Degradation of Nitrobenzene-Containing Wastewater with O3and H2O2by High Gravity Technology
Jiao Weizhou; Liu Youzhi; Liu Wenli; Li Jing; Shao Fan; Wang Chaoran
(Research Center of Shanxi Province for High Gravity Chemical Engineering and Technology, North University of China, Taiyuan 030051)
Degradation of Nitrobenzene-Containing Wastewater with O3and H2O2by High Gravity Technology
Jiao Weizhou; Liu Youzhi; Liu Wenli; Li Jing; Shao Fan; Wang Chaoran
(Research Center of Shanxi Province for High Gravity Chemical Engineering and Technology, North University of China, Taiyuan 030051)
Nitrobenzene-containing industrial wastewater was degraded in the presence of ozone coupled with H2O2by high gravity technology. The effect of high gravity factor, H2O2concentration, pH value, liquid flow-rate, and reaction time on the efficiency for removal of nitrobenzene was investigated. The experimental results show that the high gravity technology enhances the ozone utilization efficiency with O3/H2O2showing synergistic effect. The degradation efficiency in terms of the COD removal rate and nitrobenzene removal rate reached 45.8% and 50.4%, respectively, under the following reaction conditions, viz.: a high gravity factor of 66.54, a pH value of 9, a H2O2/O3molar ratio of 1:1, a liquid flow rate of 140 L/h, an ozone concentration of 40 mg/L, a H2O2multiple dosing mode of 6 mL/h, and a reaction time of 4 h. Compared with the performance of conventional stirred aeration mixers, the high gravity technology could increase the COD and nitrobenzene removal rate related with the nitrobenzene-containing wastewater by 22.9% and 23.3%, respectively.
nitrobenzene-containing wastewater; rotating packed bed; ozone;H2O2; free radicals
1 Introduction
Nitrobenzene is mainly originated from petrochemical, pharmaceutical, explosive and dyestuff industries. In most cases the nitrobenzene-containing wastewater is an organic sewage characteristic of complex composition, high toxicity, high chromaticity, and high COD value that is not readily susceptible to biodegradation and can pose great hazard to the ecology and the environment. The United States EPA has listed nitrobenzene as the priority pollutant, and China has included nitrobenzene in “The Black List” of 68 key pollutants[1-3].
The nitrobenzene-containing wastewater contains a lot of toxic substances that are, not readily biodegradable, and massive in quantity. Hence it is necessary to find out a method for treating the nitrobenzene-containing wastewater with high efficiency and low treating cost. Nowadays, the main processes for treating wastewater cover the photo-catalytic method, the absorption method, and the electrochemical method[4-10]. However, these traditional methods are of low efficiency, and the residual substances after treatment still become pollutants or hazardous materials that need to be further treated before discharge. Therefore, it is expected to further study and develop a new method for treating the nitrobenzene-containing wastewater.
In recent years the advanced oxidation process using ozone as the main oxidizing agent for treating the nitrobenzene-containing wastewater has become a hot spot of research. Ozone, a strong oxidant having a redox potential of 2.07 V, is only the second after fluorine (with a redox potential of 2.87 V) in its oxidizing ability[11]. Ozone as an oxidizing agent is extensively applied in the treatment of wastewater and can greatly improve the decolorization, mineralization rate and biodegradability of the pollutant solution and can serve as a means for wastewater treatment with definite application potential. However, the limiting step for a host of ozone oxidation processes is the mass transfer rate between the gas and liquid streams, because the ozonation technology is constrained by the mass transfer of ozone from the gas phase to the liquid phase[12-15]. In line with the double-film theory the ozone dissolvability is proportional to the mass transfer area and mass transfer coefficient, and two means can be suggested to boost the ozone dissolvability, i. e.: increasingthe ozone/water contact area, and enhancing the mixing and turbulence intensity of water to reduce the thickness of liquid film and decrease the thickness of both gas film and liquid film for reducing their resistance in an attempt to boost the ozone mass transfer coefficient to abruptly increase the ozone dissolution rate. The high gravity technology[16-17]is a novel technology for enhancing the transfer process, which functions to cut wastewater into thinner liquid film and smaller droplets to effectively increase the gas-liquid contact surface area and mass transfer efficiency that can provide ozone with an easy access to the liquid phase to enhance the ozonation ef ficiency, resulting in reduction of reaction time and energy conservation.
This paper upon comparing various methods for treating the nitrobenzene-containing wastewater has selected the ozone treating method, which is a method with most commercially applicable potential that is easy in operation and free from secondary pollution. A novel reactor capable of enhancing mass transfer through adoption of the rotating packed bed can boost the ozone utilization and reduce the treating cost. This article by targeting the nitrobenzenecontaining wastewater as the object of study has systematically investigated the degradation of nitrobenzene-containing wastewater via enhanced ozonation coupled with hydrogen peroxide by high gravity technology to provide a de finite theoretical basis for treating the nitrobenzenecontaining wastewater.
2 Experimental
2.1 Material used in experiments and methods of analysis
The nitrobenzene-containing wastewater, which was obtained from the wastewater discharged from a chemical production process, was red-brownish in color and contained nitrobenzene as the main organic compound. This wastewater had a COD of 5800 mg/L and a nitrobenzene concentration of 210 mg/L. All reagents and chemicals used in experiments were of AR grade, including hydrogen peroxide (with a purity of 30% H2O2) and ferrous sulfate heptahydrate (green vitriol). The COD of wastewater samples was determined according to the potassium bichromate method (national test method GB/ T 11914—1989), and the nitrobenzene concentration in wastewater was determined by spectrophotometry.
2.2 Experimental setup
The equipment used in experiments was a self-made cross-flow type rotating packed bed reactor with the packing made of stainless-steel ripple wire-mesh. The characteristic parameters of packing material operating under high-gravity regime are presented in Table 1.
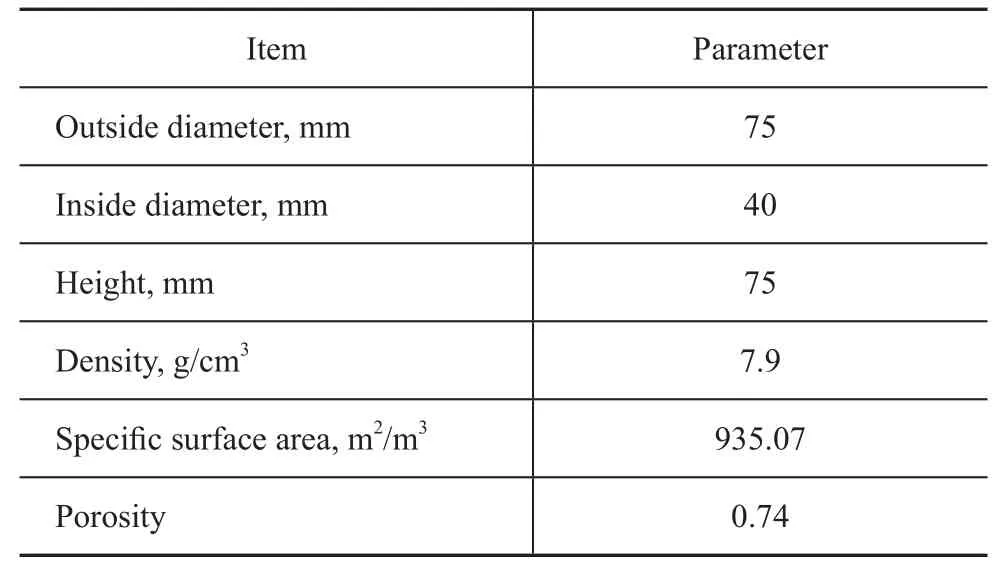
Table 1 Characteristic parameters of packing
2.3 Process flow diagram
The enperinental flow diagram for treating nitrobenzerecontaining wastewater is shown in Figure 1. Oxygen gas from the oxygen cylinder 1 is delivered to the ozone generator 2, in which ozone is formed. Ozone after measuring in the rotameter 3 is then routed to the rotating packed bed 4 from the bottom of the reactor axially through the packed bed. The wastewater in the wastewater tank 8 after being pumped by the liquid pump 7 and moved through the liquid distributor of the rotameter 6 is homogeneously sprinkled in the inner part of the packed layer and then moved under the centrifugal force along the radial direction of the packed layer. Ozone and wastewater is kept in contact with wastewater crosswise with a part of ozone reacting on the organic compounds in the packing. A part of ozone is dissolved in the wastewater and is moved along the wall of reactor prior to be collected at the bottom of the packed bed and then discharged to the wastewater tank 8. Hydrogen peroxide is added to the wastewater tank 8 to decompose ozone to form ·OH free radicals with stronger oxidation activity that can oxidize and degrade pollutants contained in the wastewater. Then the nitrobenzene-containing wastewater is again circulated through the liquid pump 7 to the rotating packed bed for treatment and the ozone stream after reaction ispassed through the tail-gas absorption bottle 9 before being vented into the atmosphere.
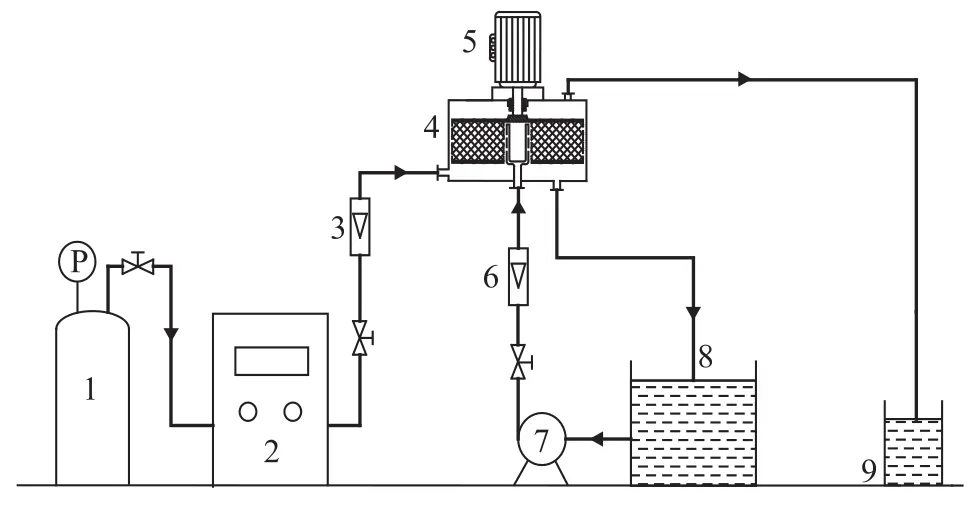
Figure 1 Experimental flow diagram for treating nitrobenzene-containing wastewater by O3/H2O2in rotating packed bed reactor
3 Results and discussion
3.1 Influence of high gravity factor on wastewater treatment efficiency
The high gravity factorβ[16], a dimensionless measure used to weigh the intensity of high gravity field, is a ratio of the centrifugal acceleration versus the gravitational acceleration as shown below:

in which,ωis angular velocity of the rotation of rotor, s-1;ris rotor radius, m;gis gravitational acceleration (=9.8 m/s2).
During the experiment the high gravity factor β of the rotating packed bed is controlled via regulating the revolution rate of a frequency converter. A dosage of 1.5 liters of nitrobenzene-containing wastewater is treated and recycled in the rotating packed bed with the pH value of water sample adjusted at 11 under the following treating conditions, i. e.: a gas flow rate of 75 L/h, a liquid flow rate of 120 L/h, and an ozone concentration of 50.0 mg/L at the reactor inlet, with 12 ml of hydrogen peroxide (30%) added in one lump to the reactor. After the water sample has been treated for 3 hours the COD removal and nitrobenzene removal from wastewater sample were checked with the results presented in Figure 2.
