The Signatures of Stable Isotopes δ15N and δ13C in Anadromous and Non-Anadromous Coilia nasus Living in the Yangtze River, and the Adjacent Sea Waters
WANG Lei, TANG Wenqiao, and DONG Wenxia
The Signatures of Stable Isotopes15N and13C in Anadromous and Non-AnadromousLiving in the Yangtze River, and the Adjacent Sea Waters
WANG Lei, TANG Wenqiao*, and DONG Wenxia
,,,201306,
Stable isotopes are increasingly used to investigate seasonal migrations of aquatic organisms. This study employed stable isotopes (13C and15N) forfrom the lower Yangtze River and the adjacent East China Sea to distinguish different ecotypic groups, ascertain trophic nutrition positions, and reflect environmental influences on.13C signatures ofsampled from Zhoushan (ZS), Chongming (CM), and Jingjiang (JJ) waters were significantly higher than those from the Poyang Lake (PYL) (<0.05). By contrast,15N signatures ofin ZS, CM, and JJ groups were significantly lower than those in PYL group (<0.05). Basing on13C and15N signatures, we could distinguish anadromous (ZS, CM, and JJ) and non-anadromous (PYL) groups. The trophic level (TL) of anadromousranged from 2.90 to 3.04, whereas that of non-anadromouswas 4.38.occupied the middle and top nutrition positions in the marine and Poyang Lake food webs, respectively.in Poyang Lake were significantly more enriched in15N but depleted in13C, suggesting that anthropogenic nutrient inputs and terrigenous organic carbon are important to the Poyang Lake food web. This study is the first to apply15N and13C to population assignment studies ofin the Yangtze River and its affiliated waters. Analysis of stable isotopes (15N and13C) is shown to be a useful tool for discriminating anadromous and non-anadromous.
Japanese grenadier anchovy;13C;15N; Changjiang River; Poyang Lake
1 Introduction
, also known as the Japanese grenadier anchovy, is a small- to medium-sized fish of the Engraulidae family that is widely distributed in offshore waters and rivers in the northwest Pacific region of Asia, including China and Japan (Whitehead., 1988). In China,is chiefly distributed in the Yellow Sea and East China Sea, and may penetrate more than 1400km up the middle reaches of the Yangtze River and its affiliated waters, including Dongting Lake and Poyang Lake (Yuan., 1980). It is both anadromous and non- anadromous (Yuan, 1987; Cheng., 2005; Yang., 2006). Every spring, the anadromous adult fish migrate from the estuaries to spawn in affiliated freshwater lakes and in the lower and middle river reaches. After spawning, the adults migrate into the estuary and adjacent waters again (Yuan, 1987; Luo., 1992). By contrast, the non-anadromous anchovies always live in freshwaters, such as the lakes of Poyang, Taihu, and Chaohu(Yuan, 1987; Zhang., 2005; Xu., 2005).
Analyses of stable isotopes, especially carbon (C) and nitrogen (N), are commonly used to investigate material circulation and energy flow within ecosystems (Peterson and Fry, 1987; Vander Zanden and Rasmussen, 2001). Measurement of stable isotopes has been used to define relationships between consumers and their food sources (Peterson and Fry, 1987; Cabana and Rasmussen, 1994), determine trophic position of consumers, and study foraging and migration (Carmichael., 2004; Best and Schell, 1996; Kline., 1998; Walker., 1999; Fuji., 2011; Lebreton., 2011). Recently, using stable isotopes to study the seasonal migration patterns of aquatic organism has attracted more attention. Walker(1999) discriminated between coastal and offshore bottlenose dolphin () populations using stable isotopes in dolphin teeth. Gu(2001) reported that Mexican sturgeon () do not feed significantly in freshwaters between the Gulf of Mexico and the coastal rivers of the southeast USA. Lebreton. (2011) demonstrated that mullets (and) feed mainly on primary consumers in salt marsh creeks in Aiguillon Bay along the French Atlantic coast during the spring migration. Bardonneta and Riera (2005) used13C signatures to report that glass eels () may use estuaries as simple migration routes and feeding habitats.
