Variations of Summer Phytoplankton Community Related to Environmental Factors in a Macro-Tidal Estuarine Embayment, Hangzhou Bay, China
ZHANG Yuexia, YU Jun, JIANG Zhibing, WANG Qin, and WANG Hui
Variations of Summer Phytoplankton Community Related to Environmental Factors in a Macro-Tidal Estuarine Embayment, Hangzhou Bay, China
ZHANG Yuexia1), 2), YU Jun2), JIANG Zhibing3), WANG Qin2), and WANG Hui4), *
1),,266100,2),310007,3),,,310012,4),100081,
To explore the distribution and composition of phytoplankton community and their responses to environmental changes, summer net-collected phytoplankton and physicochemical parameters in the Hangzhou Bay during 2004–2010 were investigated. A total of four phyla and 84 species were identified, including 67 diatom and 12 dinoflagellate species. The dominant species con- stantly consisted of the diatoms, although the dominance of dinoflagellate and cyanobacteria increased recently. Due to great spa- tio-temporal variations in environmental factors (salinity, suspended solids, and nutrient concentration), significant heterogeneities in community compositions among different years and subregions (inner and middle sections, and bay mouth) were found based on the analyses of multidimensional scaling and similarity. Canonical correspondence analysis showed that salinity and Si/N were the main variables associated with algal assemblage. Compared with the historical data since the 1980s, eutrophication (N, P, and N/P in- creased with decreasing Si/N) was exacerbated drastically. Moreover, climatic forcing and human activities resulted in a series of physical alterations, including sediment retention,temperature increase, and salinity decrease as well as reduction in water exchanges. All these changes induced obvious increases in cell density and Chl-while decreases in species diversity and diatom-dinoflagellate ratio as well as the shifting of dominant species. Therefore, the long-term phytoplankton variations were closely related to anthropo- genic and climatic perturbations in the Hangzhou Bay.
Hangzhou Bay; phytoplankton; long-term changes; eutrophication; Qiantang River; Changjiang
1 Introduction
Hangzhou Bay (HZB) is a macro-tidal estuarine em- bayment located to the south of Changjiang Estuary (CE) in the East China Sea. Besides the Changjiang River, the bay receives other riverine loading, including Qiantang, Yong, and Cao’e River. The area (with cities, Shanghai, Hangzhou, Ningbo,) around the HZB is an important populous and economic center in China, and thus the bay has been suffering from intensive human activities, such as large-scale tidal flat reclamation (Ni and Lin, 2003; Zhang., 2010) and rapid industrial and agricultural development. Particularly since the 1980s, various all the nutrients and organic matters, and other pollutants in- flowing into the HZB have drastically increased (Zhang., 2001; Han., 2003; Gao., 2011). Moreover, recent precipitation increase with decreasing evaporation (Wang., 2011, 2013) as well as the successive fre-quent water conservancy projects in the Qiantang River and Changjiang basins (Xu., 2010; Yang., 2011) have significantly altered the freshwater and sediment-associated nutrient inputs (N, P, and Si) into the bay. All these anthropogenic activities and climatic forcing have significantly altered the geographical and physicochemi- cal environment (Xie., 2009, 2013). Consequently, HZB has confronted a series of ecological challenges caused by the anthropogenic and climate-driven physico- chemical changes.
Phytoplankton is the important primary producer in estuarine/coastal ecosystem that plays a key role in the biogeochemical processes in riverine-seawater interface (Cloern and Dufford, 2005). It is also highly sensitive to environmental destabilizations and thus used in the as- sessment of ecological conditions and estuarine/coastal environmental changes (Zhou., 2008; Jiang., 2014). Therefore, its community variations are always paid great attention by researchers. Previous studies (Zhou., 2008; Wang and Wu, 2009; Li., 2010; Jiang., 2010; Jiang, 2013, 2014) have found signify- cant phytoplankton community alterations (, abundance increase, diatom-dinoflagellate ratio decrease, dominant species shifting, and frequent harmful algal blooms) in the CE and Xiangshan Bay adjacent to the HZB, owing to intensive human- and climate-driven changes. Phyto- plankton community in the HZB is also subjected to in- creased physicochemical stresses. However, distribution and composition of phytoplankton community associated with environmental factors in the HZB are not well documented (Zhang., 2001; Cai, 2006; Wang., 2008; Zhou., 2010). Moreover, the variations in phy- toplankton community response to environmental changes have not been examined.