It can be seen from Figure 2 that with an increasing high gravity factorβthe COD removal rate and nitrobenzene removal rate at first increased and then gradually decreased. When the high gravity factorβwas less than 65.44, increase in the high gravity factor could apparently enhance the removal of both COD and nitrobenzene, because the oxidation of pollutants by O3/H2O2was stilled controlled by mass transfer of ozone from gas phase to the liquid phase. In an alkaline environment hydrogen peroxide could catalyze the decomposition of ozone to form more ·OH radicals with the reaction mainly going on through indirect oxidation reaction. The ·OH radicals could react on pollutants with a higher reaction rate, making the oxidation reaction obviously constrained by the mass transfer between the gas phase and the liquid phase. Since the transfer of ozone from gas phase to the liquid phase is a liquid film controlled process, the increasing high gravity factor in the rotating packed bed caused an ever increasing rotational velocity to enhance the shear action on wastewater and increase the turbulence of gas and liquid, making the gas/liquid interface quickly renewed to significantly reduce the thickness of liquid film formed thereby and the size of liquid droplets. This process could increase the contact area between gas and liquid to boost the mass transfer rate of ozone from gas phase to the liquid phase, leading to increased oxidation efficiency. When the high gravity factor β was greater than 65.44, both the COD removal rate and nitrobenzene removal rate dropped, which might be caused by the fact that a much higher gravity factor could lead to a reduced average gas/liquid contact time, so that the pollutants in wastewater was discharged without taking part in the re-action with ·OH radicals and the wastewater treating eff iciency was reduced. Hence at the experimental conditions an appropriate high gravity factor was specified as 65.44.
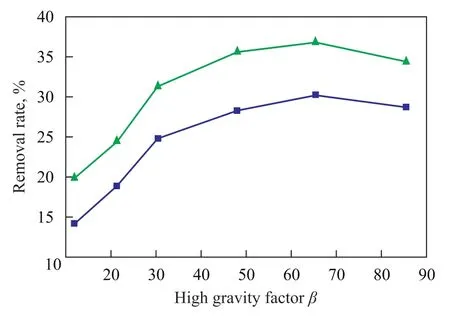
Figure 2 Influence of high gravity factor on efficiency for treating nitrobenzene-containing wastewater
3.2 Influence of pH value on wastewater treatment efficiency
It can be learned from the reaction mechanism that the key for oxidation of pollutants is the indirect oxidation of pollutants by ·OH radicals, and ozone is more readily to form ·OH radicals in the alkaline solution. This experiment intended mainly to study the influence of pH value in the alkaline condition on the oxidative treating of nitrobenzene-containing wastewater by O3/H2O2. During the experiment, the caustic soda solution was used to adjust the pH value of wastewater. A dosage of 1.5 L of nitrobenzene-containing wastewater was recycled in the rotating packed bed reactor for treating under the following reaction conditions: a high gravity factor of 65.44, a gas flowrate of 75 L/h, a liquid flow rate of 120 L/h, and an ozone concentration at the reactor inlet of 50.0 mg/L, with 12 ml of hydrogen peroxide (30%) added to the reactor in one lump. After the wastewater sample had been treated for 3 hours, the COD removal and nitrobenzene removal from wastewater sample at different pH values of the wastewater were checked with the results presented in Figure 3.
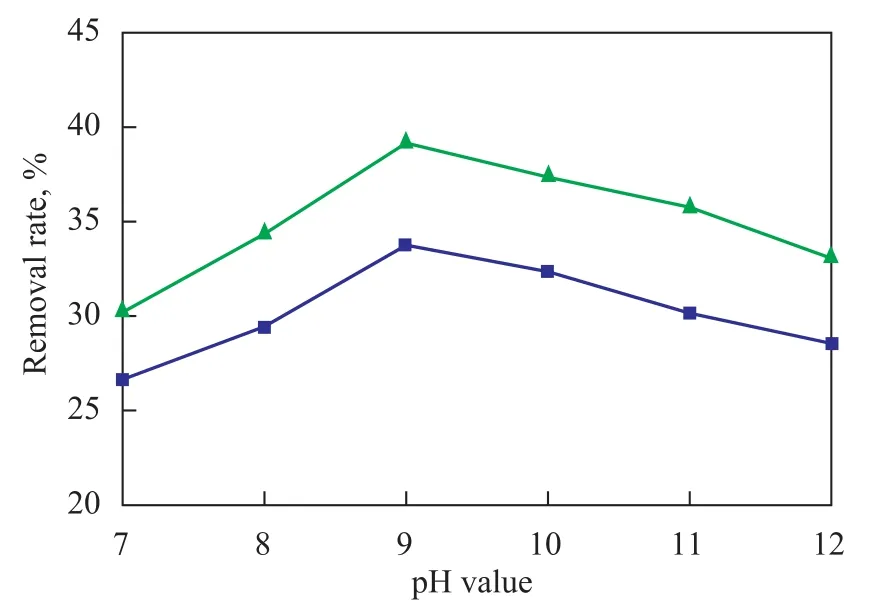
Figure 3 Influence of pH value on efficiency for treating nitrobenzene-containing wastewater
It can be seen from Figure 3 that when the pH value of wastewater increased from 7 to 9, the COD removal rate and nitrobenzene removal rate could increase from 26.7% and 30.25% to 33.8% and 39.2%, respectively. The COD and nitrobenzene removal rate reached maximum at a pH value of 9.0, and then dropped with further increase in the pH value of wastewater, because the pH value of wastewater could greatly influence the formation of ·OH radicals. At first coupled with the increase in the pH value of solution, the OH-ions could stimulate the ozone decomposition to yield more ·OH radicals, and furthermore an increasing pH value coupled with increase in the concentration of OH-ions could stimulate the formation of ·OH radicals. In the O3/H2O2reaction system theconcentration is a key factor affecting the chain reaction of ·OH radicals. High concentration ofradicals is in favor of the formation of ·OH radicals. We can learn from the reaction equations (2) and (3) as shown below:
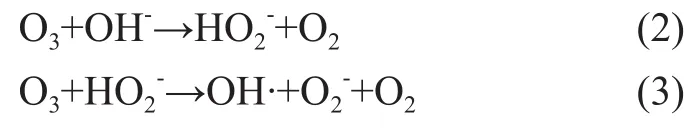
When the pH of wastewater is relatively low, the concentration of H+ions in the reaction system is quite high, which can prevent the reaction from moving towards the positive direction, and a reduced concentration of2radicals is not conducive to the formation of ·OH radicals. With increase in the pH value of wastewater solution, hydrogen peroxide is more prone to decompose intoradicals, that can accelerate the rate of formation of ·OH radicals resulted from catalytic decomposition of ozone by H2O2to speed up the oxidation rate. When the pH value of wastewater is excessively high, the concentration of various free radicals formed in the chain reactions is too high, and the probability of free radicals collision also increases which can lead to extinction of these free radicals. Furthermore, the excess OH-ions can react onradicals to form relatively inactiveions, as shown by the following equation (4):
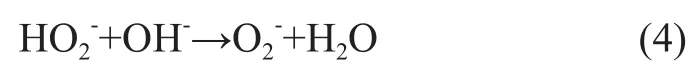
Thereby the ·OH chain reaction is terminated to reduce the number of ·OH radicals formed to pose a negative impact on the oxidation efficiency. Therefore during the oxidation process involving the oxidants O3/H2O2the pH value of wastewater solution should not be excessively high, and under the experimental conditions the initial pH value of the wastewater solution was specified as 9.0.
