UGT1A1*6基因多态性与伊立替康毒性关系的meta分析
郭晓玉,李智,曲秀娟,刘云鹏
(中国医科大学附属第一医院肿瘤内科,沈阳 110001)
·论著·
UGT1A1*6基因多态性与伊立替康毒性关系的meta分析
郭晓玉,李智,曲秀娟,刘云鹏
(中国医科大学附属第一医院肿瘤内科,沈阳 110001)
目的通过整合相关文献进行meta分析以得出UGT1A1*6基因多态性与伊立替康毒性的关系,指导临床治疗。方法通过PubMed数据库及手工搜索相关文献,制定文章纳入排除标准,纳入文章进行质量评价,提取数据后,应用STATA12.0软件进行分析。结果共纳入12篇文章进行分析,UGT1A1*6突变型较野生型发生粒细胞缺乏的风险率显著提高(OR=2.37,95% CI 1.58~3.55,P=0.001),其中纯合突变型和杂合突变型较野生型粒细胞缺乏发生风险率高,纯合突变型(OR=5.09,95%CI 2.74~9.45,P<0.001)较杂合突变型(OR=2.07,95%CI 1.37~3.13,P=0.001)风险率更高;而在腹泻方面,UGT1A1*6突变型较野生型发生腹泻的风险率高(OR=1.48,95%CI 0.86~2.55,P=0.153),但无统计学差异,而其中纯合突变型腹泻发生风险率显著增高(OR=3.51,95%CI 1.33~9.25,P=0.011),杂合突变型(OR=1.22,95%CI 0.68~2.22,P=0.503)腹泻发生风险率增高,但结果无统计学差异。结论UGT1A1*6基因多态性可以预测伊立替康的毒性,尤其是粒细胞缺乏的发生率。
UGT1A1*6;基因多态性;伊立替康;毒性;meta分析
伊立替康自20世纪90年代问世以来已广泛应用于结肠癌、直肠癌、肺癌等实体瘤治疗。大量临床试验证明,伊立替康可明显提高患者的总生存期[1~4],但因其毒性(主要为Ⅲ~Ⅳ度粒细胞缺乏和腹泻)较大,其应用受到一定限制[5]。伊立替康是喜树碱半人工合成物,主要代谢部位为肝脏。经静脉注射后,在体内经羧酸酯酶(carboxylesterase,CE)转化为7-乙基-10-羟基喜树碱(7-Ethyl-10-hydroxycamptothecin,SN-38)。后者为拓扑异构酶Ⅰ抑制剂,抑制DNA单链断裂后的修复,干扰DNA复制和转录,发挥细胞毒效应。SN-38进而经尿苷磷酸葡萄糖醛酸转移酶(uridine diphosphate glucuronosyltransferase 1,UGT1)A1灭活为葡萄糖醛酸产物SN-38G后,通过胆汁排泄入肠,在肠道细菌β-葡萄糖醛酸酶的作用下转换为SN-38而引发肠黏膜损伤及迟发性腹泻。肠道内的UGT1A1酶又可再度催化SN-38为SN-38G解毒[6]。可见,伊立替康的毒性与其主要的药物代谢酶UGT1A1有关,而其酶活性的高低又受UGT1A1基因多态性的影响。因此,UGT1A1的基因多态性与伊立替康的不良反应密切相关[7~10]。
美国食品及药物管理局(Food and Drug Administration,FDA)已于2005年批准UGT1A1*28用于预测伊立替康的不良反应。但是,UGT1A1*28在白种人群中突变率较高,约为30%~40%,在亚洲人群中的发生率仅为7%~14%,而UGT1A1*6突变在亚洲人群中的发生率却高达13%~24%。UGT1A1*6的多态性表现为第1外显子区211G>A突变,形成3种基因型:野生型(G/G)、杂合突变型(A/G)和纯合突变型(A/A)[11~13]。
目前,UGT1A1*6基因多态性与伊立替康的毒性关系并没有一个明确结果,而相关研究的样本量都较小,研究的毒性反应不统一[11~30],且有些研究结果相悖[22,26,30,31],为研究UGT1A1*6基因多态性与伊立替康引起的Ⅲ~Ⅳ度粒细胞缺乏和腹泻的关系,本文整合相关文献进行meta分析。