The present study focused on the variations of net-col- lected phytoplankton in the HZB during 2004–2010 com- bined with historical data since the 1980s, which allowed investigation into the long-term changes in the phyto- plankton community during the past 30 years. The influ- ences of environmental factors on phytoplankton were also examined. Our objectives are 1) to understand the distribution and composition of the phytoplankton com- munity in relation to environmental parameters in the HZB; 2) to explore the long-term changes in phytoplank- ton assemblage under increasing anthropogenic and cli- matic perturbations; and 3) to supply a scientific founda- tion for environmental protection and further study on this estuarine bay.
2 Materials and Methods
2.1 Study Area
The HZB is a large funnel-shaped and shallow (with an average depth of about 10m) estuarine bay located in the East China Sea, immediately south of the CE and east of the Zhoushan archipelago (Fig.1). The semidiurnal tide is the main driving force behind the horizontal water flow in the HZB, with the M2constituent being the dominant tidal component (ECBCC, 1992). The average tide am- plitude is 3–4 m at the mouth and 4–6 m further upstream. The main river system discharging freshwater and sedi- ment directly into the HZB is Qiantang River, followed by Cao’e and Yong River. The Qiantang River has an average runoff of 163.7×108m3yr−1and an average sedi- ment load of 203.5×104tyr−1recorded at Lanxi station (data from the website: http://www.irtces.org/nishagb_2012. asp). The CE is situated immediately north of the HZB. The average water discharge from Changjiang is 8927×108m3yr−1, with a sediment load of 3.85×108tyr−1re- corded at Datong station (data from the website: http://www.irtces.org/nishagb_2012.asp). A part of water and sediment from the CE enters the HZB and imposes pro- found influences on both hydrodynamics and sedimenta- tion of the HZB (Su and Wang, 1989). The Changjiang Diluted Water (CDW) plays an important role in both the circulation and sediment transport inside the bay (Su and Wang, 1989; Xie., 2013). The plume front serves as a guide for sediment transport and it is closely related to the rapid accretion at the south shore of the bay (Xie., 2009, 2013).
2.2 Sample Collection and Analysis
A total of 12 stations (Fig.1) were set in HZB in the summer during 2004–2010. Phytoplankton samples were collected vertically from bottom to surface using a plank- ton net with a mesh size of 76μm (Jiang., 2013). All collection, preservation, and identification of the phyto- plankton samples were conducted according to the speci- fication GB 17378.7-2007. Surface salinity, dissolved in- organic nitrogen (DIN), phosphorus (DIP), silicate (DSi), chemical oxygen demand (COD), and suspended solids (SS) were measured according to the specification GB 17378.4-2007.
2.3 Data Analysis
The indices of Shannon diversity () and Pielou evenness () were calculated through the PRIMER 6.0. Dominance () was calculated according to the following equation:f×n/×100%,wherenis the number (cells) of individuals of species,is the total number of indi- viduals, andfis the frequency of speciesoccurring in all the samples. Only those species with≥2% were consid- ered to be the dominants. The eutrophication index () was calculated according to the following equation:=DIN×DIP×COD/4500, where the units of DIN, DIP, and COD are mgL−1. Two-way ANOVA was used to test sig- nificant differences in the phytoplankton community and environmental variables (, salinity, SS, COD, DIN, DIP, DSi, N/P, and Si/N) among different years and subregions (inner, middle section, and mouth of the bay). Prior to ANOVA, phytoplankton variables and physicochemical parameters were tested for normality (Kolmogorov-Smirnov test) and homogeneity (Levene test), and all were log transformed where necessary. Statistically significant differences (<0.05) among treatments were further as- sessed using the Student-Newman-Keuls test. The Kruskal-Wallis test was performed on data that did not satisfy the assumptions of normality and homogeneity.
Non-metric multidimensional scaling (NMDS) was per- formed to determine spatial patterns in community struc- ture using PRIMER 6.0 software. Species abundance was log(+1) transformed and standardised before estimation using Bray-Curtis similarities between sample pairs. A two-way analysis of similarity (ANOSIM) was used to test significant differences among the phytoplankton com- munities in the different subareas and years. Phytoplank- ton species (excluded the rare species with the abundance contribution<0.01%andoccurrencefrequency<5%)abun- dance and environmental data were applied to canonical correspondence analysis (CCA) using CANOCO 4.5 soft- ware.