To better elucidate the ecological characteristics ofin the Yangtze River estuary and its affiliated waters, we measured13C and15N signatures in anadromous and non-anadromous anchovies during the migratory period. This study attempted to clearly distinguish anadromous and non-anadromousthrough stable isotope analysis, evaluate their trophic positions in different ecosystems, and describe environmental influences on13C and15N signatures ofthis species.
2 Materials and Methods
2.1 Study Site and Sampling
specimens were collected from four sites along the Yangtze River and the adjacent East China Sea from April to May 2013 (Fig.1). The sampling sites included: Zhoushan (ZS) in the East China Sea, which is an important anchovy foraging and wintering ground; Chongming (CM) in the Yangtze River estuary and Jingjiang (JJ) in the lower reach of the Yangtze River, which are important anchovy migration routes and fishing grounds; and Poyang Lake (PYL) in the middle reach of the Yangtze River, which is the largest freshwater lake in China and an important anchovy freshwater habitat and spawning ground (Yuan, 1987; Zhang., 2005). All specimens were sampled using gill nets (20mm to 40mm mesh size; 2m to 3m height; 150m to 200m length). To collect samples for the ZS, CM, and JJ groups, gill nets were placed before high tide and removed during the slack water period, with a time period of approximately 1.5h to 2h. To collect samples for the PYL group, gill nets were placed and then removed 1.5h to 2h later. All specimens were maintained at −20℃ in aportable refrigerator and subsequently frozen at −80℃ until further analysis.

Fig.1 Location of the study sites of C. nasus in the Yangtze River, adjacent East China Sea, and Poyang Lake. A, Yangtze River and adjacent East China Sea. B, Poyang Lake. ZS, Zhoushan; CM, Chongming; JJ, Jingjiang; PYL, Poyang Lake.
2.2 Stable Isotope Measurement
The dorsal muscle tissues of each fish were dissected and represented on sample. These tissues were oven dried at 60℃ for 48h to constant weights and then were ground with an agate mortar and pestle to a fine powder. All samples were acidified to remove carbonates by adding 1 molL−1hydrochloric acid drop by drop until no CO2was released, following the method described by Jacob. (2005). The acidified samples were directly re-dried at 60℃ without rinsing to minimize the loss of dissolved organic matter. Then they were ground again, and retained in desiccators prior to stable isotope analysis. Finally, 2mg of powdered samples was weighed into a clean tin capsule to simultaneously measure carbon and nitrogen isotope ratios (C:N), which were also measured during stable isotope analysis.
All samples were sent to the Instrumental Analysis Center of Shanghai Jiaotong University for signature analyses of13C and15N using the Elemental Analysis-Stable Isotope Ratio Mass Spectrometer (Vario EL III/Isoprime, Elementar Analysensysteme GmbH) (Hanau, Germany). Organic analytical standards (casein and wheat flour) were obtained from Elemental Microanalysis Ltd. (Devon, UK) and analyzed for every eight to ten unknown samples in each analytical sequence, allowing instrument drift to be corrected if required. Stable isotope ratios were expressed innotation as parts per thousand (‰) according to the following equation:

whereis13C or15N andis the corresponding ratio of13C/12C or15N/14N ratio. The standard reference materials were the Pee Dee Belemnite standard for C and atmospheric N2for N. The analytical precision of these measurements was 0.2‰ for both13C and15N.
2.3 Data Analysis
A one-way ANOVA was performed to examine the differences in13C and15N values among the four Japanese grenadier anchovy groups. A hierarchical cluster analysis was also carried out on stable N and C isotope values to identify different populations (Le Loc’h and Hily, 2005; Grall., 2006). The site station map and figures were drawn using Arcview GIS 3.0 and OriginPro 8.0 software, respectively, and statistical analysis was conducted using SPSS 15.0.