1 材料与方法
1.1 文献检索
以“UGT1A1”,“UGT1A1*6”,“irinotecan”为关键词在PubMed数据库检索截至2013年12月的所有文献,并通过手动检索相关文献。
1.2 纳入与排除标准
纳入标准:与UGT1A1*6基因多态性和伊立替康毒性关系有相关性、使用英语发表的、发表于同行审阅的杂志的文章纳入;排除标准:非英文发表、系统综述或病例报道、未提供UGT1A1*6各基因型粒细胞缺乏或腹泻发生的具体人数的文章排除。
1.3 质量评价与数据提取
对纳入文献应用The Newcastle-Ottawa Scale(NOS)量表,由两名人员分别独立对文献进行评价,不同意见讨论处理。提取作者、年份、地域、研究人数、肿瘤类型、化疗方案、伊立替康剂量,UGT1A1*6各基因型发生Ⅲ~Ⅳ度粒细胞缺乏及腹泻的人数及总人数。
1.4 统计学方法
用STATA12.0软件meta分析板块,对UGT1A1* 6不同基因型发生Ⅲ~Ⅳ度细胞缺乏及腹泻的优势比(odds ratio,OR)及95%可信区间(95%confidence interval,95%CI)进行分析,当P<0.05时,结果有统计学意义。应用Chi-square-based Q检验对异质性(I2)定量分析,若I2>50%,认为有明显异质性,我们将采用随机效应模型进行分析,若无明显异质性,将采用固定效应模型进行分析。最后,使用漏斗图进行发表偏倚评价。
2 结果
2.1 基本信息
根据检索策略,初步检索文献327篇,排除不相关文献281篇,日文文献3篇,无具体不良反应人数文献26篇,无具体不良反应类型文献5篇,最后共12篇文献[14,15,18~23,25,28,30,35]符合标准,共包括1 100例,其中野生型(G/G)707例、杂合突变型(A/G)340例,纯合突变型(A/A)53例,其中与严重粒细胞缺乏相关文献11篇,与严重腹泻相关6篇。应用NOS量表,由2名人员分别独立对文献进行评价。提取作者、年份、地域、研究人数、肿瘤类型、化疗方案、伊立替康剂量等相关信息,见表1。
2.2 UGT1A1*6基因多态性与伊立替康毒性——粒细胞缺乏关系
UGT1A1*6基因多态性与严重粒细胞缺乏关系的相关文献11篇,结果与森林图见图1、图2,UGT1A1*6突变型较野生型发生粒细胞缺乏的风险率显著增高(OR=2.37,95%CI 1.58~3.55;P<0.001),其中纯合突变型和杂合突变型较野生型粒细胞缺乏发生风险率均增高,纯合突变型(OR= 5.089,95%CI 2.742~9.446,P<0.001)较杂合突变型(OR=2.068,95%CI 1.366~3.129,P=0.001)风险率更高,差异均有统计学意义,且纯合突变型(I2= 0.0%,P=0.562)与杂合突变型(I2=37.2%,P= 0.102)均无显著异质性。
2.3 UGT1A1*6基因多态性与伊立替康毒性——腹泻关系
而在腹泻方面,UGT1A1*6基因多态性与严重腹泻关系的相关文献6篇,结果与森林图见图3、图4,UGT1A1*6突变型较野生型发生腹泻的风险率有提高(OR=1.48,95%CI 0.86~2.55,P=0.153),但无统计学差异,而其中纯合突变型腹泻发生风险率显著提高(OR=3.51,95%CI 1.33~9.25,P=0.011),结果有统计学差异,杂合突变型(OR=1.22,95%CI 0.68~2.22,P=0.503)腹泻发生风险率有提高,但结果无统计学差异,纯合突变型(I2=0.0%,P=0.748)与杂合突变型(I2=0.0%,P=0.715)均无显著异质性。