3 Results
3.1 Environmental Variables
Both the salinity and SS decreased gradually from 2005 to 2010, while the COD, nutrients (DIN, DIP, and DSi), andincreased persistently in all the subregions of the HZB (Figs.2 and 3). Table 1 shows all the environmental parameters representing significant (<0.05) temporal differences. Except the COD, N/P, and Si/N, all the other variables showed significant (<0.01) spatial differences among the inner, middle section, and mouth of the HZB. The levels of SS, COD, and nutrients decreased with in-creasing salinity (Fig.2).
3.2 Taxonomic Composition
A total of four phyla (, Bacillariophyta, Dinophyta, Cyanophyta, and Chlorophyta), 43 genera, and 84 species of net-collected phytoplankton were indentified. Diatoms displayed the highest species number (67), followed by dinoflagellates (12). Diatoms dominating the phytoplank- ton community ranged in 75.0%–93.9% of the species number and 86.6%–99.0% of the total abundance during 2004–2010. Phytoplankton in HZB showed a complicated speciescomposition,includingfreshwater(,spp.), brackish (,), coastal (,), and offshore species (,) along the estuarine salinity gradient.

Table 1 Results of the two-way ANOVA (F) or Kruskal- Wallis (H) test for phytoplankton community and environmental parameters
Notes: SS: suspended solids; COD: chemical oxygen demand; DIN: dissolved inorganic nitrogen; DIP: dissolved inorganic phosphorus; DSi: dissolved silicate; Chl-: chlorophyll;: Shannon diversity index;: Pielou evenness index.nsNo significance,*<0.05,**<0.01,***<0.001.

Fig.2 Summer environmental parameters in different subregions in the Hangzhou Bay during 2004–2010.
3.3 Biomass and Species Diversity
Both the cell density and chlorophyll(Chl-) increased gradually from 2004 to 2010, while the species diversity and evenness decreased constantly (Fig.4). Table 1 shows all these variables representing significant (<0.05) temporal differences, but there was no significant difference among different subregions in the HZB.

Fig.4 Phytoplankton community parameters in different subregions of the Hangzhou Bay in summer during 2004–2010.
3.4 Dominant Species
A total of 13 dominant species were found in the HZB during 2004–2010 (Table 2).The dominant species in dif- ferent years always consisted of diatoms (,genera,,,,, and) except the year of 2009 (domi- nated by thespp.). The dominance ofincreased gradually with decreasing(Fig.5).

Table 2 Dominance (%) of the dominant species in the Hangzhou Bay
Note: ‘–’, not detected.
3.5 Community Composition Analysis
NMDS and ANOSIM showed a significant difference (Global=0.472;=0.001) in phytoplankton community in different years (Fig. 6). In addition, ANOSIM showed visible (Global=0.298;=0.001) assemblage differ- ences among different subareas in all years although there was no significant (=0.151;=0.065) difference be- tween the inner and middle sections.
3.6 Canonical Correspondence Analysis (CCA)
The CCA showed higher significant scores (=0.002) for Axis 1 and all canonical axes, thus the ordination re- sults are believable. These 8 environmental variables (sa- linity, SS, COD, DIN, DIP, DSi, N/P, and Si/N) in CCA explained the total variation of 25.8% in the phytoplankton community. The species-environment coefficients of Axis 1 and 2 were 0.796 and 0.829, indicating a significant relationship between the 8 environmental factors and the 75 samples. Fig.7 demonstrates that salinity was the primary factor associated with the phytoplankton assem- blage in 2004–2010.
