污染。这种方法虽然被广泛使用,但其缺点是耗时长、回收率低,并不适合大量的纯化,为了解决这个问题,研究者们相继提出了不同的方法。Guyonnet等[24]提出了三步法提纯Ⅱa类乳酸菌细菌素和抗李斯特细菌素灭菌的温度和程度,来削弱杂质肽的颜色,再通过阳离子交换色谱和反相色谱纯化乳酸菌细菌素,这种方法产率虽然不稳定(10%~60%),但乳酸菌细菌素的纯度可以达到95%。Uteng等[25]提出了两步法提纯Ⅱa类乳酸菌细菌素,主要是利用SPSepharose Fast Flow阳离子交换柱吸附带正电荷的细菌素,然后用1mol/L NaCl洗脱,最后利用反相色谱回收活性峰,样品回收率从原来的10%~20%增加到90%~100%。Lasta Samar等[26]利用两步法分离纯化Lactococcus lactis BMG6.14产生的细菌素,首先利用硫酸铵沉淀提取出细菌素的粗提物,然后,利用两步反相色谱得到细菌素纯品,取得了较好的回收效果。免疫性亲和性色谱也是一个快速和纯度较高的新型纯化细菌素的方法,但这种方法需要找到合适的抗体,有待于未来进一步研究和开发。

表1 硫酸铵沉淀及固相提取salivaricin CRL 1328[15]Table 1 The extraction of salivaricin CRL 1328 with ammonium sulfate precipitation and solid phase extraction[15]
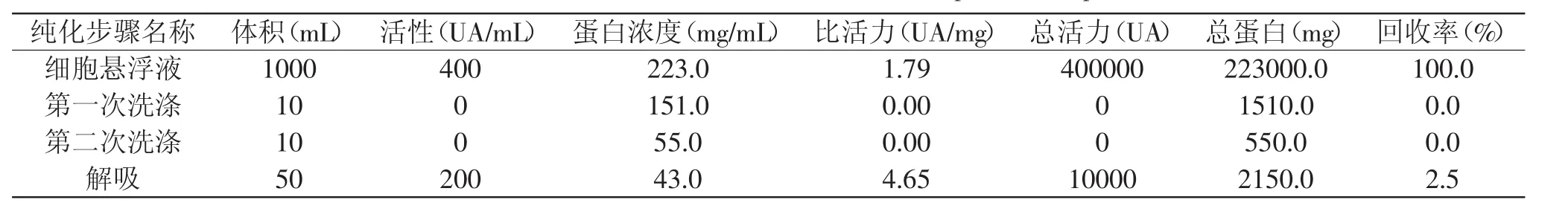
表2 细胞膜吸附提取salivaricin CRL 1328[12]Table 2 The extraction of salivaricin CRL 1328 with absorption-desorptionmethod[12]
目前已经报道的乳酸菌细菌素的分离纯化方法见表3~表6。
2 乳酸菌细菌素的检测
在整个分离纯化过程中,细菌素抗菌能力的检测起着至关重要的作用。目前相应的方法主要有琼脂扩散法和点种法、微量稀释法、SDS-PAGE和免疫化学法。
2.1 琼脂扩散法和点种法
目前,使用最广泛的检测方法是琼脂扩散法和点种法,两种方法都是将各个阶段的样品放在带指示菌的培养基平板上,通过抑菌圈的大小来判断抑菌能力。不同之处在于,第一种方法是将各个阶段的样品接到牛津杯内或打孔器打的孔内;而第二种方法是将各个阶段的样品点在敏感细胞上[49]。周配东等[50]利用双层琼脂扩散法在豆腐乳中筛选出一株具有广谱抗菌活性的乳酸菌,经16S rDNA鉴定该菌株为植物乳杆菌。岳喜庆等[51]利用琼脂扩散法在自制的发酵肉、酸菜汁、黄瓜汁、大麦汁、玉米汁等以及市售鲜奶、传统干酪、红茶中筛选出一株对单核细胞增生李斯特菌和大肠杆菌均有抑制作用的乳酸菌,经排除有机酸、过氧化氢等干扰及蛋白酶敏感后,确定产生的抑菌物质为蛋白类物质。韩雪等[52]利用琼脂扩散法从多种发酵材料中筛选出5株对枯草芽孢杆菌和大肠杆菌均有抑制作用的细菌素。A Tosukhowong等[53]利用琼脂扩散法分离出一株对Listeriamonocytogen有抑制作用的乳球菌。
2.2 微量稀释法
微量稀释法是将一定浓度的指示菌和浓缩的乳酸菌发酵上清液混合在96孔平板内,然后放在培养箱中培养,通过检测每个孔发酵前后的OD值变化来判断乳酸菌的抑菌能力,此方法具有样品用量小、操作简单等优点,缺点是平行性不是很高。

表3 Ⅰ乳类酸菌细菌素的纯化方法Table 3 The separation and purificationmethod of ClassⅠLAB bacteriocins
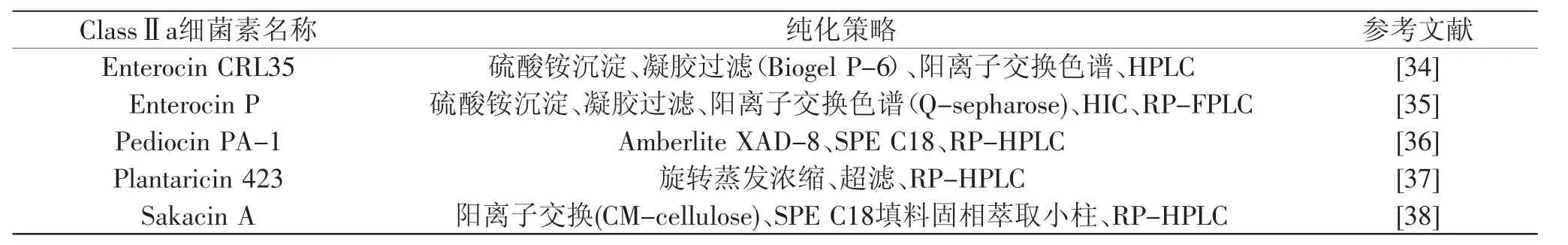