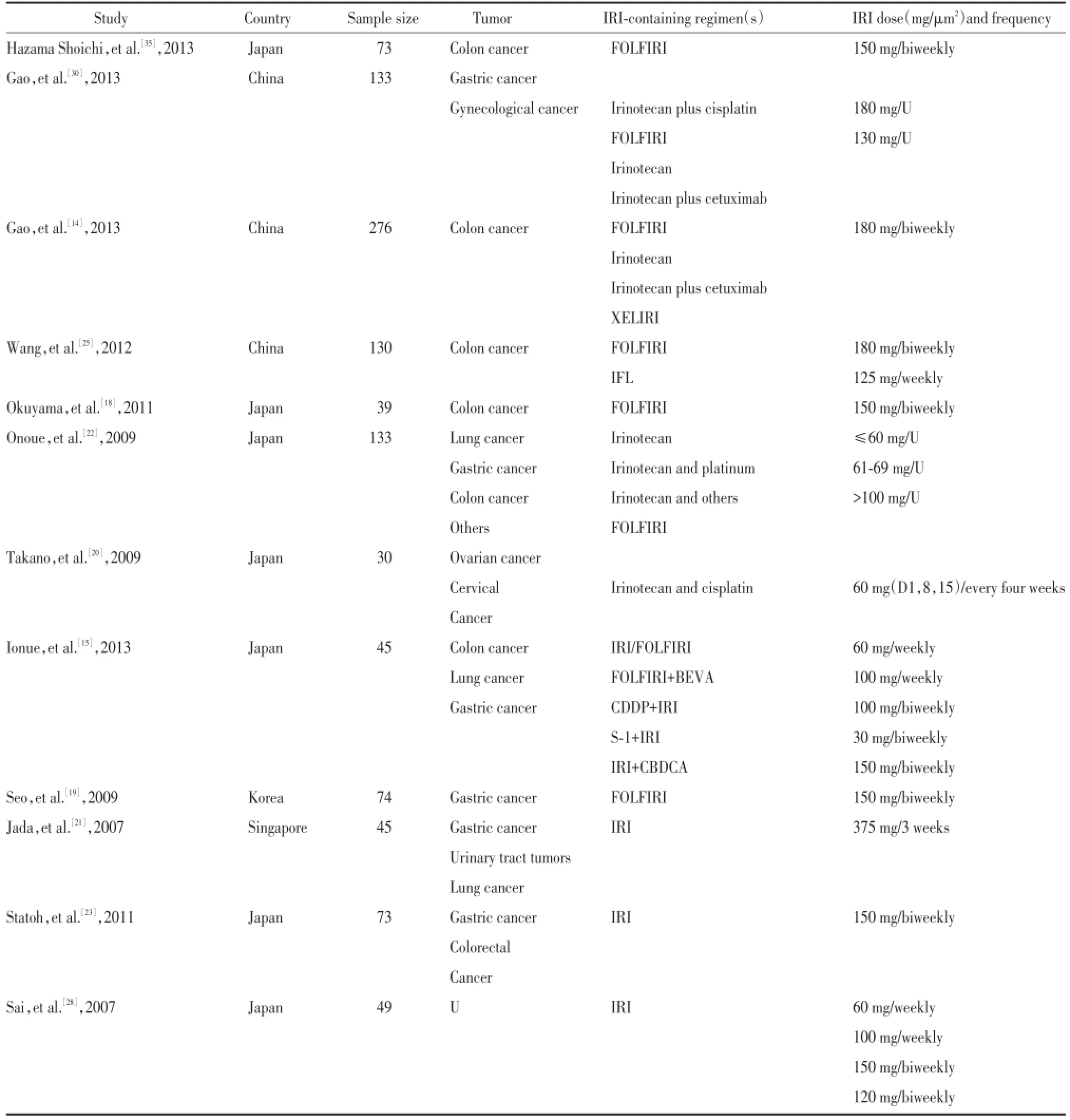
表1 基本信息表Tab.1 Main characteristics of the studies included in the meta-analysis
3 讨论
UGT1A1*6多态性对伊立替康毒性的影响主要体现在粒细胞缺乏上,对腹泻的影响并不明显。我们考虑,粒细胞缺乏主要为伊立替康的血液学毒性反应,因此受UGT1A1*6多态性影响较大,而腹泻可能与患者既往接受手术、放疗等多种因素有关,因此受UGT1A1*6多态性影响较小。
伊立替康主要代谢部位为肝脏。在体内经CE转化为SN-38发挥细胞毒效应。SN-38进而经UGT1A1酶灭活为葡萄糖醛酸产物SN-38G后,通过胆汁排泄入肠。因此,伊立替康毒性不仅与UGT1A1酶有关,而是多种酶共同作用的结果。目前有研究[2]表明,ABCB1 C3435T多态性与伊立替康代谢有关,进一步研究应用伊立替康不良反应的人群的基因表达,可更加全面准确预测伊立替康的毒性。
综上所述,UGT1A1*6多态性对伊立替康毒性有影响,无论粒细胞缺乏还是腹泻,UGT1A1*6突变型均较野生型有更大的毒性风险,且纯合突变型风险更大,因此UGT1A1*6多态性可以预测伊立替康的毒性反应,从而指导临床用药。

图1 粒细胞缺乏AA:GGFig.1 AA:GG neutropenia
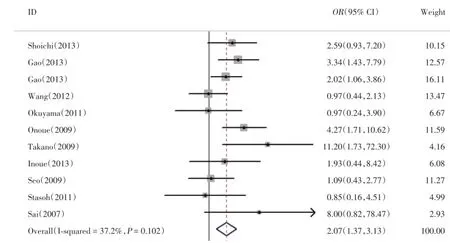
图2 粒细胞缺乏GA:GGFig.2 GA:GG neutropenia

图3 腹泻AA:GGFig.3 AA:GG diarrhea
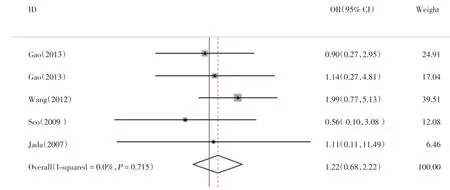
图4 腹泻GA:GGFig.4 GA:GG diarrhea
[1]Rothenberg ML,Eckardt JR,Kuhn JG,et al.PhaseⅡtrial of irinotecan in patients with progressive or rapidly recurrent colorectal cancer[J].Clin Oncol,1996,14(4):1128-1135.
[2]Lara PN Jr,Natale R,Crowley J,et al.PhaseⅢtrial of irinotecan/cisplatin compared with etoposide/cisplatin in extensive-stage smallcell lung cancer:clinical and pharmacogenomic results from SWOG S0124[J].Clin Oncol,2009,27(15):2530-2535.
[3]Lim WT,Lim ST,Wong NS,et al.CPT-11 and cis¬platin in the treatment of advanced gastric cancer in Asians[J].J Chemother,2003,15(4):400-405.
[4]Yamamoto K,Kokawa K,Umesaki N,et al.Phase I study of combination chemotherapy with irinotecan hydrochlo¬ride and nedaplatin for cervical squamous cell carcinoma:Japanese gynecologic oncology group study[J].Oncol Rep,2009,21(4):1005-1009.