3.3 Influence of ozone concentration on wastewater treatment efficiency
During the experiment the electric power discharge in theozone generator was regulated to generate different ozone concentrations. A dosage of 1.5 L of nitrobenzene-containing wastewater, the pH value of which was adjusted to 9.0, was circulated in the rotating packed bed reactor for treating under the following reaction conditions: a high gravity factor of 65.44, a gas flowrate of 75 L/h, a liquid flow rate of 120 L/h, with 12 ml of hydrogen peroxide (30%) added in one lump to the reactor. After the wastewater sample had been treated for 3 hours, the COD removal and nitrobenzene removal from wastewater sample at different ozone concentrations in the wastewater were checked with the results presented in Figure 4.
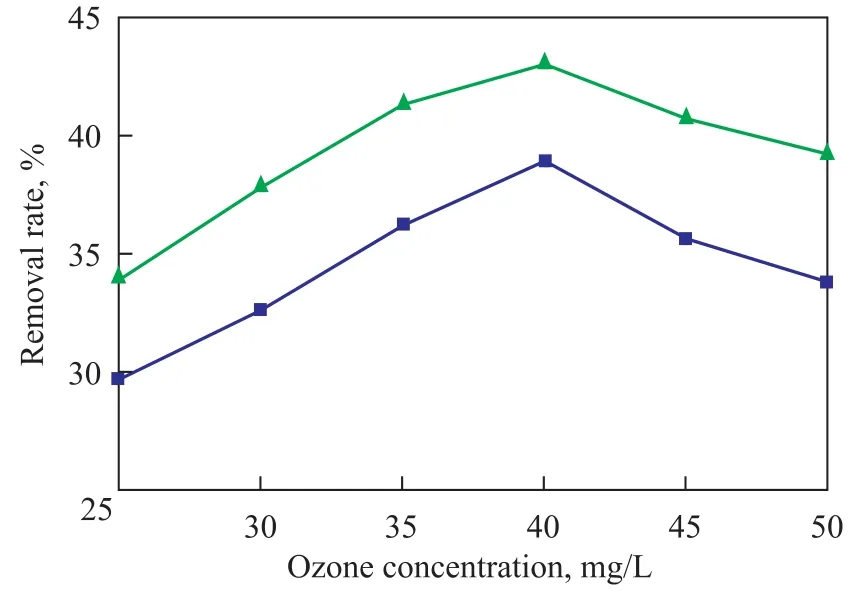
Figure 4 Influence of ozone concentration on efficiency for treating nitrobenzene-containing wastewater
It can be seen from Figure 4 that at a continuously increasing ozone concentration the COD removal rate and nitrobenzene removal rate at first increased and then gradually decreased. Both the COD removal rate and nitrobenzene removal rate reached maximum at an ozone concentration of 40 mg/L. Since the reaction of ozone on organic compounds in the wastewater involved the gasliquid reaction, the increase in ozone concentration in gas phase could push on mass transfer and increase mass transfer rate to continuously increase the ozone concentration in liquid phase to boost the amount of ·OH radicals generated thereby, resulting in an increasing removal of COD and nitrobenzene. When the ozone concentration reached a definite level, the ·OH radicals in wastewater could basically meet the needs for oxidation of organic compounds in wastewater, and further increase in ozone concentration could lead to decreased removal of COD and nitrobenzene, because excess ozone could not be dissolved in water and could react on ·OH radicals in wastewater as depicted by Equation (5) that could consume a part of ·OH radicals to reduce the oxidation efficiency. It was verified that at experimental conditions the appropriate ozone concentration should be 40.0 mg/L.
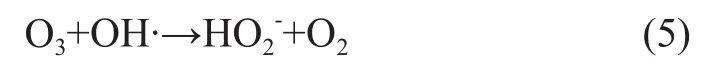
3.4 Influence of H2O2/O3molar ratio on wastewater treatment efficiency
The general reaction equation of wastewater treatment using the O/HO oxidation method is as follows:
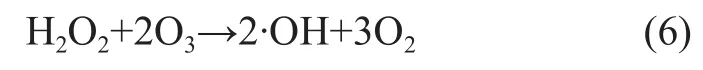
It can be learned from the above equation that the theoretic H2O2/O3molar ratio should be 0.5. When the H2O2/ O3molar ratio is less than 0.5, ozone cannot be effectively converted to ·OH radicals, and when the H2O2/O3molar ratio is greater than 0.5, there would be surplus hydrogen peroxide. However, during treating of wastewater there might be some free radical initiators and scavengers which can result in varying H2O2/O3molar ratios. Therefore, when the H2O2/O3method is adopted to treat nitrobenzene-containing wastewater the amount of added hydrogen peroxide should be determined through experiments at a fixed amount of added ozone.