3 Results
3.1 Morphological Characteristics
Body length and weight,maxillary/head length (M/H), and sampling dates are presented in Table 1. The body lengths of the ZS, CM, and JJ groups were close and ranged from 30.30cm to 33.18cm, and the body weights of the three groups ranged from 100.70g to 131.66g. The body length and body weight of the PYL group were only 22.10cm and 38.15g, respectively. Based on otoliths, the fourgroups were 2 to 3 years old.
The maxillary/head length (M/H) of the four groups ranged from 0.916 to 1.157. The ZS, CM, and JJ groups exhibited higher M/H ratios than the PYL group (<0.05), and no significant difference in M/H ratio (>0.05) was found among the three groups (Table 1). The four groups could be categorized into long maxillary group (M/H, 1.148–1.157) and short maxillary group (M/H, 0.916).

Table 1 Body length, body weight,maxillary/head length (M/H), and sampling date of the C. nasus in the Yangtze River and East China Sea
Notes: Values in the same column with different superscripts were significantly different (<0.05, mean±SD).
3.213C and15N Signatures
Dual isotope values (13C and15N) of the fourgroups are plotted in Fig.2. The13C and15N values of the four groups ranged from −28.73‰ to −18.19‰ and from 8.06‰ to 17.22‰, respectively. No significant difference in13C and15N values was found among the ZS, CM, and JJ groups (>0.05) (Table 2). The PYL group had more enriched15N and more depleted13C values than the ZS, CM, and JJ groups (<0.05).

Fig.2 Dual isotope plots for δ13C and δ15N values of the four C. nasus groups sampled from the Yangtze River and adjacent East China Sea. ZS, Zhoushan; CM, Chongming; JJ, Jingjiang; PYL, Poyang Lake.
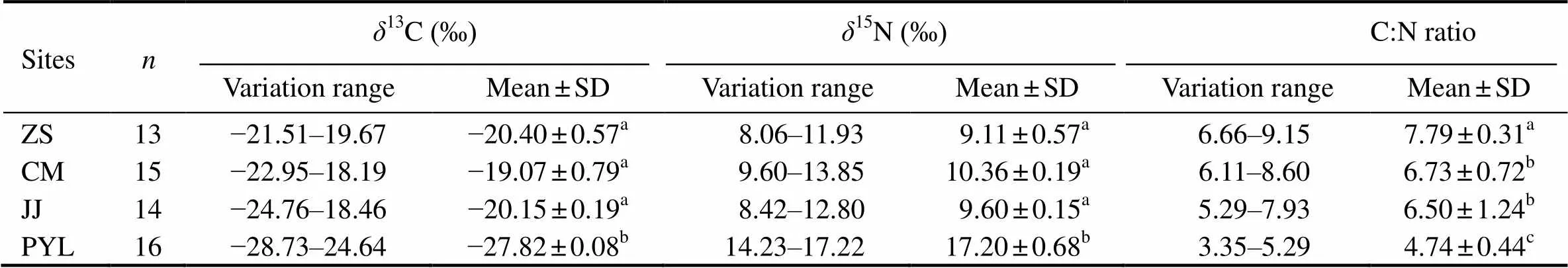
Table 2 Stable isotope signatures and C:N ratios of the four C. nasus groups sampled from the Yangtze River and adjacent East China Sea. ZS, Zhoushan; CM, Chongming; JJ, Jingjiang; PYL, Poyang Lake; n, number of individuals.Values in the same column with different superscripts were significantly different (P<0.05)
3.3 Hierarchical Cluster Analysis
According to the hierarchical cluster analysisbased on15N and13C values of the fourgroups, indi-viduals were classified into two groups, namely, the ZS, CM, and JJ groups (squared Euclidean distance, 0.238) and the PYL group (squared Euclidean distance, 8.338– 8.804), which corresponded to the anadromous and non- anadromous groups, respectively (Fig.3).