[5]de Forni M,Bugat R,Chabot GG,et al.Phase I and pharmacokinetic study of the camptothecin derivative irinotecan,administered on a weekly schedule in cancer patients[J].Cancer Res,1994,54(16):4347-4354.
[6]Iyer L,King CD,Whitington PF,et al.Genetic predisposition to the metabolism of irinotecan(CPT-11).Role of uridine diphosphate glucuronosyltransferase isoform 1A1 in the glucuronidation of its active metabolite(SN-38)in human liver microsomes[J].Clin Investig,1998,101(4):847-854.
[7]Toffoli G,Cecchin E,Corona G,et al.The role of UGT1A1*28polymorphism in the pharmacodynamics and pharmacokinetics of irinotecan in patients with metastatic colorectal cancer[J].Clin Oncol,2006,24(19):3061-3068.
[8]Cote JF,Kirzin S,Kramar A,et al.UGT1A1 polymorphism can predict hematologic toxicity in patients reated with irinotecan[J].Clin Cancer Res,2007,13(11):3269-3275.
[9]Rouits E,Charasson V,Petain A,et al.Pharmacokinetic and pharmacogenetic deter¬minants of the activity and toxicity of irinotecan in metastatic colorectal cancer patients[J].Br J Cancer,2008,99(8):1239-1245.
[10]Marcuello E,Altes A,Menoyo A,et al.UGT1A1 gene variations and irinotecan treat¬ment in patients with metastatic colorectal cancer[J].Br J Cancer,2004,91(4):678-682.
[11]Ando Y,Chida M,Nakayama K,et al.The UGT1A1*28 allele is relatively rare in a Japanese population[J].Pharmacogenetics,1998,8(4):357-360.
[12]Mackenzie PI,Bock KW,Burchell B,et al.Nomencla¬ture update for the mammalian UDP glycosyltransferase(UGT)gene superfamily[J].Pharmacogenet Genomics,2005,15(10):677-685.
