Purification of Crude Glycerol from Waste Cooking Oil Based Biodiesel Production by Orthogonal Test Method
Cai Tianfeng; Li Huipeng; Zhao Hua; Liao Kejian
(School of Petrochemical Engineering, Liaoning Shihua University, Fushun 113001)
Purification of Crude Glycerol from Waste Cooking Oil Based Biodiesel Production by Orthogonal Test Method
Cai Tianfeng; Li Huipeng; Zhao Hua; Liao Kejian
(School of Petrochemical Engineering, Liaoning Shihua University, Fushun 113001)
Purification of original crude glycerol obtained from biodiesel production was conducted in a laboratory scale equipment by means of a combined chemical and physical treatment method based upon repeated cycles of acidification of liquid phase to the desired pH value by using 5.85% H3PO4solution for pH value adjustment, and the mixture was kept at 70 ℃ for 60 min to make phase separation for obtaining a glycerol-rich middle phase. The yield of crude glycerol reached 81.2%. Subsequently, upon reaction of the obtained glycerol phase with 0.03% of sodium oxalate at 80 ℃ for 30 min the impurity removal rate was equal to 19.8%. The fraction boiling between 164 ℃ and 200 ℃ was collected by vacuum distillation followed by decolorization with 2% of active carbon at 80 ℃ for two times to yield the product glycerol with an acceptable purity of 98.10%.
crude glycerol; sodium oxalate; biodiesel;purification; glycerol-rich layer
1 Introduction
Nowadays, biodiesel has been attracting the world-wide attention because of its good fuel properties such as higher oxygen content for improved combustion, reduced sulphur and aromatics content, as well as lower toxicity level and net CO2emissions relative to fossil diesel[1-3]. However, the main problem is its high production cost, which has been prompting the development and utilization of glycerol derived from biodiesel.
Glycerol, or propan-1,2,3-triol, is an important commercial by-product of the biodiesel production process based on transesterification of triglycerides originated from spent or fresh vegetable oils or fats and waste oil with alcohols, like methanol and ethanol, in the presence of a homogeneous base catalyst like NaOH or KOH[4-5]or an acid catalyst[6]. In general, the production of every 10 kg of biodiesel can yield approximately one kilogram of crude glycerol (10%)[7], and currently the world’s capacity for biodiesel production is increasing dramatically. Any further increase in biodiesel production ratio will signif icantly boost the quantity and surplus of crude glycerol and partially purified glycerol in the environment. In contrast to the surplus of glycerol and impure glycerol, the high purity glycerol is an important industrial feedstock for applications in the food, cosmetic and pharmaceutical industries, as well as other more minor uses, but it is costly to refine crude glycerol[8]. The conventional process used for recovering or purifying crude glycerol is based upon the chemical and physical treatment. It is reported that chemical treatment at a low pH value is a better option as it can increase the glycerol yield and reduce the ash content in the recovered crude glycerin. However, the matter organic non glycerol (MONG) content is slightly increased. This treatment can also increase the recovered salt and reduce the crude glycerin concentration without affecting the recovery of crude fatty acids. However it is relatively suitable for recovering crude glycerin from a dilute glycerol source (containing 10%—20% glycerol) with a high NaCl (60%—70%) concentration, such as that found in glycerol residues. It has been reported[9-10]that the combined processes of chemical and physical treatment (acid protonation, ether and ethanol extraction, filtration and distillation) were used to recover from glycerol pitch the products glycerol and diglycerol with high purity and low contaminants.In this work, the purification of crude glycerol via transesterification reaction of a waste used-oil delivered from a biodiesel plant was carried out by using a combination of the chemical and vacuum distillation treatment with complex reaction, characteristic of easy operation, low operating cost, high efficiency and reusable solvent. The technological conditions were investigated followed by separation, desalination, and removal of impurities and decolorization of crude glycerol. The effect of pH value in the acidification stage of the purification system on the yield of the purified crude glycerol was also explored.