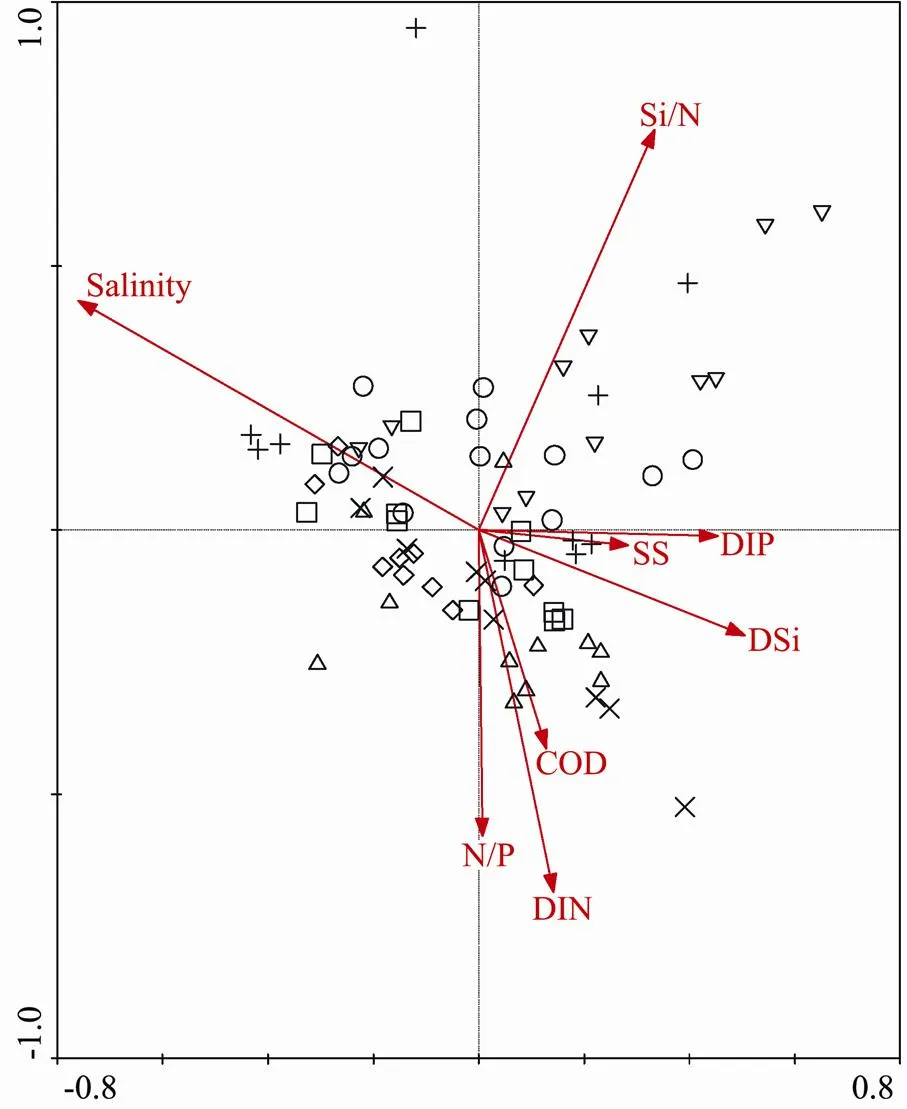
Fig.7 CCA ordination of phytoplankton samples with environmental variables. The symbols indicates sam- pling years which are the same as Fig.6a.
4 Discussions
4.1 Relationship Between Phytoplankton Distribution and Environmental Factors
The HZB in summer is mainly influenced by the run- offs from Qiantang River and Changjiang, CDW, and Taiwan Warm Current (ECBCC, 1992). Therefore, the phytoplankton in HZB showed a complicated species composition, including the freshwater, brackish, coastal, and offshore species, based on the salinity gradient. How- ever, the use of a 76-µm net to collect phytoplankton samples in this study resulted in loss of the small-sized phytoplankton species (Jiang., 2013, 2014), and thus relatively low total species number.
The present study found that the phytoplankton com- munity was absolutely dominated by diatoms (the domi- nant species mainly consisted of,, and), both in species number and cell density. These results are consistent with the compo- sition of net-collected microalgal community in the adja- cent Xiangshan Bay (Jiang., 2013). This finding supports the empirically based principle that diatoms re- spond rapidly to episodic high-light and high-nutrient conditions (Cloern and Dufford, 2005). However, the dominance of dinoflagellate and other taxonomic groups increased recently with decreasing diatom density. This might be attributed to the recent decreasing trend in Si/N.