[13]Han JY,Lim HS,Shin ES,et al.Comprehensive analysis of UGT1A polymorphisms predictive for pharmacokinetics and treatment outcome in patients with non-small-cell lung cancer treated with irinotecan and cisplatin[J].Clin Oncol,2006,24(15):2237-2244.
[14]Gao J,Zhou J,Li Y,et al.UGT1A1 6/28 polymorphisms could predict irinotecan-induced severe neutro¬penia not diarrhea in Chinese colorectal cancer patients[J].Med Oncol,2013,30(3):604.
[15]Inoue K,Sonobe M,Kawamura Y,et al.Polymorphisms of the UDP-glucuronosyl transferase 1A genes are associated with adverse events in cancer patients receiving irinotecan-based chemotherapy[J].Tohoku J Exp Med,2013,229(2):107-114.
[16]Choi YH,Kim TW,Kim KP,et al.A phaseⅡstudy of clinical outcomes of 3-week cycles of irinotecan and S-1 in patients with previously untreated meta-static colorectal cancer:influence of the UGT1A1 and CYP2A6 polymorphisms on clinical activity[J].Oncology,2012,82(5):290-297.
[17]Nakamura Y,Soda H,Oka M,et al.Randomized phaseⅡtrial of irinotecan with paclitaxel or gemcitabine for non-small cell lung cancer:association of UGT1A1*6and UGT1A1*27with severe neutro-penia[J].Thorac Oncol,2011,6(1):121-127.
[18]Okuyama Y,Hazama S,Nozawa H,et al.Prospective phase II study of FOLFIRI for mCRC in Japan,including the analysis of UGT1A1 28/6 poly¬morphisms[J].Jpn J Clin Oncol,2011,41(4):477-482.
[19]Seo BG,Kwon HC,Oh SY,et al.Comprehensive analysis of excision repair com¬plementation group 1,glutathione S-transferase,thymidylate synthase and uridine diphosphate glucuronosyl transferase 1A1 polymorphisms predictive for treatment outcome in patients with advanced gastric cancer treated with FOLFOX or FOLFIRI[J].Oncol Rep,2009,22(1):127-136.
[20]Takano M,Kato M,Yoshikawa T,et al.Clinical significance of UDP-glucuronosyltransferase 1A1*6 for toxicities of combi¬nation chemotherapy with irinotecan and cisplatin in gyneco-logic cancers:a prospective multi-institutional study[J].Oncology,2009,76(5):315-321.
[21]Jada SR,Lim R,Wong CI,et al.Role of UGT1A1*6,UGT1A1*28 and ABCG2 c.421C>A polymorphisms in irinotecan-induced neu¬tropenia in Asian cancer patients[J].Cancer Sci,2007,98(9):1461-1467.
[22]Onoue M,Terada T,Kobayashi M,et al.UGT1A1*6polymorphism is most predictive of severe neutropenia induced by irinotecan in Japanese cancer patients[J].Int J Clin Oncol,2009,14(2):136-142.
[23]Satoh T,Ura T,Yamada Y,et al.Genotype-directed,dose-finding study of irinotecan in cancer patients with UGT1A1*28 and/or UGT1A1*6 polymorphisms[J].Cancer Sci,2011,102(10):1868-1873.
[24]Takahara N,Nakai Y,Isayama H,et al.Uridine diphos¬phate glucuronosyl transferase 1 family polypeptide A1 gene(UGT1A1)polymorphisms are associated with toxicity and effi¬cacy in irinotecan monotherapy for refractory pancreatic cancer[J].Cancer Chemother Pharmacol,2013,71(1):85-92.
[25]Wang Y,Shen L,Xu N,et al.UGT1A1predicts outcome in colorectal cancer treated with irinotecan and fluorouracil[J].World J Gastroenterol(WJG),2012,18(45):6635-6644.
[26]Sunakawa Y,Ichikawa W,Fujita K,et al.UGT1A1*1/*28and*1/* 6 genotypes have no effects on the efficacy and toxicity of FOLFIRI in Japanese patients with advanced colorectal cancer[J].Cancer Chemother Pharmacol,2011,68(2):279-284.
[27]Ando Y,Saka H,Ando M,et al.Polymorphisms of UDP-glucuronosyltransferase gene and irinotecan toxicity:a pharmacogenetic analysis[J].Cancer Res,2000,60(24):6921-6926.