2 Experimental
2.1 Separation of crude glycerol
300 g of waste oil after deacidification was added into a biodiesel reactor that utilized waste used-oil as the raw material for biodiesel production. 3.6 g of sodium methoxide was dissolved in methanol that had been preheated to 60 ℃. Then the hot mixture was added into the reactor and in a few minutes the solution phase was separated into two distinct layers, the top layer of which was biodiesel with the bottom layer consisting of crude glycerol. The top layer was removed by slow decantation and the bottom layer was then separated and filtered.
2.2 Puri fication of crude glycerol
During the puri fication step in a laboratory-scale experiment, 30 g of crude glycerol was acidi fied via the addition of 5.85% H3PO4solution at 70 ℃ for 60 min for adjusting the liquid mixture to the desired pH value, and the mixture was left for a while until the solution phase separated into three distinct layers. The top layer consisted of free fatty acids, and the middle layer was a glycerol-rich solution, while the bottom layer was composed of inorganic salts. The top layer was removed by slow decantation and then the middle layer was obtained and filtered followed by degassing and drying to yield the semi-product of glycerol. Subsequently, the separated glycerol phase was mixed with 0.03% sodium oxalate solution at 80 ℃for 30 min to remove the residual impurities followed by filtration to obtain a glycerol-rich solution. Subsequently, the purified crude glycerol was then obtained through vacuum distillation for collecting the fractions boiling between 164 ℃ and 200 ℃[11]followed by decolorization with 2% of active carbon at 80 ℃ for two times[12]. Then the sample was characterized by IR spectroscopy. Ash content was analyzed according to the standard method ISO 2098—1972 by burning one gram of glycerol in a muffle furnace at 750 ℃ for 3 h.
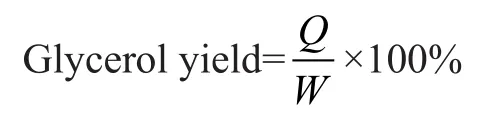
in whichQ(g) is the mass of obtained glycerol after being acidified with H3PO4, andW(g) is the mass of glycerol after purification.

in whichG(g) is the mass of glycerol after being subjected to complex reaction with sodium oxalate.
2.3 Determination of glycerol concentration
2.3.1 Quantitative determination of glycerol content with H5IO6titration
The glycerol content was measured quantitatively by means of titration with H5IO6. An excess amount of H5IO6was added to the sample in a 500 mL flask to initiate the oxidation-reduction reaction between C3H8O3and H5IO6. The glycerin was oxidized to HCHO and HCOOH, while H5IO6was reduced to HIO3according to the reaction (1).
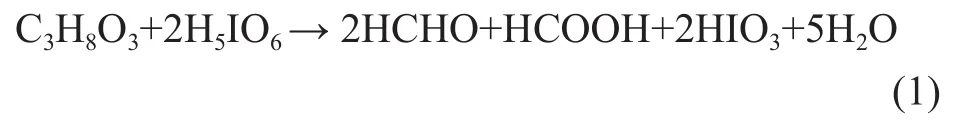
Under strong acidic conditions, large amount of KI was oxidized quantitatively to I2by HIO3and the remaining H5IO6was titrated by standard Na2S2O3solution using starch as the indicator according to reactions (2), (3) and (4). Then a blank experiment was carried out using 25 mL of water instead of glycerol samples.

2.3.2 Qualitative determination of glycerin
A few drops of glycerol sample were added into 0.5 g of KHSO4. The mixture was heated, and was left for a while until an irritating odor of acrolein was recognized, which could verify the existence of glycerol.
3 Results and Discussion
3.1 Acidification and desalting of crude glycerol
3.1.1 Investigation of single factor
3.1.1.1 Effect of pH value on glycerol yield
The original crude glycerol separated from biodiesel that was derived from waste cooking oil was a dark brown liquid with lower density and viscosity compared with commercial glycerol. Indeed, it was found to be a colloidal mixture composed of glycerol, a small amount of biodiesel, methanol, water, remaining sodium methoxide, and by-product soap. Inorganic matter, such as sodium salts, was originated from the catalyst used in the transesterification process, while the water content might be attributed to the absorption of moisture from the surrounding environment during the production process. By far the largest amount of contaminant, i.e.: matter organic nonglycerol, was generated by the contamination of soap, methanol and methyl esters in the glycerol residue formed during the biodiesel production process. Therefore, the original crude glycerol was acidified firstly by addition of H3PO4to adjust the solution to the desired pH value[13], and then the impurities were removed by centrifuging to separate substances according to different densities.