The SS concentrations in the inner and middle sections were much higher than those in the mouth of the HZB (Fig.2), due to the higher flow velocity, stronger tidal resuspension, and abundant sediment input from the Qiantang River (ECBCC, 1992; Xie., 2013). Other- wise, the freshwater discharges provided high levels of nutrients (DIN, DIP, and DSi) and COD in the HZB along the salinity gradient (Fig.2). However, the light limitation (due to the extremely high SS concentration) and change- able osmotic stresses in the upstream estuarine bay re- strained the phytoplankton growth and primary produc- tion (Fig.4), although the inner and middle sections pre- sented highlevel (Fig.3). Many studies found that high turbidity is an important physical process that limits light availability for estuarine primary productivity, such as the cases of the CE (Zhu., 2009) and Guadiana Estuary (Domingues., 2011). In contrast, high cell density and Chl-were found in the mouth in our study with rela- tively high nutrients and deep light penetration (Fig.2), due to exchanges with the external clear water impacted by the Taiwan Warm Current.
CCA showed that salinity was the primary variable as- sociated with the phytoplankton community (Fig.7). This supports the empirically-based principle that salinity varying geographically is the most important factor which determines the phytoplankton composition and distribution in the estuarine waters (, Van Damme., 2009). The hydrological (circulation, SS, and salinity) and nu- trient conditions in the HZB were significantly different from the inner section to mouth, leading to the obvious spatial differences in assemblage compositions (Fig.6). According to the NMDS and ANOSIM, the phytoplank- ton also showed spatial heterogeneity along the salinity gradient, driven by the different environmental factors among different subareas (Cloern and Dufford, 2005; Jiang., 2013).
4.2 Long-Term Changes in Phytoplankton Under Increasing Human and Climatic Perturbations
The estuarine HZB, as a transition zone between river and ocean environments, is subject to riverine (, dis- charges of sediment and freshwater associated with huge nutrients) and marine (, tides, waves, and saline water intrusion) mutual impact. The changes in phytoplankton community in the HZB are profoundly impacted by the above-mentioned environmental factors, especially the runoff, sediment, nutrients, and water exchange (Van Damme., 2009). Yu. (2013) simulated that the phytoplankton dynamics in the estuarine Chesapeake Bay were controlled by the river flow associated with terri- genous materials, according to a 3-D physical-biogeo- chemical model based on ROMS.
Large-scale dam construction in the Qiantang River and Changjiang basins (Xu., 2010; Yang., 2011) retained huge sediments, while the recent precipitation increase with decreasing evaporation (Wang., 2011, 2013) enhanced the freshwater discharges from Qiantang River (Fig.8). These anthropogenic and climatic changes resulted in an increase in nutrients and reduction in SS and salinity (Figs.2 and 9), which profoundly influenced the phytoplankton community variations. For example, the turbidity reduction (transparency enhancement) com- bined with eutrophication exacerbation (Figs.2 and 3) in this seriously light-limited HZB promoted the algal growth. Fig.10 shows that phytoplankton cell density and Chl-increased gradually during 1982–2012. In addition, the temperature increase caused by global warming might have amplified the microalgal biomass. Jiang. (2014) found that summer SST in the CE increased significantly at a rate of 0.49℃/decade during 1982 to 2009.
Due to the extensive use of chemical fertilizers and the large discharge of industrial wastewater and domestic sewage, the riverine N and P loadings increased persis- tently, which induced continual elevation in DIN and DIP in the HZB, as well as drastic alteration in nutrient ratios (significant increase in N/P and decrease in Si/N) since 1980s (Fig.9). The results are similar to those in the pre- vious studies on the adjacent CE (Zhou., 2008; Jiang., 2010; Li., 2010; Gao., 2012; Jiang., 2014). Meanwhile, DSi concentration in the HZB did not change a lot, although the sediments from Changjiang and Qiantang River decreased sharply (Fig.8). Gao. (2012) concluded that the sharply reduced sediments do not nec- essarily mean the weathering rates of silicate rocks have also decreased. Moreover, large-scale tidal flat reclama-tion (Ni and Lin, 2003; Zhang., 2010) changed the hydrodynamic environment (tide, flow field, residual currents,), reduced the water exchange and residence time, prolonged the nutrient-lingering period, and thereby aggravated the eutrophication (Jiang., 2013). This adverse factor would exacerbate harmful algal blooms in the HZB mouth as well as the adjacent CE and Zhoushan archipelago in terms of frequency, range, persistence, and destructive capability (Zhou., 2008; Wang and Wu, 2009; Li., 2010).