[28]Sai K,Saito Y,Sakamoto H,et al.Importance of UDP-glucuronosyltransferase 1A1*6 for irinotecan toxicities in Japanese cancer patients[J].Cancer Lett,2008,261(2):165-171.
[29]Akie K,Oizumi S,Ogura S,et al.PhaseⅡstudy of irinotecan plus S -1 combination for previously untreated advanced non-small cell lung cancer:Hokkaido Lung Cancer Clinical Study Group Trial(HOT)0601[J].Oncology,2011,81(2):84-90.
[30]Gao J,Zhou J,Li Y,et al.Associations between UGT1A1*6/*28 polymorphisms and irinotecan-induced severe toxicity in Chinese gastric or esophageal cancer patients[J].Med Oncol,2013,30(3):630.
[31]Ando Y,Ueoka H,Sugiyama T,et al.Polymorphisms of UDP-glucuronosyltrans¬ferase and pharmacokinetics of irinotecan[J].Ther Drug Monit,2002,24(1):111-116.
[32]Fukushima M,Sakamoto K,Ohshimo H,et al.Irinotecan overcomes the resistance to 5-fluorouracil in human colon cancer xenografts by down-regulation of intratu¬moral thymidylate synthase[J].Oncol Rep,2010,24(4):835-842.
[33]Ide H,Kikuchi E,Hasegawa M,et al.Therapeutic enhancement of S-1 with CPT-11 through down-regulation of thymidylate synthase in bladder cancer[J].Cancer Med,2013,2(4):488-495.
[34]Raida M,Schwabe W,Hausler P,et al.Prevalence of a common point mutation in the dihydropyrimidine dehydrogenase(DPD)gene within the 5′-splice donor site of intron 14 in patients with severe 5-fluorouracil(5-FU)-related toxicity compared with controls[J].Clin Cancer Res,2001,7(9):2832-2839.
[35]Hazama S,Mishima H,Tsunedomi R,et al.UGT1A1*6,1A7*3,and 1A9*22 genotypes predict severe neutropenia in FOLFIRI-treated metastatic colorectal cancer in two prospective studies in Japan[J].Cancer Sci,2013,104(12):1662-1669.
(编辑 武玉欣)
Association of UGT1A1*6Polymorphismswith Irinotecan-induced Toxicities:AMeta-analysis
GUOXiao-yu,LIZhi,QUXiu-juan,LIU Yun-peng
(DepartmentofMedicalOncology,The FirstHospital,China MedicalUniversity,Shenyang 110001,China)
Objective To conduct a meta-analysis of literatures to explore the relationship of UGT1A1*6gene polymorphism and irinotecan toxicity,so as to guide clinical treatment.MethodsPapers were searched by PubMed database and manual search.The inclusion and exclusion criteria of studies were formulated and the methodologies quality was assessed,data were extracted and the statistical analysis was made using STATA12.0 software.ResultsAtotalof12 articles were included according to the inclusion and exclusion criteria.Patients with mutated UGT1A1*6showed an increased risk for neutropenia compared to wild UGT1A1*6(OR=2.37,95%CI 1.58-3.55,P=0.001).Both homozygous and heterozygous mutation showed an increased risk for neutropenia compared to wild type and the homozygous mutation(OR=5.09,95%CI 2.74-9.45,P<0.001)showed an even higher risk for neutropenia compared to the heterozygous mutation(OR=2.07,95%CI 1.37-3.13,P=0.001).For severe diarrhea,mutated UGT1A1*6showed an increased risk compared to wild type(OR=1.48,95%CI 0.86-2.55,P=0.153),though without statistical significance.The homozygous mutation performed a significantly increased risk(OR=3.51,95%CI 1.33-9.25,P=0.011)and the heterozygous mutation also showed increased risk,however,the difference between them was notstatistically significant.ConclusionUGT1A1*6polymorphisms can predictirinotecan toxicity,especially forincidence ofneutropenia.
UGT1A1*6;polymorphisms;irinotecan;toxicity;meta-analysis
R73-31
A
0258-4646(2015)07-0596-06
国家自然科学基金(81372485);辽宁省科学技术计划(2011404013-1;2012225001);辽宁省高等学校杰出青年学者成长计划(LJQ2011082);高等学校博士学科点联合资助(20112104110005)
郭晓玉(1988-),女,医师,硕士.
刘云鹏,E-mail:cmuliuyunpeng@hotmail.com
2014-12-16
网络出版时间:

