A Preliminary Analysis of Trace-Elemental Signatures in Statoliths of Different Spawning Cohorts for Dosidicus gigas off EEZ Waters of Chile
LIU Bilin, CHEN Xinjun, *, FANG Zhou, HU Song, and SONG Qian
A Preliminary Analysis of Trace-Elemental Signatures in Statoliths of Different Spawning Cohorts foroff EEZ Waters of Chile
LIU Bilin1), 2), 3), 4), 5), CHEN Xinjun1), 2), 3), 4), 5), *, FANG Zhou1), HU Song1), and SONG Qian1)
1),,201306,2),201306,3),,201306,4),,201306,5),201306,
We applied solution-based ICP-MS method to quantify the trace-elemental signatures in statoliths of jumbo flying squid,, which were collected from the waters off northern and central Chile during the scientific surveys carried out by Chinese squid jigging vessels in 2007 and 2008. The age and spawning date of the squid were back-calculated based on daily increments in statoliths. Eight elemental ratios (Sr/Ca, Ba/Ca, Mg/Ca, Mn/Ca, Na/Ca, Fe/Ca, Cu/Ca and Zn/Ca) were analyzed. It was found that Sr is the second most abundant element next to Ca, followed by Na, Fe, Mg, Zn, Cu, Ba and Mn. There was no significant relationship between element/Ca and sea surface temperature (SST) and sea surface salinity (SSS), although weak negative or positive tendency was found. MANOVA analysis showed that multivariate elemental signatures did not differ among the cohorts spawned in spring, autumn and winter, and no significant difference was found between the northern and central sampling locations. Classification results showed that all individuals of each spawned cohorts were correctly classified. This study demonstrates that the elemental signatures instatoliths are potentially a useful tool to improve our understanding of its population structure and habitat environment.
jumbo flying squid; element/Ca; off EEZ waters of Chile; solution-based ICP-MS; environmental variables
1 Introduction
(d’Orbigny, 1835), commonly known as jumbo flying squid, is generally found in the eastern Pacific tropical-subtropical area between 40˚N to 45˚S (Nigmatullin., 2001). It inhabits from the sea surface down to depth of 1200m (Nigmatullin., 2001).fishery is year-round, and is one of the most important fisheries in the Chilean waters (Rocha and Vega, 2003; Zúñiga., 2008; Hu., 2011). In the oceanic waters off Chile, Chinese squid jigging vessels began to target this squid in 2004 (Chen and Zhao, 2005; Chen., 2011). And then three scientific surveys foroff the exclusive economic zone (EEZ) waters of Chile were made by Chinese squid jigger vessels during 2006 to 2008 (Chen., 2011). The fishery biology ofin the central-south area off Chile have been studied for yielding the information about age, growth, size structure and reproductive activity (Ibáñez and Cubillost, 2007; Qian., 2008; Wang and Qian, 2008; Liu., 2010a, b; Argüelles., 2012; Yan., 2012).
Trace elements composition of calcified structures is considered as a convenient natural tag in investigating population structure and reconstructing the living environment (Thorrold., 2002). In the last two decades, various techniques have been developed to analyze microchemistry of calcified tissues. Solution-based ICP-MS analyzes elements of the whole tissues, which is different from the other methods such as Proton-induced X-ray emission (PIXE; Ikeda., 1995; Durholtz., 1997), electron microprobe analysis (EPMA; Yatsu., 1998; Bettencourt and Guerra, 2000) and laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS; Thorrold and Shuttleworth, 2000; Zumholz., 2007b) that are based on small part of the structure. Following an application of element analysis in teleost fish otoliths (Fowler., 1995; Gillanders and Kingsford, 1996; Patterson., 1999; Swearer., 2003), the Solution- based ICP-MS was first used to analyzestatolith (Arkhipkin., 2004).
Statolith is considered as a useful calcareous structure recording the information experienced by cephalopod over their life cycle. Elemental signature in statolith is considered as a potential complement to more common genetic methods in determining cohort and population structure (Arkhipkin, 2005). Arkhipkin. (2004) suggested that the statolith elements in Patagonian longfin squidvaried significantly in different geographic and spawning groups. Ikeda(2003) reported that Sr/Ca in statolith of Japanese common squidcollected from the Sea of Japan showed significant geographic differences. Warner. (2009) found that geographic differences in statolith elemental signature were distinct enough to identify the California market squidpopulation. However, no significant difference in Sr/Ca was found in statolith of seven star flying squidfrom the waters of Antarctic Polar Frontal Zone and Patagonian Shelf Edge (Rodhouse., 1994). Ontogenetic variation in 4 trace element (88Sr,137Ba,24Mg,23Na) concentrations and their ratios to Ca were measured in statoliths of the jumbo flying squid off EEZ of Chilean and Peruvian waters using LA-ICP-MS (Liu., 2011). It was found that Sr/Ca and Mg/Ca were good indicators for distinguishing squid from autumn and winter spawning seasons (Liu., 2011). Ikeda. (2002b) revealed that Sr concentration did not significantly differ between the El Niño year and non-El Niño year. Instead, the Strontium concentration in statoliths offrom Peruvian waters (high salinity) was significantly higher than those from Costa Rican waters (low salinity) regardless of El Niño.
In this study, we used an inductively coupled plasma mass spectrometry (ICP-MS) to determine trace-elemen- tal signatures in statoliths ofoutside the EEZ waters of Chile. The objectives of this study were to determine the element composition of their statoliths, evaluate if elemental compositions varied seasonally, and examine the relationship between elemental composition versus ambient water temperature and salinity. The results derived in this study may lead to the development of an alternative way to evaluatestock structure off Chile and to improve understanding of their habitat requirements in different life history stages in oceanic ecosystems.
2 Materials and Methods
2.1 Sampling
The sampled(14 individuals with 10 females and 4 males) used in this study were collected outside the EEZ waters of Chile during the scientific surveys carried out by Chinese jigging vessels in 2007 and 2008 (Table 1; Fig.1). The sampled squids were sexed and measured on the fishing vessels for mantle length (ML) to the nearest 1mm. Their maturity stages were determined with naked eyes according to a scale of stages I-V developed by Lipinski (1979). Statoliths were extracted for each specimen and stored in 90% alcohol for further analysis. The CTD (SBE 37-SM) was deployed at each sampling location to measure sea surface temperature (SST) and sea surface salinity (SSS) (Table 2). Detailed information on all specimens is shown in Table 1.