Fig.8 Long-term changes in annual average runoff and sediment of (a) Qiantang River and (b) Changjiang (data from the website: http://www.irtces.org/nishagb_2012.asp).

Fig.9 Long-term changes of nutrient parameters in the Hangzhou Bay in summer (other data from personal data; ECBCC, 1992; Liu et al., 2001; Zhang et al., 2002; personal data).

Fig.10 Long-term changes in summer phytoplankton community parameters in the Hangzhou Bay (other data from Liu and Ning, 1994; Liu et al., 2001; Cai, 2006; Wang et al., 2008; SOA, 2012, 2013; personal data).
Fig.5 and Table 3 show that the dominance of non-group livingdecreased gradually, while that of small-size chain-forming species (,,, and) increased, especially the. This change might refer to eutrophication exacerbation and runoff increase (salinity decrease). As an extremely high siliceous group, the sharp decline of Si/N inevitably inhibited the growth and re- production of, while the relatively low siliceous diatoms (,, and) profited from this condition. These species, as-strategists, can rapidly multiply under increasing eutro- phication, particularly the(Liu., 2002). Besides, as estuarine species,spp. gradually takes the advantage of community competition in the tur- bid estuary with low salinity, such as the CE. Many field investigations (, Guo and Yang, 1992; Luan., 2006) found that this microalgae population generally dominates (>95% in total cell density) and blooms in the principal part of CDW with the salinity <23. The labora- tory experimental study by Li. (2005) supports this finding. They found that the optimal growth salinity ofis 19.2 with the suitable range of 14–23. Thus, on the conditions of decreasing salinity (<20), the domi- nance ofspp. in the HZB has increased ob- viously.

Table 3 Long-term changes in summer dominant species in the Hangzhou Bay
Under increasing Si-limitation (Si/N decrease) in the HZB, both the richness and cell density of the diatom species decreased gradually, while the dominance of dinoflagellate and other taxonomic groups (mainly the cyanobacteria) increased (Fig.11). Similarly, diatom-dinoflagellate ratio in species and cell number in the ad- jacent CE (Zhou., 2008; Li., 2010; Jiang., 2014) and Xiangshan Bay (Jiang., 2013) decreased significantly under the decreasing Si/N stresses. Besides, CDW shrinkage (caused by recent sharp reduction in runoffs from Changjiang in Fig.8; Jiang., 2014) combined with increasing intrusion of Taiwan Warm Current (Tang., 2009) brought about abundant off- shorein summer (Table 3), in despite of the salinity reduction in the HZB. However, due to the abso- lutely dominance by several chain-forming species, the phytoplankton community diversity and evenness de- creased evidently (Fig.10). This abnormal phenomenon was also found in the CE (Li., 2010).

Fig.11 Long-term changes in summer phytoplankton community composition in the Hangzhou Bay.
5 Conclusions
The summer net-collected phytoplankton community in HZB was dominated by diatoms during 2004–2010, mainly consisting of genera,, and. Planktonic assemblages showed sig- nificant spatial heterogeneity along the salinity gradient. Increasing human activities and climatic forcing resulted in a series of physicochemical alterations, including eu- trophication exacerbation, light limitation alleviation, and salinity reduction. These changes induced an obvious increase in algal biomass with decreasing species diver- sity and diatom-dinoflagellate ratio as well as the shifting of dominant species. The present study confirmed that long-term changes in phytoplankton community in the HZB were closely related to the anthropogenic and cli- matic perturbations. However, the present use of a net with 76-µm mesh size resulted in loss of the small-sized phytoplankton species. We should obtain more informa- tion from water samples of microalgae with small cell sizes, and examine the data from net-collected samples to better understand the responses of phytoplankton to envi- ronmental changes in future.
Acknowledgements
The data that this paper used was provided by the Ma- rine Monitoring and Forecasting Center of Zhejiang. This work was funded by the National Basic Research Pro- gram of China (2010CB428903), the National Marine Public Welfare Research Project of China (201305009 and 201305043-3), the National Natural Science Founda- tion of China (41206103), and Basic Scientific Research of Second Institute of Oceanography, SOA (JG1222).
Cai, Y. H., 2006. Study on the phytoplankton diversity in the Hangzhou Bay. Master thesis. Ocean University of China.