After the addition of H3PO4solution to the original crude glycerol until a desired final pH value of the solution was attained, the liquid phase was separated into three distinct layers automatically, with the inorganic salt layer located at the bottom, the glycerol-rich layer located in the middle and the free fatty acid layer located at the top. The inorganic salt layer and the free fatty acid layer were removed by gravitational decantation and filtration. Figure 1 shows the weight fraction of middle layer at different pH values investigated thereby. It can be seen from Fig. 1 that increasing the pH value of the solution during the acidification step from 3.0 to 5.0 or 6.0 could lead to an increase in the volume of glycerol-rich layer from 40% to 70%, but a further increase of pH value would concomitantly decrease the glycerol yield. However, a too strong acidic condition may only corrode the equipment and promote the esterification reaction of glycerol with acid, resulting in low glycerol yield. On the contrary, weak acidic condition may cause difficulties to the final product separation process. Therefore, the best separation process was carried out at a pH value of 5 or 6.
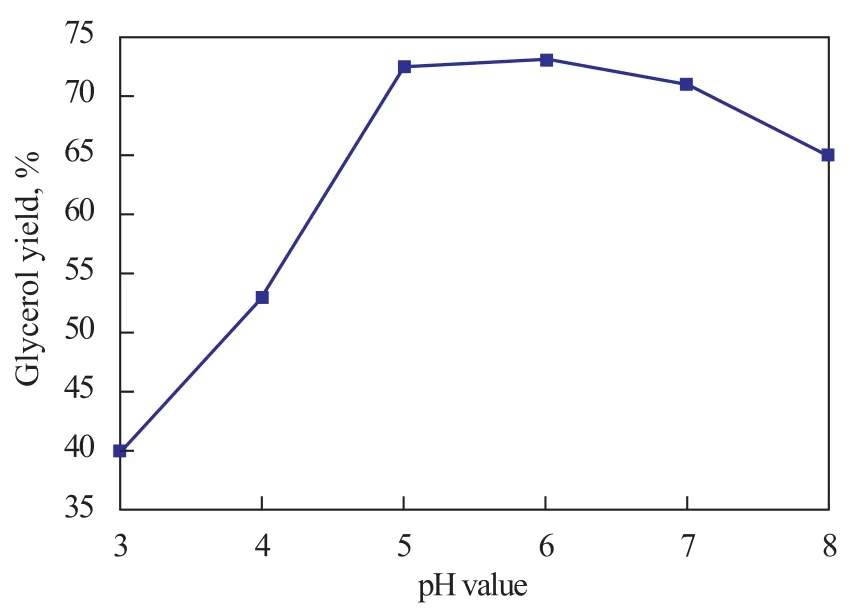
Figure 1 Glycerol yield of the middle layer obtained from the H3PO4acidification stage of glycerol purification at different pH values
3.1.1.2 Effect of reaction temperature on glycerol yield
As it can be seen from Figure 2, increasing the reaction temperature from 40 ℃to 70 ℃ led to an increase in the glycerol yield from 70% to 77%, but a concomitantly decreased yield could be seen when temperature was higher than 70 ℃, which might be attributed to the fact that soap upon reacting on glycerol at 70 ℃ could decompose completely into fatty acids and triglyceride or monoglycerides to cause the occurrence of esterification reaction in the system.

Figure 2 Effect of reaction temperature on glycerol yield
3.1.1.3 Effect of reaction time on glycerol yield
The experiment was carried out to study the effect of reaction time on glycerol yield in the presence of 5.85% H3PO4at a pH value of 5 in the solution at 70 ℃.It can be seen from Figure 3 that increasing the reaction time from 40 min to 60 min led to an increase in the glycerolyield from 12.5% to 15.2%. No significant change was observed when reaction time was longer than 60 min.