Fig.1 sampling locations off the north and central waters off Chile.

Table 1 Summary information of D. gigas sampled from the north and central waters off Chile

Table 2 Mean values of temperature and salinity in the twelve sampling locations at sea surface and depths of 50m, 100m, 150m, 200m, 250m and 300m
2.2 Age Estimation and Hatching Date Calculation
A total of 14 pared statoliths were extracted. The left statolith was prepared for age determination, and the right statolith for the element analysis. The age of statolith was estimated using the method developed by Dawe and Natsukari (1991). The number of increments in each statolith was counted independently by two readers, and the average value was used if the range of counts was within 10% of the mean. The smallest squid was 199 days old and the oldest one was 365 days old. Back-calculated hatching dates were distributed in austral spring (September and October), summer (January), autumn (March, April, May) and winter (June, July, August) (Table 1).
2.3 Elemental Signature Analysis
The statoliths initially were cleaned in ultrapure water for 5min and then air-dried under a Class 100 laminar flow hood for 24h. After drying, statoliths were weighed to the nearest 10μg and saved in polyethylene vials for further analysis. Weighed statoliths were digested in ultrapure 70% nitric acid and then diluted in 5% HNO3for subsequent analysis. Trace-elemental signatures were determined by the ICP-MS at the State Key Laboratory of Geological Processes and Mineral Resources, China University of Geosciences, using Agilent 7500a ICP-MS. The trace-elemental signatures with relative error less than 5% and value at least 3 times higher than detection limit were accepted by software ICPMSDataCal V9.2 for further analysis.
2.4 Water Temperature and Salinity
Monthly sea surface temperature and salinity (SST and SSS) of northern and central sampling area were down- loaded from http://iridl.ldeo.columbia.edu, and integrated SST/SSS was calculated as mean temperature/salinity experienced by a given squid during the time period from the day of hatching to the day when it was caught.
2.5 Statistical Analysis
To evaluate possible spatial., sampling locations) and temporal (., spawning cohorts) variability in statolith elemental signatures, an analysis of variance (ANOVA) and multivariate analysis of variance (MANOVA) were performed after the normality and homogeneity test. We used principal component analysis (PCA) and canonical discriminate analysis (CDA) to examine differences in multivariate elemental signature between the northern and central areas and among spring, autumn and winter- spawned cohorts.
3 Results
3.1 Elemental Concentrations
Elemental concentrations were expressed as the ratios compared to Ca, because Ca was abundant with constant values instatolith. Of the analyzed elements in the statolith, Sr was the second most abundant element after Ca, followed by other elements in the order of Na, Fe, Mg, Zn, Cu, Ba and Mn (Table 3). Values of Mg/Ca, Zn/Ca and Cu/Ca tended to have larger standard deviations (SD; Table 3).
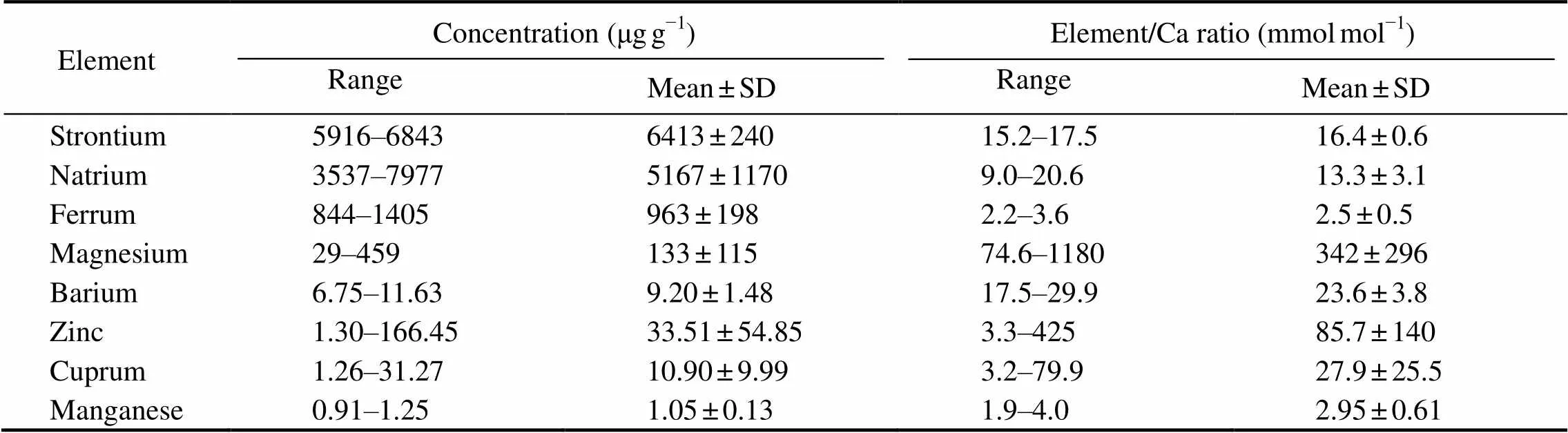
Table 3 Elemental concentrations and ratios to Ca of D. gigas statoliths from sampling locations of northern and central waters off Chile
3.2 Element/Ca Correlation with SST and SSS
Sr/Ca, Cu/Ca and Zn/Ca were found to have negative correlations with SST (Figs.2A, G, H), while Mg/Ca, Mn/Ca, Na/Ca and Fe/Ca showed positive correlations with SST (Figs.2C, D, E, F), although neither of the correlation was significant (>0.05). There was no significant relationship between element/Ca and SSS (Figs.2a, c, e, g, i, k, m, o). Sr/Ca and Cu/Ca showed weak negative relations with SSS (>0.05; Figs.2a, g), while Mn/Ca, Na/Ca, Fe/Ca and Zn showed weak positive relations with SSS (>0.05; Figs.2d, e, f, h).

Fig.2 A–F, a–f Correlations between elements/Ca versus SST and SSS (Sping, diamonds; summer, squares; autumn, triangles; winter, cycles).
Fig.2 G, H, g, h Correlations between elements/Ca versus SST and SSS (Sping, diamonds; summer, squares; autumn, triangles; winter, cycles).
3.3 Elemental Signatures Variation in Geographic Locations and Spawning Cohorts
The Fe/Ca and Mn/Ca ratios in the statolith differed significantly between the sexes (<0.05, ANOVA), while no significant sexual differences were found for the other element concentrations. The MANOVA analysis showed that multivariate elemental signatures instatoliths did not differ among spring-, autumn- and winter-spawned cohorts, and between northern and central locations (>0.05, Table 4).

Table 4 Results of two-way MANOVA of element signatures (element/Ca) in statoliths of jumbo flying squid from spring-, summer-, autumn- and winter-spawned cohorts
Notes: The value is Pillai’s statistic,significant at=0.05; ns, no significant.