Cloern, J. E., and Dufford, R., 2005. Phytoplankton community ecology: Principles applied in San Francisco Bay., 285: 11-28.
Domingues, R. B., Anselmo, T. P., Barbosa, A. B., Sommer, U., and Galvão, H. M., 2011. Light as a driver of phytoplankton growth and production in the freshwater tidal zone of a turbid estuary., 91 (4): 526-535.
Editorial Committee of the Bay Chorography in China (ECBCC), 1992.. China Ocean Press, Beijing, 166-233.
Gao, L., Li, D. J., and Zhang, Y. W., 2012. Nutrients and par- ticulate organic matter discharged by the Changjiang (Yang- tze River): Seasonal variations and temporal trends., 117 (G4), G04001.
Gao, S. Q., Chen, J. F., Jin, H. Y., Wang, K., Lu, Y., Li, H. L., and Chen, F. J., 2011. Characteristics of nutrients and eutro- phication in the Hangzhou Bay and its adjacent waters., 29 (3): 36-47.
Guo, Y. J., and Yang, Z. Y., 1992. Quantitative variation and ecological analysis of phytoplankton in the estuarine area of the Changjiang River., 33: 167-189.
Han, Z. C., Dai, Z. H., and Li, G. B., 2003.. China Water Power Press, Beijing, 122-131.
Jiang, T., Yu, Z. M., Song, X. X., Cao, X. H., and Yuan, Y. Q., 2010. Long-term ecological interactions between nutrient and phytoplankton community in the Changjiang Estuary., 28 (4): 887-898.
Jiang, Z. B., Liu, J. J., Chen, J. F., Chen, Q. Z., Yan, X. J., Xuan, J. L., and Zeng, J. N., 2014. Responses of summer phyto- plankton community to drastic environmental changes in the Changjiang (Yangtze River) Estuary during the past 50 years., 54: 1-11.
Jiang, Z. B., Zhu, X. Y., Gao, Y., Chen, Q. Z., Zeng, J. N., and Zhu, G. H., 2013. Spatio-temporal distribution of net-col- lected phytoplankton community and its response to marine exploitation in Xiangshan Bay., 31 (4): 762-773.
Li, J. T., Zhao, W. H., Yang, D. F., and Wang, J. T., 2005. Effect of turbid water in Changjiang (Yangtze) Estuary on the growth of., 29 (1): 34-37.
Li, Y., Li, D. J., Tang, J. L., Wang, Y. M., Liu, Z. G., and He, S. Q., 2010. Long-term changes in the Changjiang Estuary plankton community related to anthropogenic eutrophication., 13 (1): 66-72.
Liu, D. Y., Sun, J., Chen, Z. T., and Wei, T. D., 2002. Effect of N/P ratio on the growth of a red tide diatom., 2002 (2): 39-44.
Liu, Z. L., and Ning, X. R., 1994. Phytoplankton standing stock and primary production in the front of Hangzhou Bay., 12 (4): 58-66.
Liu, Z. L., Ning, X. R., and Cai, Y. M., 2001. Primary produc- tivity and standing stock of the Hangzhou Bay to the Zhou- shan fishing ground during autumn., 23 (2): 93-99.
Luan, Q. S., Sun, J., Shen, Z. L., Song, S. Q., and Wang, M., 2006. Phytoplankton assemblage of Yangtze River Estuary and the adjacent East China Sea in summer., 5 (2): 123-131.
Ni, Y. Q., and Lin, J., 2003. The effects of regulation and recla- mation in Qiantang Estuary on Hangzhou Bay., 21 (3): 73-77.
State Oceanic Administration (SOA), 2012..
State Oceanic Administration (SOA), 2013..
Su, J. L., and Wang, K. S., 1989. Changjiang River plume and suspended sediment transport in Hangzhou Bay., 9 (1): 93-111.
Tang, X. H., Wang, F., Chen, Y. L., and Li, M. L., 2009. Warm- ing trend in northern East China Sea in recent four decades., 27 (2): 185-191.
Van Damme, S., Struyf, E., Maris, T., Cox, T., and Meire, P., 2009. Characteristic aspects of the tidal freshwater zone that affect aquatic primary production In:. Barendregt, A.,, eds., Backhuys, 123-136.
Wang, J. H., and Wu, J. Y., 2009. Occurrence and potential risks of harmful algal blooms in the East China Sea., 407 (13): 4012-4021.