Fig.3 Bivariate plot of δ15N vs. δ13C values (mean ± SD) of the four C. nasus groups sampled from the Yangtze River and adjacent East China Sea.The groups of taxa (circled) were selected from the results of the hierarchical cluster analysis. ZS, Zhoushan; CM, Chongming; JJ, Jing- jiang; PYL, Poyang Lake.
3.4 Linear Regression Analysis
Linear regression analysis confirmed significant relationships between15N values and body weights for the anadromous group (=0.520,<0.005), but no significant linear relationships were observed between13C values and body weights for the anadromous group (= 0.128,=0.419; Figs.4a and c). No significant linear relationships were found in the non-anadromous group with regard to15N (=0.023,=0.931) and13C (=0.020,=0.941) values and body weights (Figs.4b and d).
4 Discussion
4.1 Anadromous and Non-Anadromous Groups
According to13C and15N values, allcould be classified into two groups (Fig.3), namely, the de-pleted13C/enriched15N group (PYL group) and the enriched13C/depleted15N group (including ZS, CM, and JJ groups). No significant difference was found among the CM, JJ, and ZS groups, suggesting that the isotope composition ofhasn’t been changed, and the anadromous Yangtze River groups (CM and JJ groups) have the same marine carbon and nitrogen pool as the ZS group. Consequently, in this study these three groups were classified as anadromous group. The PYL group was markedly different from the anadromous group with regard to13C and15N values, and was considered as non- anadromous.
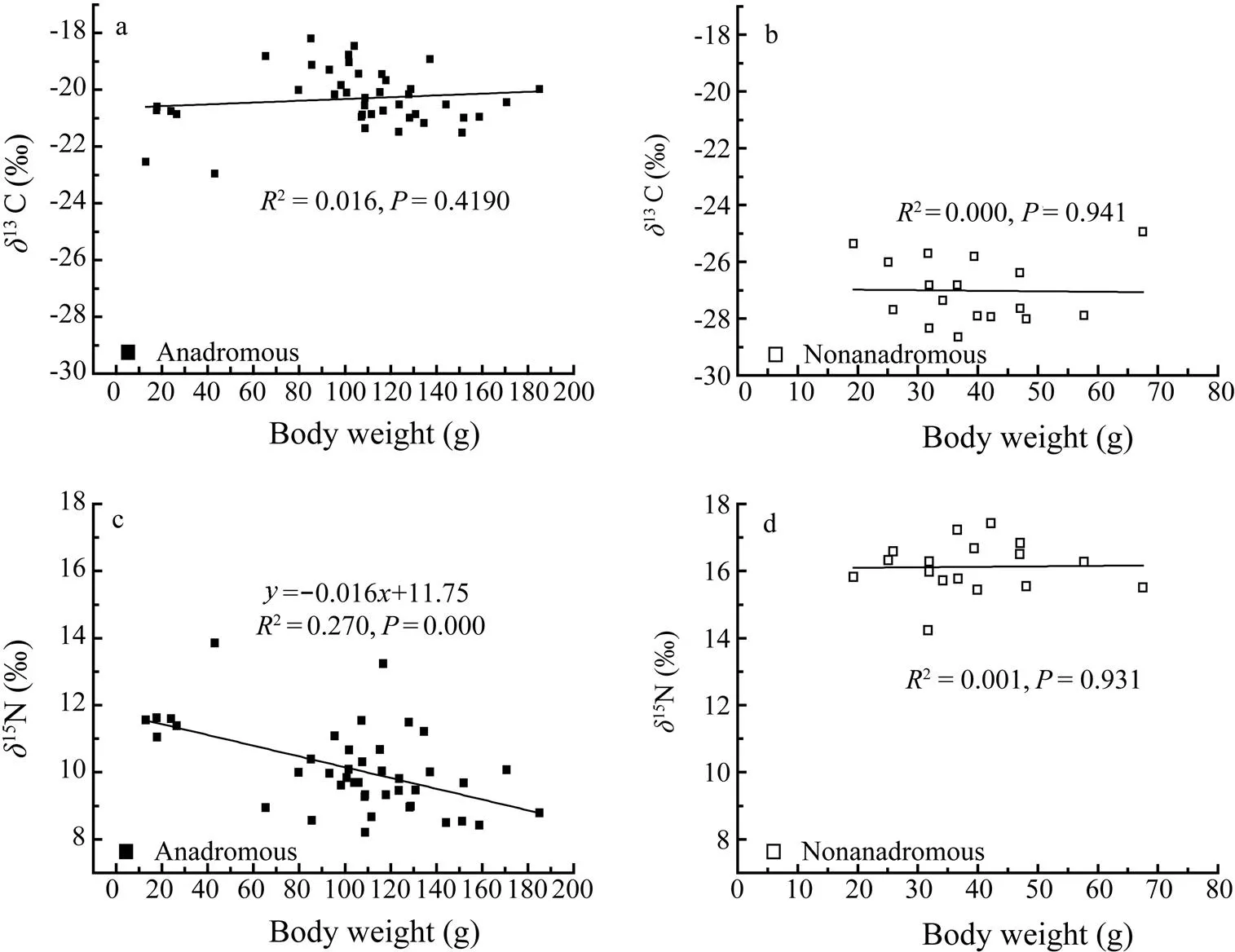
Fig.4 Relationships between δ13C and δ15N values and body weights of anadromous(a, c) and non-anadromous (b, d) C. nasus sampled from the Yangtze River and adjacent East China Sea.
The ratio of maxillary/head length (M/H) is the key to discriminate differentecotypes (Chen and Tang, 2011; Wang., 2012). The M/H values in this study were partitioned into long and short maxillary groups, and the obtained data agreed with those results reported in previous papers (Xu., 2009; Chen and Tang, 2011; Wang., 2012). The