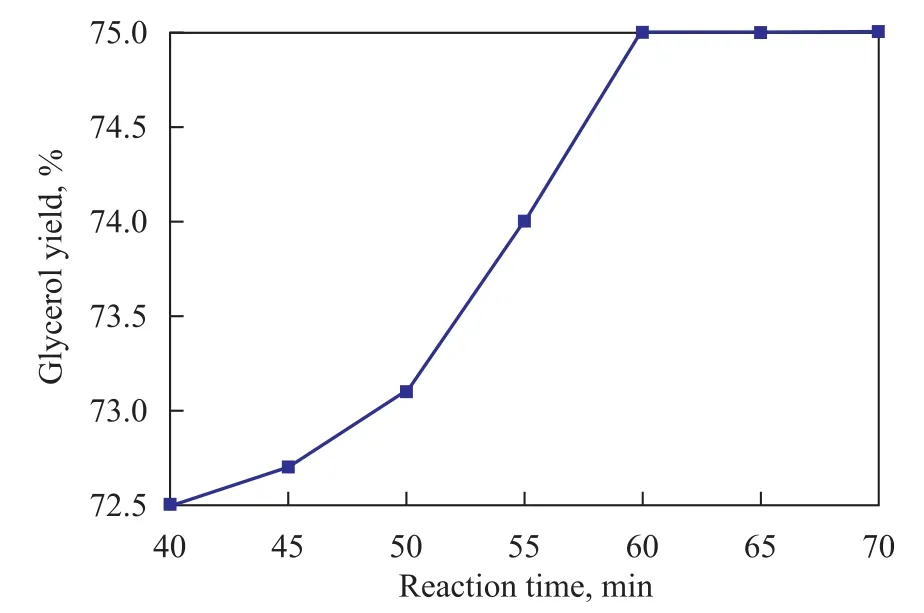
Figure 3 Effect of reaction time on glycerol yield
The optimal conditions were specified as follows: a desired pH value of 5 or 6 using 5.85%H3PO4to adjust the pH value of the solution, a reaction temperature of 70 ℃, and a reaction duration of 60 min.
3.1.2 Orthogonal test
Table 1 lists the factors and levels of orthogonal test for acidification of crude glycerol, and the test results.

Table 1 Arrangement and results of L9(34) orthogonal test
It can be seen from the data depicted in Table 1 that the reaction temperature had a greatest impact on the glycerol yield followed by the reaction time and the pH value. The optimal acidification conditions were found to be as follows: a reaction time of 60 min, a reaction temperature of 70 ℃, and a pH value of 5 in the presence of 5.85% H3PO4, with the acceptable yield of the semi-product glycerol equating to 81.2%.
3.2 Removal of impurities in semi-product glycerol via the sodium oxalate complex reaction
Sodium oxalate complex reaction was carried out to remove the residual impurities contained in the semiproduct glycerol. Reaction temperature, reaction time and dosage of sodium oxalate were chosen as three important factors affecting the impurities removal rate. Table 2 summarizes the levels, factors and results of orthogonal test.

Table 2 Arrangement and results of L9(34) orthogonal test
It can be seen from Table 2 that the reaction temperature had a greatest impact on impurities removal rate than the dosage of sodium oxalate and the reaction time. The optimal conditions were obtained after the reaction was conducted at 80 ℃ for 30 min using 0.03% of sodium oxalate to achieve an acceptable impurities removal rate of 19.8%. Then the recovery of crude glycerin from glycerol residues was carried out by a simple distillation at 164—200 ℃with an acceptable purity of 98.10% glycerol.
3.3 IR characterization
The purified glycerol was characterized by IR spectrometry and compared to commercial glycerol as shown in Fig 4. The main functional groups were clearly identified, including the O—H bond stretching at 3 350 cm-1, the C—H bond stretching at 2 880 cm-1and 2 930 cm-1, the C—O bond stretching at 2 100 cm-1, the C—O—H bond bending from 1 400 cm-1to 1 460 cm-1, the C—O bond stretching from 1450 cm-1to 1 100 cm-1, and the O—H bond bending at 920 cm-1. However, the spectra of the purified glycerol showed a weak peak at 1 740 cm-1, indicating to the presence of C=O compounds of an ester of carboxylic acid or fatty acid.