During the experiment the addition of hydrogen peroxide was controlled in order to change the H2O2/O3molar ratio. A dosage of 1.5 L of nitrobenzene-containing wastewater, the pH value of which was adjusted to 9.0, was circulated in the rotating packed bed reactor under the following reaction conditions: a high gravity factor of 65.44, a gas flowrate of 75 L/h, a liquid flow rate of 120 L/h, and an ozone concentration of 40 mg/L, with 12 ml of hydrogen peroxide (30%) added in one lump to the reactor. After the water sample had been treated for 3 hours, the COD removal and nitrobenzene removal from wastewater sample at different H2O2/O3molar ratios were checked with the results presented in Figure 5.
It can be seen from Figure 5 that with an increasing amount of added H2O2at lower H2O2/O3molar ratios the COD and nitrobenzene removal rate was increased continuously. When the H2O2/O3molar ratio increased from 0.16 to 1.0, the COD and nitrobenzene removal rate increased to 40.8% and 44.2%, respectively, from 25.46% and 29.6%, because hydrogen peroxide functioned as a promoter for decomposition of ozone to form ·OH freeradicals. With a continuously increasing amount of added hydrogen peroxide, the amount of ·OH radicals also increased to effectively enhance the oxidative degradation of organic compounds in wastewater. When the H2O2/ O3molar ratio exceeded 1.0, the removal of both COD and nitrobenzene dropped, because excess hydrogen peroxide could react upon ·OH radicals to consume some ·OH radicals, andions formed during dissociation of hydrogen peroxide could also react on ·OH radicals as shown below:
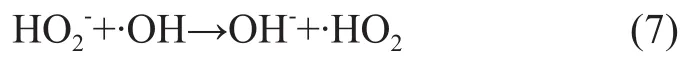
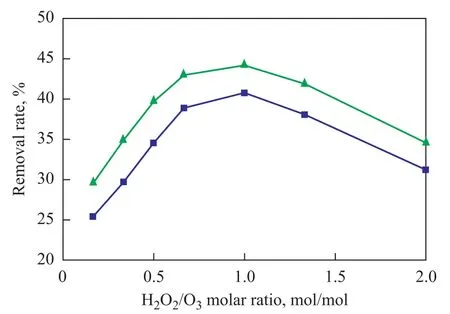
Figure 5 Influence of H2O2/O3molar ratio on efficiency for treating nitrobenzene-containing wastewater
Therefore excess amount of hydrogen peroxide could lead to decrease of ·OH radicals in wastewater to negatively affect the oxidative degradation of organic compounds, leading to reduced wastewater treatment ef ficiency. Under our experimental conditions the optimal H2O2/O3molar ratio should be 1:1.
3.5 In fluence of liquid flowrate on wastewater treatment ef ficiency
During the experiment the liquid flow was adjusted by means of a rotameter. A dosage of 1.5 L of nitrobenzenecontaining wastewater, the pH value of which was adjusted to 9.0, was circulated in the rotating packed bed reactor to be treated under the following reaction conditions: a high gravity factor of 65.44, a fixed gas flowrate of 75 L/h, and an ozone concentration of 40 mg/L, with 18 ml of hydrogen peroxide (30%) added in one lump to the reactor. After the water sample had been treated for 3 hours, the removal of COD and nitrobenzene from wastewater sample at different liquid flowrates was checked with the results presented in Figure 6.
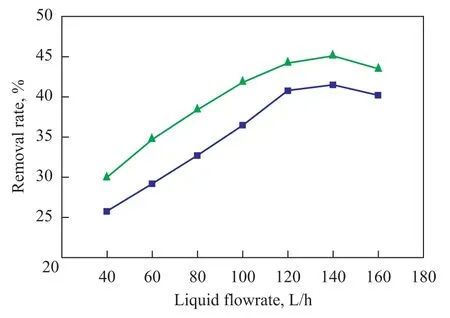
Figure 6 Influence of liquid flow rate on efficiency for treating nitrobenzene-containing wastewater
It can be seen from Figure 6 that there was an optimal value for COD and nitrobenzene removal with the increase in liquid flowrate. The wastewater treatment ef ficiency reached a best value when the liquid flowrate was around 140 L/h, which could achieve a COD removal rate of 41.5% and a nitrobenzene removal rate of 45.1%. At a fixed super gravity factor the centrifugal force did not change, and the wastewater-ozone contact time in the rotating packed bed was basically not changed. However, with an increasing liquid flowrate the liquid circulation frequency increased to definitely increase the liquidgas contact frequency, resulting in enhanced oxidation efficiency because of increased contact of gas with the liquid. Meanwhile when the liquid flowrate was low, the packing in the rotating packed bed was not fully wetted and could not enhance the gas-liquid mass transfer rate. With an increase of liquid flow the liquid film flow speed and wetting degree on the packing surface were increased to boost the liquid film mass transfer coef ficient and effective speci fic surface area that could facilitate the gasliquid mass transfer rate, resulting in a continuously increased removal of COD and nitrobenzene. When the liquid flowrate increased to 120 L/h, the increase in COD and nitrobenzene removal rate tended to level off and the removal of COD and nitrobenzene reached a maximum at a liquid flowrate of 140 L/h. A further increase in the liquid flowrate would reduce the wastewater treating efficiency, because an excessively large liquid flow would increase the liquid film thickness that could increase the resistance of liquid film against mass transfer to sti fle the gas-liquid mass transfer ef ficiency. By taking into accountthe overall situation, the appropriate liquid flowrate under experimental conditions should be 140 L/h.
3.6 Influence of H2O2feeding mode on wastewater treatment efficiency
A dosage of 1.5 L of nitrobenzene-containing wastewater, the pH value of which was adjusted to 9.0, was circulated in the rotating packed bed reactor and was treated under the following reaction conditions: a high gravity factor of 65.44, a gas flowrate of 75 L/h, a liquid flowrate of 140 L/ h, and an ozone concentration of 40 mg/L, with 18 ml of hydrogen peroxide (30%) added to the reactor. When the water sample was being treated for 3 hours, the H2O2was fed into the reactor according to different modes, which included: (1) adding 18 mL of hydrogen peroxide in one lump at the start of reaction; (2) adding each batch of 9 mL of H2O2in every 1.5 h; and (3) adding each batch of 6 mL of H2O2in every 1.0 h.
The influence of different modes for hydrogen peroxide feeding on removal of COD and nitrobenzene from wastewater sample was examined with the results presented in Figure 7.
It can be seen from Figure 7 that the effect of adding batches of hydrogen peroxide into the reactor in multiple times was better than that achieved by feeding hydrogen peroxide in one lump. After the wastewater sample was treated with hydrogen peroxide introduced in three times, the COD and nitrobenzene removal was higher by 2.1% and 3.1%, respectively, than that achieved by the case using the same amount of hydrogen peroxide introduced in one lump. Upon analyzing the outcome it is supposed that when hydrogen peroxide was fed in one lump, massive ·OH free radicals were formed instantly at the start of reaction. However, the ·OH radicals would be subjected to extinction reaction when they reached a definite concentration in water, and surplus hydrogen peroxide species could also react on ·OH radicals, which could lead to drastic reduction of ·OH radicals at the later stage of oxidation reaction to affect the oxidative degradation of pollutants in wastewater. On the other hand, upon feeding hydrogen peroxide in multiple batches in the course of reaction the ·OH radicals were timely replenished to maintain their concentration to a proper extent following their continuous consumption by the oxidation reaction, which was favorable to the sustainable oxidation of pollutants by ·OH radicals and could avoid extinction reaction of ·OH radicals to enhance the oxidative degradation of pollutants. It is also learned from Figure 7 that feeding hydrogen peroxide in three batches to the reaction system showed a better effect than introducing hydrogen peroxide in two batches. Under our experimental conditions, the reaction was carried out through feeding hydrogen peroxide in three batches, with each batch equating to 6 mL/h of hydrogen peroxide.
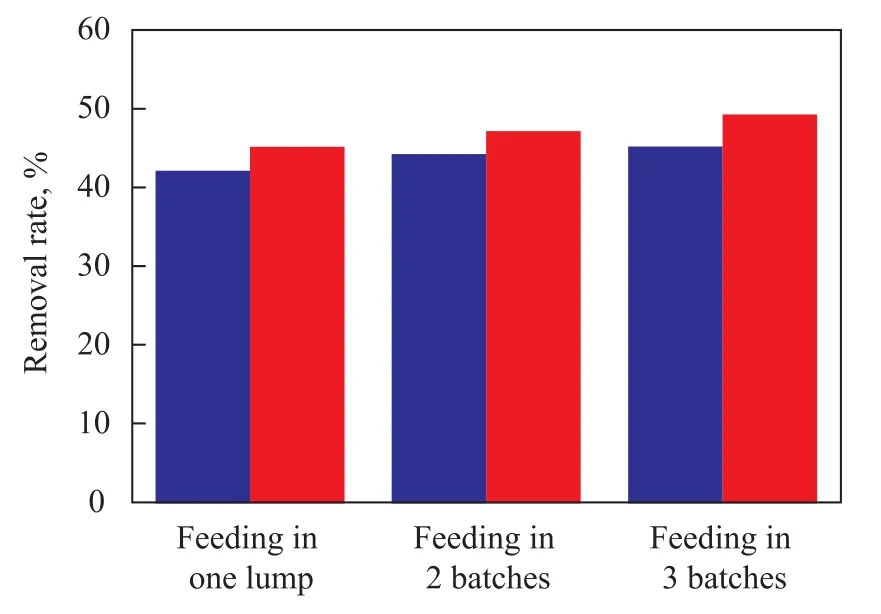
Figure 7 Influence of H2O2feeding mode on efficiency for treating nitrobenzene-containing wastewater
3.7 Influence of treating duration on wastewater treatment efficiency
A dosage of 1.5 L of nitrobenzene-containing wastewater, the pH value of which was adjusted to 9.0, was circulated in the rotating packed bed reactor for treating under the following conditions: a high gravity factor of 65.44, a fixed gas flowrate of 75 L/h, a liquid flow rate of 140 L/h, and an ozone concentration of 40 mg/L, with a total of 30 ml of hydrogen peroxide (30%) added in multiple batches of 6 m/h apiece to the reactor. After the water sample had been treated for 5 hours, the changes in COD and nitrobenzene removal from wastewater sample at different treating times were checked with the results presented in Figure 8.
It can be seen from Figure 8 that the removal of COD and nitrobenzene increased with an increasing treating time with the increase of pollutants removal rate gradually leveling off. After 4 hours of treatment the COD and nitrobenzene removal rate did not change further, because at the initial stage of reaction the concentrationof organic compounds in wastewater was quite high and the utilization of ·OH radicals resulted from decomposition of O3following the addition of hydrogen peroxide was relatively high with good oxidation efficiency which could quickly reduce the COD and nitrobenzene concentration in wastewater. With the extension of reaction time the concentration of organic compounds in wastewater decreased with time and the pH value also dropped continuously with gradual consumption of chemical reagents, which resulted in a decreasing amount of ·OH free radicals along with a plummeting reaction rate. An extended reaction time would not help improve the treating efficiency except the increased wastewater treatment cost. Therefore the optimal wastewater treatment duration should be 4 hours.
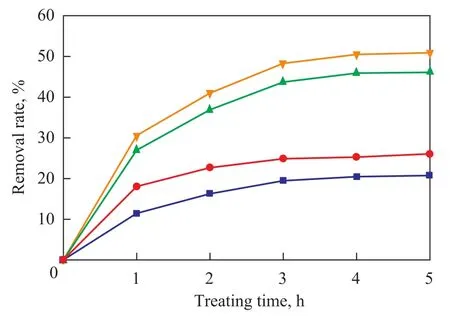
Figure 8 Influence of treating time on efficiency for treating nitrobenzene-containing wastewater
In the meantime, the difference between the single ozone treating method was compared with the O3/H2O2treating method on their efficiency for treating the nitrobenzenecontaining wastewater. In the course of treating the wastewater for 4 h with the addition of hydrogen peroxide along with ozone used as the oxidizing agent, the COD removal rate in wastewater could increased from 20.41% to 45.8%, and the nitrobenzene removal rate could increased to 50.4% from 25.2%. However, the effect of O3/ H2O2method on treating wastewater for one hour was better than that achieved by the single ozone method for treating the wastewater for 5 hours, indicating that hydrogen peroxide could greatly enhance the formation of ·OH free radicals to significantly enhance the degradation of nitrobenzene as compared to the case using single ozone as the oxidizing agent. After the treatment the nitrobenzene removal rate was higher than the COD removal rate, showing that the full mineralization of nitrobenzene in wastewater was even slower than its degradation, and in the course of its degradation a lot of byproducts formed in the degradation process could not be readily degraded by ·OH free radicals, and after treatment the COD value of wastewater was still quite high. For instance, after the wastewater had been treated for 4 h, the COD removal rate was 45.8%, while the nitrobenzene removal rate reached 50.4%.
3.8 Comparison between the rotating packed bed and the aeration stirred unit on treating efficiency using the O3/H2O2method
A dosage of 1.5 liters of nitrobenzene-containing wastewater was put respectively in the stirred aeration unit and in the rotating packed bed reactor to be subjected to treatment under the same conditions as shown below: a wastewater pH value of 9.0, a rotational velocity of 1400 r/min, a fixed gas flowrate of 75 L/h, an ozone concentration of 40.0 mg/L, and a total amount of 30 ml of H2O2(30%) added in five batches of 5 mL/h apiece. The wastewater sample was treated for 5 hours with the liquid flowrate in the rotating packed bed adjusted to 140 L/h. The removal of COD and nitrobenzene in the two reactors was examined at different time intervals with the results presented in Figure 9.It can be seen from Figure 9 that under the same treating conditions the efficiency for treating wastewater in the rotating packed bed was much better than the stirred aeration unit, and could increase the removal of COD and nitrobenzene by 22.9% and 23.3%, respectively as compared to that achieved by the stirred aeration unit, because the rotating packed bed could drastically enhance the gasliquid mass transfer rate and increase the ozone utilization rate, resulting in an improved efficiency for oxidation of wastewater. Hence the rotating packed bed as a means for treating wastewater using the ozone method has the advantage that can never be replaced by any other method.
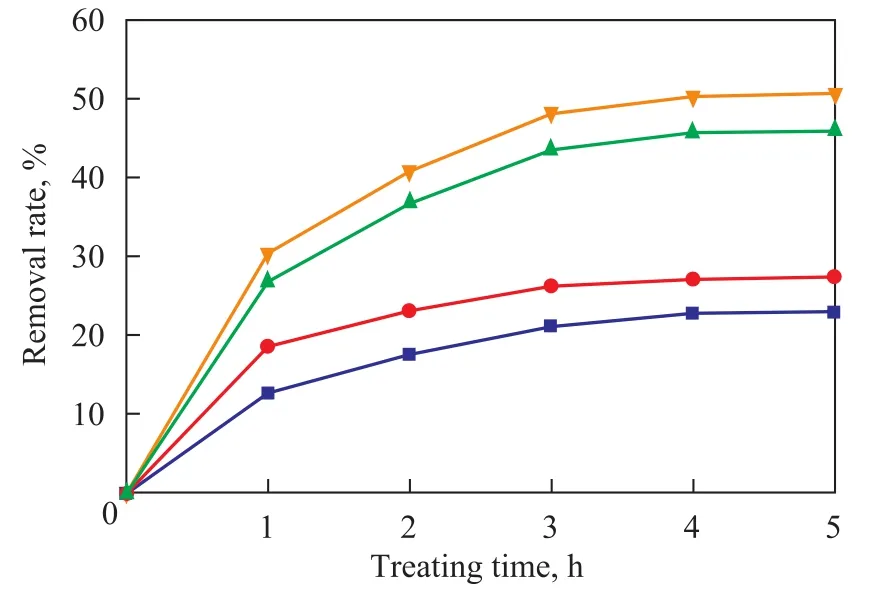
Figure 9 Comparison of two reactors on their efficiency for treating nitrobenzene-containing wastewater
4 Conclusions
1) The O3/H2O2method for treating nitrobenzene-containing wastewater in rotating packed bed reactor showed better effect as compared to the treating method using O3as the oxidizing agent, as the former method could increase the removal of COD and nitrobenzene from wastewater by 24.3% and 24.8%, respectively, more than the latter case, which revealed that H2O2and O3had good synergistic effect.
2) Upon studying the factors influencing the O3/H2O2method for treating nitrobenzene-containing wastewater in rotating packed bed reactor, the appropriate process regime for treatment of nitrobenzene-containing wastewater is as follows: a high gravity factor of 65.44, a pH value of 9.0 in water sample, a H2O2/O3molar ratio of 1:1, a liquid flow rate of 140 L/h, an ozone concentration of 40 mg/ L, and a treating duration of 4h, with hydrogen peroxide (30%) added in multiple batches of 6 mL/h apiece to the reactor. Under these process conditions the COD removal from wastewater reached 45.8% and the nitrobenzene removal reached 50.4%.
3) Under the same treating conditions the difference between the rotating packed bed reactor and the stirred aeration reactor in their efficiency for treating nitrobenzenecontaining wastewater using the O3/H2O2oxidation method was examined. The former was much better than the latter, indicating to a better ozone utilization rate achieved by the rotating packed bed reactor capable of enhancing oxidative degradation of wastewater. The O3/ H2O2method for treating wastewater in rotating packed bed reactor has a broad prospect in the domain of water treatment.
Acknowledgement:The project is financially supported by the National Natural Science Foundation of China (21206153) and Science and Technology Development Program Fund of Taiyuan City (120164053).
[1] Zhou Wenming, Fu Deqian, Sun Zongguang. Determination of black list of China’s priority pollutants in water[J]. Research of Environmental Science, 1991, 4 (6): 9-12 (in Chinese)
[2] Juneja T R, Bala A, Kumar P, et al. Mutagenicity of nitrobenzyl derivative: Potential bioreductive anticancer agents[J]. Mutation Research, 1995, 348: 137-145
[3] Keith L H, Telliard W A. Priority pollutants: Ⅰ-A perspective view[J]. Environ Sci Technol, 1979, 13(4): 416-423
[4] An Fuqiang, Feng Xiaoqin, Gao Baojiao. Adsorption mechanism and property of a novel adsorption material PAM/ SiO2towards 2,4,6-trinitrotoluene[J]. Journal of Hazardous Materials, 2009, 168(1): 352-357
[5] Zhang Dan, Guo Weinan, Yu Jiang. Novel process for bulk photocatalytic treatment of organic wastewater [J]. CIECS Journal, 2011, 62(4): 1077-1083 (in Chinese)
[6] Kyung-Duk Zoh, Daniels J I, Knezovich J P, et al. Treatment of hydrolysates of the highly explosive hexahydrol,3,5-trinitro- l,3,5-triazine and octahydro-l,3,5,7-tetranitrol,3,5,7-tetrazocine using biological denitrification[J]. Water Environment Research, 1999, 71(2):148-155
[7] Su Yuanbo, Li Qingbiao, Wang Yuanbeng, et al. Treatment of cyanide/silver containing electroplating wastewater by means of electrolysis with pulse power source [J]. CIECS Journal, 2009, 60(9): 2308-2313 (in Chinese)
[8] Chang Hai, Lü Xiaoping. Study on ultrasonic degradation of mixed carbon tetrachloride and methyl orange wastewater [J]. Journal of Chemical Engineering of Chinese Universities, 2011, 25(1): 155-160 (in Chinese).
[9] Brenner A, Ronen Z, Abeliovich A, et al. Use of hexahydrol,3,5-trinitro-l,3,5-triazine as a nitrogen source in biological treatment of munitions wastes[J].Water Environment Research, 2000, 72(4): 469-475
[10] Wang Guizhen, Li Lixin, Li Yongzhen, et al. Preparation of active carbon from raw bamboo and study on its adsorp-tion of phenolic wastewater [J]. Journal of Chemical Engineering of Chinese Universities, 2010, 24(4): 700-704 (in Chinese)
[11] Wu Xikang. Technology for Treating Organic Chemical Wastewater [M]. Beijing: Chemical Industry Press, 1985 (in Chinese)
[12] Ouyang Jiting, Liu Xiaochun, Feng Changgen, et al. Study on treatment of TNT solution by ozone under ultraviolet illumination [J]. Journal of Beijing Institute of Technology, 1999, 19(5):656-659 (in Chinese)
[13] Wen Zhongyue, Jiang Xuanzhen, Liu Weiping. Degradation of para-chlorophenol in wastewater via high-voltage pulse electric discharge coupled with ozonation[J]. Environmental Science, 2002, 23(2): 73-76 (in Chinese)
[14] Lin Chiachang, Liu Wentzong. Ozone oxidation in a rotating packed bed[J]. Chem Technol Biotechnol, 2003, 78: 138-141
[15] Chen Yihung, Chang Chingyuan, Su Weiling, et al. Ozonation of CI reactive black 5 using rotating packed bed and stirred tank reactor[J]. J Chem Technol Biotechnol, 2005, 80: 68-75
[16] Jiao Weizhou, Liu Youzhi, Qi Guisheng. Gas pressure drop and mass transfer characteristics in cross-flow rotating packed bed with porous plate packing [J]. Ind Eng Chem Res, 2010, 49 (8): 3732-3740
[17] Gao Jing, Chang Lingfei, Liu Youzhi. A novel high gravity electrochemical reactor with multi-concentric cylindrical electrodes and electrochemical treatment of phenol wastewater[J]. China Petroleum Processing & Petrochemical Technology, 2012, 14(2): 71-75
[18] Rajan S, Kumar M, Ansari M J, et al. Limiting gas liquid flows and mass transfer in a novel rotating packed bed (HiGee)[J]. Ind Eng Chem Res, 2011, 50 (2): 986-997
[19] Jiao Weizhou, Liu Youzhi, Shao Fan, et al. Degradation of wastewater containing nitrobenzene by high gravity-ultrasonic/oxidation/electrolysis technology[J]. China Petroleum Processing & Petrochemical Technology, 2012,14(3): 97-102
Recieved date: 2012-08-23; Accepted date: 2012-10-10.
Dr. Jiao Weizhou, Telephone: +86-351-3921986; E-mail: jwz0306@126.com.
- 中国炼油与石油化工的其它文章
- Alumina Supported Vanadium Oxide Catalysts for Residue Hydrotreating
- Highlights on Planned Grassroots Styrene Units and Expansion of Existing Styrene Units in China
- Hydrocarbon Composition of Different VGO Feedstocks and Its Correlation with FCC Product Distribution
- Purification of Crude Glycerol from Waste Cooking Oil Based Biodiesel Production by Orthogonal Test Method
- A Novel Modeling, Simulation and Optimization Approach of Crude Oil Cold Stripping Process
- Et3NHCl-AlCl3Ionic Liquids as Catalyst for Alkylation of Toluene with 2-Chloro-2-methylpropane