data also confirmed that the anadromous group had a higher M/H ratio than the non-ana- dromous group in Poyang Lake. The M/H ratios were consistent with our cluster results of stable isotope signatures of. Therefore, the stable isotope method could be used to determinehabitat utilization and discriminate amongliving in different ecotypes.
4.2 Trophic Position
The signatures of15N were particularly useful in assessing the trophic position of consumers, as demonstrated by the following equation:

whereis the trophic level andis the trophic enrichment factor. When the baseline was the producer, the value ofwas 1, whereas when the baseline was the primary consumer, the value ofwas 2 (Post, 2002). Theis generally considered to be 3.4‰ in marine food webs (Minagawa and Wada, 1984; Vander Zanden and Rasmussen, 1999; Post, 2002; Le Loc’h and Hily, 2005). The15N values of(6.05‰) (Cai., 2005) were applied to the baseline for marine-food source groups (ZS, CM, and JJ groups), and the15N values of suspended particulate organic matter (5.7‰) were applied to the baseline for the Poyang Lake group (Wang., 2009). The TLs of the ZS, CM, and JJ groups were 2.90, 3.26, and 3.04, respectively, and theof the PYL group was 4.38.
For thein the marine food web, our obtained data agreed with those of previous reports, and the15N value andwere 11.35‰ and 3.13, respectively (Cai., 2005). Isotope results were consistent with previous stomach content analysis, indicating that anchovies fed on both zooplankton and small planktivorous fish, such as mysid shrimp,cladocerans, copepods, shrimp, and even small fish (Zhuang., 2012). In the coastal food web, theof consumers ranged from 1.43 to 4.30 (Cai., 2005), andwas an intermediate carnivorous fish, occupying the middle nutrition position in the marine food web. For thein freshwater Poyang Lake, theofwas 4.38. This finding supports previous reports (Wang., 2009) that theofin Poyang Lake ranged from 4.0 to 4.2. Theof consumers in Poyang Lake ranged from 1.5 to 4.2, andoccupied the top nutrition position in the Poyang Lake food web.