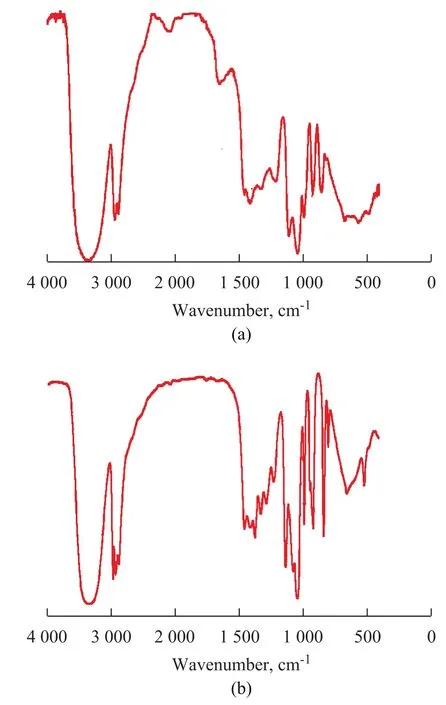
Figure 4 IR spectra of the purified glycerol with the acidification stage performed at a pH value of 5.0 (a) and a commercial glycerol solution (b)
3.4 Properties of glycerol after purification
The properties of waste cooking oil based glycerol after purification in comparison with commercial glycerol are listed in Table 3, and it can be seen that the performance of purified crude glycerol reached basically the quality level of commercial glycerol.
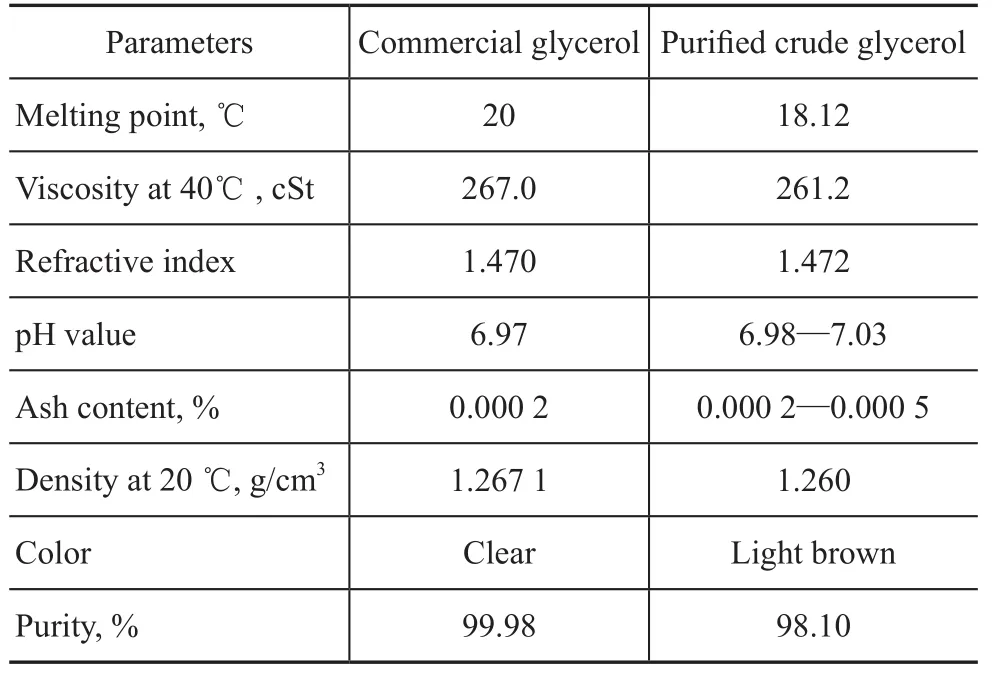
Table 3 The properties of waste cooking oil based glycerol after purification in comparison with commercial glycerol
4 Conclusions
1) The purification of glycerol from a crude glycerol derived from a waste cooking oil based biodiesel production was achieved using the combined approach of chemical treatment and vacuum distillation. The original high content of ash, water and other impurities in crude glycerol was successfully removed.