表4 Ⅱa类乳酸菌细菌素的纯化方法Table 4 The separation and purificationmethod of ClassⅡa LAB bacteriocins

表5 Ⅱb类乳酸菌细菌素的纯化方法Table 5 The separation and purificationmethod of ClassⅡb LAB bacteriocins

表6 Ⅱc类乳酸菌细菌素的纯化方法Table 6 The separation and purificationmethod of ClassⅡc LAB bacteriocins
2.3 SDS-PAGE
SDS-PAGE蛋白质变性电泳是检测部分纯化和完全纯化细菌素分子量的有力手段[54],缺点主要是在染色期间,疏水性小肽容易在聚丙烯酰胺凝胶上扩散开来,导致没有条带。为了克服这个问题,很多学者做了相应的研究,Bhunia等[55-60]提出了凝胶覆盖法或生物量法,其原理是用带指示菌的培养基覆盖染色和不染色的胶,结果最下边的那条带如果产生抑菌圈,就说明这条带具有抑菌活性,这种方法能够直接检测细菌素抑菌能力,但是其取决于纯化过程中细菌素的产量及染色方法(考马斯亮蓝、银染法、SYPRO-Ruby荧光染色、新蛋白凝胶荧光染色),新蛋白凝胶荧光染色不仅具有染色的优势,而且能够从胶上切割下条带,有利于下一步质谱分析及序列分析,而且按照标准步骤直接将条带转移到PVDF膜上也是可能的。
2.4 免疫化学
免疫化学法是一种新兴的定量检测细菌素抑菌活性的方法,具有效率高、敏感和专一的优点,但却不容易找到合适的抗体,且成本较高,不适宜大规模应用。
以上乳酸菌细菌素抑菌能力的检测手段各有各的优缺点,还不是很完善,还需要进一步开发一种效率高、专一、成本低的检测方法,从而有利于发现新的细菌素,更好地为乳酸菌细菌素在医疗及食品行业的应用服务。
3 展望
乳酸菌细菌素的分离纯化在过去二十年已经完成了一个非常重要的阶段,但总结起来,目前,乳酸菌的分离纯化仍然需要进一步提高,首先,分离过程中尽量缩短时间和利用简单的工艺来保证乳酸菌细菌素的活性;其次,乳酸菌细菌素的收率和纯度尚不能同时达到要求,来进行大规模工业化生产,最后,分离纯化过程所附带的环境污染问题仍需考虑。相信随着未来乳酸菌分离纯化工艺的进一步完善,更多的乳酸菌细菌素将被合法使用,更多的乳酸菌细菌素将进行大规模的工业化生产,乳酸菌细菌素将会广泛地应用到食品保藏、防腐、医疗等各个领域。
[1]Fim land G,Johnsen L,Dalhus B,et al.Pediocin-like antimicrobial peptides(class IIa bacteriocins)and their immunity proteins:biosynthesis,structure,andmode ofaction[J].JPept Sci,2005(11):688-696.
[2]Lawton E M,Ross RP,Hill C,Cotter P D.Two-peptide lantibiotics:amedical perspective[J].Mini Rev Med Chem,2007(7):1236-1247.
[3]Castellano P,Belfiore C,Fadda S,et al.A review of bacteriocinogenic lactic acid bacteria used as bioprotective cultures in fresh meat produced in Argentina[J].Meat Sci,2008(9):483-499.
[4]Carolissen-Mackay V,Arendse G,Hastings JW.Purification of bacteriocins of lactic acid bacteria:problems and pointers[J]. Int JFood Microbiol,1997,34:1-16.
[5]Suarez A M,Azcona J I,Rodriguez JM,et al.One-step purification of nisin A by immunoaffinity chromatography[J]. Appl Environ Microbiol,1997,63:4990-4992.
[6]Parente E,Ricciardi A.Production,recovery and purification of bacteriocins from lactic acid bacteria[J].Appl Microbiol Biotechnol,1999,52:628-638.
[7]Guyonnet D,Fremaux C,Cenatiempo Y,et al.Method for rapid purification of class IIa bacteriocins and comparison of their activities[J].Appl Environ Microbiol,2000,66:1744-1748.
[8]UtengM,Hauge H H,Brondz I,etal.Rapid two-step procedure for large-scale purification of pediocin-like bacteriocins and other cationic antimicrobial peptides from complex culture medium[J].Appl Environ Microbiol,2002,68:952-956.
[9]Berjeaud J M,Cenatiempo Y.Purification of antilisterial bacteriocins[J].Methods Mol Biol,2004,268:225-233.
[10]Saavedra L,Castellano P,Sesma F.Purification ofbacteriocins produced by lactic acid bacteria[J].Methods Mol Biol,2004a,268:331-336.
[11]JSambrook,E F Fritsch,TManiatis.A Laboratory Manual edited by Cold Spring Laboratory Press[J].Molecular Cloning,1989.
[12]Ramakrishnan Srinivasan,Dinesh K.Kumawat,Sunil Kumar,et al.Purification and characterization of a bacteriocin from Lactobacillus rhamnosus L34[J].Ann Microbiol,2012(8):132-133.
[13]N Ravi SankarV,Deepthi Priyanka,P Srinivas Reddy,et al.Purification and Characterization of Bacteriocin Produced by Lactobacillus plantarum Isolated from Cow Milk[J].International Journal of Microbiological Research,2012(2):133-137.
[14]Soumaya Messaoudi, Gilles Kergourlay, Michèle Dalgalarrondo,et al.Purification and characterization of a new bacteriocin active against Campylobacter produced by Lactobacillus salivarius SMXD51[J].Food Microbiology,2012:1-6.
[15]E Vera Pingitore,E Salvucci,F Sesm,et al.Different strategies for purification of antimicrobial peptides from Lactic Acid Bacteria(LAB)[J].Communicating Current Research and Educational Topics and Trends in Applied Microbiology,2007:557-568.
[16]Burianek L L,Yousef A E.Solvent extraction of bacteriocins from liquid cultures[J].Lett ApplMicrobiol,2000,31:193-197.
[17]Taylor T M,Davidson P M,Zhong Q.Extraction of nisin from a 2.5%commercialnisin productusingmethanoland ethanol solutions[J].JFood Prot,2007,70:1272-1276.
[18]Dong Min Chung,Ki Eun Kim,Seong-Yeop Jeong,et al. Rapid concentration of some bacteriocin-like compounds using an organic solvent[J].Food Science And Biotechnology,2011(20):1457-1459.
[19]Yang R,Johnson M C,Ray B.Novelmethod to extract large amounts of bacteriocins from lactic acid bacteria[J].Appl Environ Microbiol,1992,58:3355-3359.
[20]Daba H,Lacroix C,Huang J,et al.Simple method of purification and sequencing of a bacteriocin produced by Pediococcus acidilactici UL5[J].JAppl Bacteriol,1994,77:682-688.
[21]Coventry M J,Gordon J B,Alexander M.A food-grade process for isolation and partial purification of bacteriocins of lactic acid bacteria that uses diatomite calcium silicate[J].Appl Environ Microbiol,1996,62:1764-1769.
[22]L Daoudi,C Turcotte,C Lacroix,et al.Production and characterization of anti-nisin Zmonoclonal antibodies:suitability for distinguishing active from inactive forms through a competitive enzyme immunoassay[J].Appl Microbiol Biotechnol,2001,56:114-119.
[23]陈琳,孟祥晨.超滤法分离植物乳杆菌KLDS1.0391发酵液中的细菌素[J].食品科学,2011,32(5):198-201.
[24]Guyonnet D,Fremaux C,Cenatiempo Y,et al.Method for rapid purification of class IIa bacteriocins and comparison of their activities[J].Appl Environ Microbiol,2000,66:1744-1748.
[25]Uteng M,Hauge H H,Brondz I,et al.Rapid two-step procedure for large-scale purification ofpediocin-likebacteriocins and other cationic antimicrobial peptides from complex culture medium[J].Appl Environ Microbiol,2002,68:952-956.
[26]Piard JC,Muriana PM,Desmazeaud M J,et al.Purification and partial characterization of lacticin 481,a lanthioninecontaining bacteriocin produced by Lactococcus lactis subsp. lactis CNRZ 481[J].Appl Environ Microbiol,1992,58:279-284.
[27]Lasta Samar,OuzariHadda,Andreotti Nicolas,et al.Lacticin LC14,a New Bacteriocin Produced by Lactococcus lactis BMG6.14:Isolation,Purification and Partial Characterization[J]. Infectious Disorders-Drug Targets,2012(12):316-325.
[28]McAuliffe O,Ryan M P,Ross R P,et al.Lacticin 3147,a broad spectrum bacteriocin which selectively dissipates the membrane potential[J].Appl Environ Microbiol,1998,64:439-445.
[29]Suarez A M,Azcona J I,Rodriguez JM,et al.One-step purification of nisin A by immunoaffinity chromatography[J]. Appl Environ Microbiol,1997,63:4990-4992.
[30]Coughlin R T,Crabb JH.Patent US6794181.2002[P].
[31]Cotter PD,Hill C.PatentWO/2009/135945.2009[P].
[32]Zendo T,Koga S,Shigeri Y,et al.Lactococcin Q,a novel two-peptide bacteriocin produced by Lactococcus lactis QU4[J]. Appl Environ Microbiol,2006,72:3383-3389.