Wang, Y. J., Ding, Y. J., Ye, B. S., Liu, F. J., and Wang, J., 2013. Contributions of climate and human activities to changes in runoff of the Yellow and Yangtze rivers from 1950 to 2008., 56 (8): 1398-1412.
Wang, Y. J., Liu, B., Su, B. D., Zhai, J. Q., and Gemmer, M., 2011. Trends of calculated and simulated actual evaporation in the Yangtze River Basin., 24: 4494-4507.
Wang, Y. L., Yuan, Q., and Shen, X. Q., 2008. Distribution status and change tendency of phytoplankton during summer in Changjiang Estuary and adjacent waters., 27 (2):169-172.
Xie, D. F., Pan, C. H., Cao, Y., and Zhang, B. H., 2013. Decadal variations in the erosion/deposition pattern of the Hangzhou Bay and their mechanism in recent 50a., 35 (4): 121-178.
Xie, D. F., Wang, Z. B., Gao, S., and De Vriend, H. J., 2009. Modeling the tidal channel morphodynamics in a macro-tidal embayment, Hangzhou Bay, China., 29 (15): 1757-1767.
Xu, K., Milliman, J. D., and Xu, H., 2010. Temporal trend of precipitation and runoff in major Chinese rivers since 1951., 73: 219-232.
Yang, S. L., Milliman, J. D., Li, P., and Xu, K., 2011. 50000 dams later: Erosion of the Yangtze River and its delta., 75: 14-20.
Yu, Q. Y., Wang, Y., Tang, X. X., and Li, M., 2013. River flow control on the phytoplankton dynamics of Chesapeake Bay., 12 (1): 103-114.
Zhang, H. G., Sui, Y. Z., and Huang, W. G., 2010. Patterns of reclamation land use of Hangzhou Bay with remote sensing in the last two decades.. Michel, U., and Civco, D. L., eds., Proceedings of SPIE Vol. 7831, 78311E, 6pp.
Zhang, J., Shi, Q. S., Wu, X. Y., Ye, X. R., and Zhu, G. H., 2002. Distribution characteristic analysis of main pollution factor in rainy season in the Hangzhou Bay., 20 (4): 35-41.
Zhang, S. Y., Shao, J. B., and Dai, X. J., 2001. Studies on eu- trophication and phytoplankton diversity in Hangzhou Bay., 25 (6): 512-517.
Zhou, M. J., Shen, Z. L., and Yu, R. C., 2008. Responses of a coastal phytoplankton community to increased nutrient input from the Changjiang (Yangtze) River., 28 (12): 1483-1489.
Zhou, Y., Zhao, C. J., Gao, Y. S., Long, H., and Yu, J., 2010. Variation and distribution characteristics of phytoplankton in ecology-monitoring area of Hangzhouwan Bay from 2005 to 2008., 28 (2): 28-35.
Zhu, Z. Y., Ng, W. M., Liu, S. M., Zhang, J., Chen, J. C., and Wu, Y., 2009. Estuarine phytoplankton dynamics and shift of limiting factors: A study in the Changjiang (Yangtze River) Estuary and adjacent area., 84 (3): 393-401.
(Edited by Xie Jun)
DOI 10.1007/s11802-015-2483-6
ISSN 1672-5182, 2015 14 (6): 1025-1033
© Ocean University of China, Science Press and Springer-Verlag Berlin Heidelberg 2015
(September 12, 2013; revised March 2, 2014; accepted October 1, 2015)
* Corresponding author. E-mail: wangh@nmefc.gov.cn
 Journal of Ocean University of China2015年6期
Journal of Ocean University of China2015年6期
- Journal of Ocean University of China的其它文章
- Inversion Study on Pollutant Discharges in the Bohai Sea withthe Adjoint Method
- Different Responses of Sea Surface Temperature in the North Pacific to Greenhouse Gas and Aerosol Forcing
- The Change Features of the West Boundary Bifurcation Line of the North Equatorial Current in the Pacific
- Wave Pressure Acting on V-Shaped Floating Breakwater in Random Seas
- Dynamic Response of a Riser Under Excitation of Internal Waves
- An Effective Method of UV-Oxidation of Dissolved Organic Carbon in Natural Waters for Radiocarbon Analysis by Accelerator Mass Spectrometry