2) The optimal acidification conditions were found to be as follows: a reaction time of 60 min, a reaction temperature of 70 ℃, and a pH value of 5 in the presence of 5.85% H3PO4, with the acceptable yield of semi-product glycerol reaching 81.2%.
3) The optimal impurities removal conditions were obtained at a reaction temperature of 80 ℃ and a reaction time of 30 min when 0.03% of sodium oxalate was used to achieve an acceptable impurities removal rate of 19.8%. Then the recovery of crude glycerin from glycerol residues was carried out by a simple distillation procedure at 164—200 ℃to attain an acceptable glycerol purity of 98.10%.
Acknowledgements:We acknowledge the financial support from Scientific Research Foundation for Doctoral Program of Liaoning Province (20081104).
[1] Kalam M A, Husnawan M, Masjuki H H. Exhaust emission and combustion evaluation of coconut oil-powered indirect injection diesel engine[J]. Renew Energy, 2003, 28(15): 2405-2415
[2] Kalligeros S, Zannikos F, Stournas S, et al. An investigation of using biodiesel/marine diesel blends on the performance of a stationary diesel engine[J]. Biomass Bioenergy, 2003, 24(2): 141-149
[3] Labeckas G, Slavinskas S. Performance of direct-injection off-road diesel engine on rapeseed oil[J]. Renew Energy, 2006, 31(6): 849-863
[4] Fukuda H, Kondo A, Noda, H. Biodiesel fuel production by transesterification of oils[J]. J Biosci Bioeng, 2001, 92: 405-416
[5] Wang Z M, Lee J S, Park J Y, et al. Novel biodiesel production technology from soybean soapstock[J]. Korean J Chem. Eng, 2007, 24 (6): 1027-1030
[6] Wang Z M, Lee J S, Park J Y, et al. Optimization of biodiesel production from trap grease via acid catalysis[J]. Korean J Chem Eng, 2008, 25(4): 670-674
[7] Chi Z, Pyle D, Wen Z, et al. A laboratory study of producing docosahexaenoic acid from biodiesel-waste glycerol by microalgal fermentation[J]. Process Biochemistry, 2007, 42(11): 1537-1545
[8] Johnson D T, Taconi K A. The glycerin glut: Options for value-added conversion of crude glycerol resulted from biodiesel production[J]. Environ Prog, 2007, 26: 338-348
[9] Hazimah A H, Ooi T L, Salmiah A. Recovery of glycerol and diglycerol from glycerol pitch[J]. J Oil Palm Res, 2003, 15(1): 1-5
[10] Israel A U, Obot I B, Asuquo J E. Recovery of glycerol from spent soap lye by-product of soap manufacture[J]. EJournal of Chemistry, 2008, 5(4): 940-945
[11] Xin Y N. Technique for continuous distillation of crude glycerin[J]. China Oils and Fats, 2002, 27(3): 67-69(in Chinese)
[12] Chen W W, Gao Y Y, Lin X Y, et al. Technology for refining of glycerol from by-products of biodiesel[J]. China Oils and Fats, 2006, 31(5): 62-64 ( in Chinese)
[13] Yang K H, Jiang J C, Nie X A, et al. Study on preparation of biodiesel and technology for recovery and refining of glycerol from its by-product[J]. Biomass Chemical Engineering, 2006, 40(1): 1-4
Recieved date: 2012-07-20; Accepted date: 2012-09-16.
Li Huipeng, Telephone: +86-24-56860830; E-mail: zh.113@126.com.
- 中国炼油与石油化工的其它文章
- Hydrocarbon Composition of Different VGO Feedstocks and Its Correlation with FCC Product Distribution
- Commercial Applications of Paraxylene Adsorbents RAX-2000A and RAX-3000
- Et3NHCl-AlCl3Ionic Liquids as Catalyst for Alkylation of Toluene with 2-Chloro-2-methylpropane
- Alumina Supported Vanadium Oxide Catalysts for Residue Hydrotreating
- Degradation of Nitrobenzene-Containing Wastewater with O3and H2O2by High Gravity Technology
- Highlights on Planned Grassroots Styrene Units and Expansion of Existing Styrene Units in China