Fig.3 Plot of the first two principal components of multivariate element signature in statolith of squid collected from northern (solid circles) and central waters (open circles) off Chile.
Squid from the northern and central areas could not be readily distinguished in the PCA analysis (Fig.3). However, the CDA analysis showed that the winter-spawned cohort could be distinguished from the spring- and autumn-spawned cohorts on CV1, and the spring- and autumn-spawned cohorts could be separated based on the CV2 (Fig.4). Classification results showed that every individual in each group was correctly classified (Table 5).

Fig.4 Plot of the first two canonical variants of multivariate element signature in statolith of spring, summer, autumn and winter-spawned cohorts (solid squares are cohort’s centroid).
ANOVA was used to investigate the contribution of individual elements to spatial and temporal differences in the multivariate elemental signatures (Table 6). No elemental signatures were significantly different among different cohorts and between the geographic locations. Temporal/spatial interaction terms were not significant for any of the elements except for Zn/Ca.

Table 5 Classification results of spring-, summer-, autumn- and winter-spawned cohorts collected at locations of northern and central waters off Chile

Table 6 Results of two-way ANOVA of element/Ca ratios in statoliths of jumbo flying squid from spring-, summer-,autumn- and winter-spawned cohorts
Notes: SS, sum of squares; MS, mean squares;**, significant at α=0.05; ns, not significant.
4 Discussion
Direct comparisons with elements in other squid statoliths determined by ICP-MS showed that Sr/Ca value in(15.2–17.5mmolmol−1) statolith was similar to that of oceanic Ommestrephid squid.(15.8–16.6 mmolmol−1; Ma, 2010), but higher than that of costal Loliginid squid(about 8mmolmol−1; Arkhipkin., 2004).statolith tended to have a relatively higher Sr/Ca ratio than squid(8.5–10mmolmol−1; Ikeda., 2003), octopus(10.6–14.3mmolmol−1; Ikeda., 1999) and cuttlefish(about 4mmolmol−1; Zumholz., 2007a), the Sr/Ca ratios of which were determined by PIXE or LA- ICP-MS. The Ba/Ca ratio in(17.5–29.9μmolmol−1) was higher than that in.(13.6–16.5μmolmol−1; Ma, 2010) and(3–8mmolmol−1; Arkhipkin., 2004). Mn/Ca ratio was similar to that indetermined with the ICP-MS, but was almost an order of magnitude lower than that in.(22–35μmolmol−1; Ikeda., 1995) and.(22–40μmolmol−1; Ikeda., 1999) determined with the PIXE. These differences were either due to inter-species differences in elements uptakes or due to the different instrumental effects (Arkhipkin., 2004). The spatial differences in ambient waters of these squids from different geographic locations were probably another reason. Elemental signature in hard tissues of marine organisms such as corals, gastropod and fish is often considered as a thermometer for reconstructing their ambient temperature. Variability of Sr/Ca in coralske- leton depends strongly on SST (Swart., 2002). Zacherl(2003) showed a significant inverse effect of temperature on Sr incorporated into neogastropod. However, temperature reconstruction based on Sr/Ca in otolith is complicated and only performed well for fishes in cold waters (Campana, 1999). In cephalopod, temperature experienced by a small number of species belonging to Loliginids (Arkhipkin., 2004) and octopus (Ikeda., 1999) were rebuilt based on Sr concentrations. However, the relationship between Sr/Ca ratio and temperature hasn’t been established in statolith of ommastrephids (Ikeda., 2002b; Yatsu., 1998). Ommastrephidundertake extensive diel vertical migrations (from surface to about depth of 1200m; Nigmatullin., 2001), resulting in wide variation of ambient temperature in 24 hours. Thus it is difficult to relate element/Ca in the whole statolith with temperature. Experimental data implied that the range of temperature caused by diel vertical movement was not reflected in Ommastrephid.statoliths. This may result from its movement between 200m and surface (Ikeda., 2002a). Because of a wide temperature range, although it is about 2.5℃ higher in comparing temperature in El Niño with that in non-El Niño years, Sr concentrations did not significantly differ between the normal and abnormal years (Ikeda., 2002b). As expected, our study failed to establish a significant correlation between element/Ca ratio with SST, except for Cu/Ca showing a significant positive correlation with SST. However, Ikeda. (1996a) suggested that Sr, Fe and Zn concentrations in.statoliths are strongly related to integrated water temperature. Ikeda. (1996a) indicated that Sr concentration in benthic octopusstatoliths correlated negatively with bottom water temperature. Zumholz. (2007a) revealed that Ba/Ca and I/Ca in rearedstatolith was significantly related with controlled water temperature. Loliginid squids commonly found in near-bottom water layers on the continental shelf experienced narrower vertical migrations compared with Ommastrephids (Hanlon and Messenger, 1996). Sr/Ca ratio in temperatefrom Japanese waters was significantly higher than those in tropical,, andfrom the Andaman Sea and Gulf of Thailand (Ikeda., 1997).statoliths Sr/Ca and Ba/Ca showed negative, while Mg/Ca and Mn/Ca showed positive correlations with a temperature history index (Arkhipkin., 2004). We suggest that the range of changes in temperature within a short period may have influences on reconstructing element/Ca with ambient temperature.