4.3 Anthropogenic Nutrients
The natural abundance of stable nitrogen isotopes is frequently used to trace anthropogenic nutrients (Mc- Clelland., 1997; Cabana and Rasmussen, 1996; Lake., 2001). Anthropogenic nutrient inputs increase15N values in food webs (Lake., 2001; Vizzini and Mazzola, 2006; Castro., 2007; Karube., 2010; Fertig., 2013). The15N values forin Poyang Lake were higher than those previously reported (range: 14.8‰ to 15.4‰) (Wang., 2009).Despite various watershed protection efforts, our data suggested that high levels of terrestrially derived nutrients still entered Poyang Lake. The samples obtained from the Poyang Lake showed enriched15N values (17.20‰) compared with those from the coastal food resource groups (ZS, CM, and JJ ranged from 9.11‰ to 10.36‰). Approximately 7.2 million people live around Poyang Lake, which is the largest freshwater lake in China. Therefore, the terrestrial nutrient input from the surrounding cities may cause the enriched15N values in the Poyang Lake food web. Other studies (Nixon., 2007; Bannon and Roman, 2008) reported similar conclusions, suggesting that human wastewater discharge causes elevated15N values in local food webs.
Acknowledgements
The authors thank Dr. Li Zhang of the Instrumental Analysis Center of Shanghai Jiaotong University for performing the isotope analysis. We also acknowledge Dr. Weimin Quan ofthe East China Sea Fishery Research Institute for his helpful comments on the manuscript; Drs. Weijing Chen and Linhong Shen of the Administration of Fishery of Jingjiang for sample collection; and Drs. YingyingLi, GuoliZhu, CongWang, and Tianyi Xu for sample treatment. This study was financially supported by the Special Fund for Agro-scientific Research in the Public-Interest (Grant No. 201203065), the National Natural Science Foundation of China (Nos. 31172407, 1472280), and the Specialized Research Fund for the Doctoral Program of Higher Education (No. 20123104110006).
Bannon, R. O., and Roman, C. T., 2008. Using stable isotopes to monitor anthropogenic nitrogen inputs to estuaries., 18 (1): 22-30.
Bardonneta, A., and Riera, P., 2005. Feeding of glass eels () in the course of their estuarine migration: New insights from stable isotope analysis.,63(1): 201-209.
Best, P. B., and Schell, D. M., 1996. Stable isotopes in southern right whale () baleen as indicators of seasonal movements, feeding and growth., 124 (4): 483-494.
Cabana, G., and Rasmussen, J. B., 1994. Modeling food chain structure and contaminant bioaccumulation using stable nitrogen isotopes., 372 (6503): 255-257.
Cai, D. L., Li, H. Y, Tang, Q. S., and Sun, Y., 2005. Establishing trophic chart of ecosystem in the Yellow Sea and East Sea based on the stable isotope results., 35 (2): 123-130.
Carmichael, R. H., Rutecki, D., Annett, B., Gaines, E., and Valiela I., 2004. Position of horseshoe crabs in estuarine food webs: N and C stable isotopic study of foraging ranges and diet composition., 299 (2): 231-253.
Castro, P., Valiela, I., and Freitas, H., 2007. Eutrophication in Portuguese estuaries evidenced by15N of macrophytes., 351: 43-51.
Cheng, Q. Q., Lu, D. R., and Ma, L., 2005. Morphological differences between close populations discernible by multivariate analysis: A case study of genus(Teleostei: Clupeiforms)., 18 (2): 187-192.
Cheng., W. X., and Tang, W. Q., 2011. Some phenotypic varieties between different ecotypes ofin the Yangtze River., 46 (5): 33-40.
Fertig, B., O’Neil, J. M., Beckert, K. A., Cain, C. J., Needham, D. M., Carruthers, T. J. B., and Dennison, W. C., 2013. Elucidating terrestrial nutrient sources to a coastal lagoon, Chincoteague Bay, Maryland, USA., 116: 1-10.
Fuji, T., Kasai, A., Suzuki, K. W., Ueno, M., and Yamashita, Y., 2011. Migration ecology of juvenile temperate seabass Lateolabrax japonicus: A carbon stable isotope approach., 78 (7): 2010-2025.
Grall, I., Le, Loc’h, F., Guyonnet, B., and Riera, P., 2006. Community structure and food web based on stable isotopes (15N and13C) analysis of a North Eastern Atlantic maerl bed., 338 (1): 1- 15.
Gu, B., Schell, D. M., Frazer, T., Hoyer, M., andChapman, F. A., 2001.Stable carbon isotope evidence for reduced feeding of Gulf of Mexico sturgeon during their prolonged river residence period., 53 (3): 275-280.
Jacob, U., Mintenbeck, K., Brey, T., Knust, R., and Beyer, K., 2005. Stable isotope food web studies: A case for standardized sample treatment., 287: 251- 253.
Karube, Z., Sakai, Y., Takeyama, T., Okuda, N., Kohzu, A., Yoshimizu, C., Nagata, T., and Tayasu, I., 2010. Carbon and nitrogen stable isotope ratios of macroinvertebrates in the littoral zone of Lake Biwa as indicators of anthropogenic activities in the watershed., 25 (4): 847-855.
Kline, T. C., Wilson, W. J., and Goering, J. J., 1998. Natural isotope indicators of fish migration at Prudhoe Bay, Alaska., 55 (6): 1494-1502.
Lake, J. L., McKinney, R. A., Osterman, F. A, Pruell, R. J, Kiddon, J., Ryba, S. A., and Libby, A. D., 2001. Stable nitrogen isotopes as indicatiors of anthropogenic activities in small freshwater systems., 58 (5): 870-878.
Lebreton, B., Richard, P., Parlier, E. P., Guillou, G., and Blanchard, G. F., 2011. Trophic ecology of mullets during their spring migration in a European saltmarsh: A stable isotope study.,91 (4): 502-510.
Le Loc’h, F., and Hily, C., 2005. Stable carbon and nitrogen isotope analysis of Nephrops norvegicus/Merluccius merluccius fishing grounds in the Bay of Biscay (NE Atlantic)., 62 (1): 123- 132.
Luo, B. Z, Xue, P., Lu, J. W., and Huang, S. F., 1992.Impact of the Three Gorges Project on the fishery of the Changjiang estuary and adjacent waters., 33: 341-351.
McClelland, J. W., Valiela, I., and Michener, R. H., 1997. Nitrogen-stable isotope signatures in estuarine food webs: A record of increasing urbanization in coastal watersheds.,42 (5): 930-937.
Minagawa, M., and Wada, E., 1984. Stepwise enrichment of15N along food chains: further evidence and the relation between15N and animal age.,48 (5): 1135-1140.
Nixon, S. W., Buckley, B. A., Granger, S., L., Entsua-Mensah, M., Ansa-Asare, O., White, M. J., Mckinney, R. A., and Mensah, E., 2007.Anthropogenic enrichment and nutrients in some tropical lagoons of Ghana, West Africa., 17 (sp5): S144-S164.
Peterson, B. J., and Fry, B., 1987. Stable isotopes in ecosystem studies., 18: 291- 320.
Post, D. M., 2002. Using stable isotopes to estimate trophic position: Models, methods, and assumptions., 83(3): 703-718.
Sackett, W. M., and Thompson, R. R., 1963. Isotopic organic carbon composition of recent continental derived clastic sediments of eastern Gulf Coast, Gulf of Mexico., 47 (3): 525-531.