[33]Prioult G,Turcotte C,Labarre L,et al.Rapid purification of nisin Z using specific monoclonal antibody-coated magnetic beads[J].Int Dairy J,2000,10(9):627-633.
[34]FariasM E,FariasRN,de RuizHolgado AP,etal.Purification and N-terminal amino acid sequence of Enterocin CRL35,a‘pediocin-like’bacteriocin produced by Enterococcus faecium CRL35[J].Lett ApplMicrobiol,1996(22):417-419.
[35]Cintas LM,Casaus P,Havarstein L S,et al.Biochemical and genetic characterization ofenterocin P,a novel sec-dependent bacteriocin from Enterococcus faecium P13 with a broad antimicrobial spectrum[J].Appl Environ Microbiol,1997,63:4321-4330.
[36]Kaur K,Andrew LC,WishartDS,etal.Dynamic relationships among type IIa bacteriocins:temperature effects on antimicrobial activity and on structure of the C-terminal amphipathic alpha helix as a receptor-binding region[J].Biochemistry,2004,43:9009-9020.
[37]Van Reenen C A,Dicks L M,Chikindas M L.Isolation,purification and partial characterization of plantaricin 423,a bacteriocin produced by Lactobacillus plantarum[J].J Appl Microbiol,1998,84:1131-1137.
[38]Guyonnet D,Fremaux C,Cenatiempo Y,et al.Method for rapid purification of class IIa bacteriocins and comparison of their activities[J].Appl Environ Microbiol,2000,66:1744-1748.
[39]Maldonado-Barragan A,Caballero-Guerrero B,Jimenez E,et al.Enterocin C,a class IIb bacteriocin produced by E. faecalis C901,a strain isolated from human colostrum[J].Int J Food Microbiol,2009,133:105-112.
[40]Balla E,Dicks L M,Du Toit M,et al.Characterization and cloning of the genes encoding enterocin 1071A and enterocin 1071B,two antimicrobial peptides produced by Enterococcus faecalis BFE 1071[J].Appl Environ Microbiol,2000,66:1298-1304.
[41]Muriana P M,Klaenhammer T R.Purification and partial characterization of lactacin F,a bacteriocin produced by Lactobacillus acidophilus 11088[J].Appl Environ Microbiol,1991,57:114-121.
[42]Muriana P M,Klaenhammer T R.Purification and partial characterization of lactacin F, a bacteriocin produced by Lactobacillusacidophilus11088[J].ApplEnviron Microbiol,1999,57:114-121.
[43]Muriana P M,Klaenhammer T R.Purification and partial characterization of lactacin F, a bacteriocin produced by Lactobacillus acidophilus 11088[J].Appl Environ Microbiol,1991,57:114-121.
[44]Zendo T,Koga S,Shigeri Y,et al.Lactococcin Q,a novel two-peptide bacteriocin produced by Lactococcus lactis QU4[J]. Appl Environ Microbiol,2006,72:3383-3389.
[45]Jimenez-Diaz R,Ruiz-Barba J L,Cathcart D P,et al. Purification and partial amino acid sequence of plantaricin S,a bacteriocin produced by Lactobacillus plantarum LPCO10,the activity of which depends on the complementary action of two peptides[J].Appl Environ Microbiol,1995,61:4459-4463.
[46]Anderssen E L,Diep D B,Nes I F,et al.Antagonistic activity of Lactobacillus plantarum C11:two new two-peptide bacteriocins,plantaricins E F and J K,and the induction factor plantaricin A[J].Appl Environ Microbiol,1998,64:2269-2272.
[47]Maldonado A,Ruiz-Barba JL,Jimenez-Diaz R.Purification and genetic characterization ofplantaricin NC8,a novel cocultureinducible two-peptide bacteriocin from Lactobacillus plantarum NC8[J].Appl Environ Microbiol,2003,69:383-389.