Vander Zanden, M. J., and Rasmussen, J. B., 2001. Variation in15N and13C trophic fractionation: implications for aquatic food web studies., 46 (8): 2061- 2066.
Vizzini, S., and Mazzola, A., 2006. The effects of anthropogenic organic matter inputs on stable carbon and nitrogen isotopes in organisms from different trophic levels in a southern Mediterranean coastal area., 368 (2): 723-731.
Walker, J. L., Potter, C. W., and Macko, S. A., 1999. The diets of modern and historic bottlenose dolphin populations reflected through stable isotopes., 15 (2): 335- 350.
Wang, D. T., Yang, J., Jiang, T., Liu, H. B., and Shen, X. Q., 2012. A comparative study of the morphology of different geographical populations of., 36 (1): 79-90.
Wang, Y. Y., Yu, X. B., Zhang, L., and Xu, J., 2009. Food web structure of Poyang Lake during the dry season by stable carbon and nitrogen isotopes analysis., 29 (3): 1181-1188.
Whitehead, P., Nelson, G., and Wongratana, T., 1988. FAO species catalogue. Clupeoid fishes of the world (Suborder Clupeoidei). Part 2. Engraulididae., 125 (7): 460-475.
Xu, J., Xie, P., Zhang, M., and Yang, H., 2005. Variation in stable isotope signatures of seston and a zooplanktivorous fish in a eutrophic Chinese lake., 541 (1): 215-220.
Xu, Z. Q., Ge, J. C., Huang, C., Dou, H. X., Pan, J. L., and Xia, A. J., 2009. Taxonomy of short jaw tapertail anchovyby jaw length and mitochondrial Cytochrome b gene analysis., 24 (3): 243-246.
Yang, J., Arai T., Liu, H., Miyazaki, N., and Tsukamoto, K., 2006. Reconstructing habitat use ofandof the Yangtze River estuary, and ofof Taihu Lake, based on otolith strontium and calcium., 69 (4): 1120-1135.
Yuan, C. M., Qin A. L., Liu, R. H., and Lin J. B., 1980. On the classification of the anchovies,from the lower Yangtze River and the southeast coast of China., 3: 67-82.
Yuan, C. M., 1987. Spawning migration of Chinese anchovy., 12: 1-3.
Zhang, M. Y., Xu, D. P., Liu, K., and Shi, W. G., 2005. Studies on biological characteristics and change of resource ofSchlegel in the lower reaches of the Yangtze River., 14 (6): 694- 698.
Zhuang, P., Luo, G., Zhang, T., Zhang, L. Z., Liu, J. Y., Feng, G. P., and Hou, J. L., 2010. Food comparison among juvenileand other six economic fishes in the Yangtze estuary., 30 (20): 5544-5554.
(Edited by Qiu Yantao)
DOI 10.1007/s11802-015-2611-3
ISSN 1672-5182, 2015 14 (6): 1053-1058
© Ocean University of China, Science Press and Spring-Verlag Berlin Heidelberg 2015
(March 3, 2014; revised April 21, 2014; accepted August 25, 2015)
* Corresponding author. E-mail: wqtang@shou.edu.cn
 Journal of Ocean University of China2015年6期
Journal of Ocean University of China2015年6期
- Journal of Ocean University of China的其它文章
- Inversion Study on Pollutant Discharges in the Bohai Sea withthe Adjoint Method
- Different Responses of Sea Surface Temperature in the North Pacific to Greenhouse Gas and Aerosol Forcing
- The Change Features of the West Boundary Bifurcation Line of the North Equatorial Current in the Pacific
- Wave Pressure Acting on V-Shaped Floating Breakwater in Random Seas
- Dynamic Response of a Riser Under Excitation of Internal Waves
- An Effective Method of UV-Oxidation of Dissolved Organic Carbon in Natural Waters for Radiocarbon Analysis by Accelerator Mass Spectrometry