[48]Sawa N,Zendo T,Kiyofuji J,et al.Identification and characterization of lactocyclicin Q,a novel cyclic bacteriocin produced by Lactococcus sp.strain QU12[J].Appl Environ Microbiol,2009,75:1552-1558.
[49]Steinberg D,Lehrer R.Shafer W M,et al.Designer assays for antimicrobial peptides,Disputing the“one-size-Fits-All”Theory[J].Antibacterial Peptide Protocols,1997,78:169-186.
[50]周配东,潘道东,张玉千,等.产细菌素乳酸菌的筛选及其所产细菌素的特性[J].食品科学,2011,32(17):303-307.
[51]岳喜庆,闵钟熳,郭晨,等.产Ⅱa类细菌素乳酸菌的筛选及鉴定[J].江苏农业学报,2011(2):410-414.
[52]韩雪,李研东,王颖.产细菌素乳酸菌的分离与筛选[J].中国饲料,2011(7):31-32.
[53]Amonlaya Tosukhowong, Takeshi Zendo, Wonnop Visessanguan,et al.Garvieacin Q,a Novel Class II Bacteriocin from Lactococcus garvieae BCC 43578[J].Applied and EnvironmentalMicrobiology,2012,78:1619-1623.
[54]Schagger H,von Jagow G.Tricine-sodium dodecyl sulfatepolyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100ku[J].Anal Biochem,1987,166:368-379.
[55]Bhunia A K,Johnson M C,Ray B.Purification, characterization and antimicrobial spectrum of a bacteriocin produced by Pediococcus acidilactici[J].JAppl Bacteriol,1988,65:261-268.
[56]Onda T,Yanagida F,TsujiM.Production and purification of a bacteriocin peptide produced by Lactococcus sp.strain GM005,isolated from Miso-paste[J].Int JFood Microbiol,2003,87:153-159.
[57]Ball M S,Karuso P.Mass spectral compatibility of four proteomics stains[J].JProteome Res,2007(6):4313-4320.
[58]Vera Pingitore E,Salvucci E,Sesma F,et al.Different strategies for purification of antimicrobial peptides from lactic acid bacteria[J].Applied Microbiology,2007:557-568.
[59]Vera Pingitore E,Hebert E M,Sesma F,et al.Influence of vitamins and osmolites on growth and bacteriocin production by Lactobacillus salivarius CRL 1328 in a chemically defined medium[J].Can JMicrobiol,2009,55:304-310.
[60]Nock C M,Ball M S,White IR,et al.Mass spectrometric compatibility of Deep Purple and SYPRO Ruby total protein stains for high-throughput proteomics using large-format twodimensionalgelelectrophoresis[J].Rapid Commun MassSpectrom,2008(22):881-886.
Detection technique of bacteriocin from lactic acid bacteria and
research advance in separation and purification technology
LIUWen-li1,ZHANG Lan-wei1,*,John shi2,YIHua-xi1
(1.School of Food Science and Engineering,Harbin Institute of Technology,Harbin 150090,China;2.Guelph Food Research Center,Agriculture and Agri-Food Canada,Guelph N1G5C9,Canada)
Bacteriocins can be againstnumerous foodborne pathogen and spoilage organisms,hence researchers at home and aboard are interested in bacteriocins,especially,bacteriocin from lac tic acid bac teria(LAB)are focused most.However,for bacteriocin could be app lied to biotechnological fields better,it is essential to illum inate its biochem ical structure and its mode of action,which needs that bacteriocins is purified to homogeneity.The main technologies used for the purification and detection of numerous bacteriocins was described,the exp loration of bacteriocins potential in LAB was also d iscussed.
lactic acid bacteria;bac teriocin;separation;purification;detection
TS252.1
A
1002-0306(2012)22-0414-06
2012-05-14 *通讯联系人
刘文丽(1982-),女,博士研究生,研究方向:食品科学。
科技部“863”课题(2007AA10Z354)。