An analysis of the relationship between elements in statolith and ambient waters may help rebuild the life history of cephalopod (Arkhipkin, 1993). For instance, differences in Sr/Ca reflect different spawning grounds and transport routes of Tsushima and Subarcticgroups (Ikeda.,2003). Based on the analysis of Sr/Ca and statolith increments, Rodhouse. (1994) inferred thatspawned in the warmer water. Ontogenetic variations of Sr/Ca and Ba/Ca in statolith revealed that paralarvaeinhabited in areas with higher temperature compared with adult (Yatsu., 1998), and thatmigrated from the sea surface during juvenile phase to deeper waters in subadult and adult stages (Zumholz, 2005).migrated into colder waters with increased sizes, which was reflected by an increase in U/Ca and Sr/Ca from nucleus to the edge of statolith (Zumholz., 2007a). Elemental signature in statolith, such as Sr/Ca and Ba/Ca, was considered as a thermometer for reconstructing ambient water temperature in organisms’ habitats. Generally, Sr/Ca is considered to be negatively correlated with water temperature (Ikeda., 1996a, 1996b, 1999; Arkhipkin., 2004), although salinity and food supplies are also found to influence element composition in statolith (Ikeda., 1997, 2002b; Zumholz, 2006). Therefore, the quantitative relationships between statolith trace elements and environmental conditions under different growth stages are needed to improve our understanding of the life history of
Fluctuation in salinity was smaller than changes in temperature from surface to deeper waters (Table 2). Thus, diel movements ofhave no influence on salinity values. Several studies succeeded in relating statolith Sr/Ca with water salinity. The ratio varied with salinity forfrom the Arabian Sea, Indian Ocean and Pacific Ocean (Ikeda et., 1997).from the Mediterranean Sea with higher salinity have higher Sr concentrations than those ofin the central North Sea with a lower salinity (Biemann and Piatkowski, 2001). In contrast, Sr/Ca instatoliths of this study decreased with an increased salinity, although the tendency was not statistically significant (>0.05). We concluded thatfrom different waters is similar toof Peruvian and Costa Rican waters, which belong to different geographic populations. These two species showed significantly negative correlations between Sr/Ca and salinity. Differences of Sr/Ca betweenandmay be species-spe- cific rather than a result of salinity effect.
In the eastern Pacific Ocean, three groups can be identified based on ML values of adult males and females: a small group (130–260mm and 140–340mm, respectively, for males and females) predominantly in the near-equa- torial area; a medium group (240–420mm and 280–600mm, respectively for males and females) within the whole species range; and a large group (>400–500mm and 550–650 to 1000–1200mm, respectively, for males and females) occurring northward of 10˚–15˚N and southward of 10˚–15˚S (Nigmatullin., 2001). Our specimens sampled from different seasonal cohorts and geographic locations off Chilean waters might represent a single medium-sized population. No significant difference in multivariate element signatures between northern and central-geographic locations was found, which was consistent with the hypothesis that only a single population exists. Nevertheless, significant difference of multivariate element signatures in statolith of spring-, summer-, autumn- and winter-spawned cohorts seemed to contradict the single population hypothesis. One possible reason for this inconsistency is that cohorts spawned in different seasons experienced different environments over their life span.
One weakness of this study is the small sample size, which may introduce some uncertainty in the results derived from this study. We will increase sample size to further test this approach, and use LA-ICP-MS method to investigate the element signatures of different ontogenesis to trace migration pathway of different cohorts in the eastern Pacific Ocean.
Acknowledgements
This work was funded by the National Natural Science Foundation of China (Nos. 41306127 and 41276156), National Science Foundation of Shanghai (13ZR1419 700), the innovation Program of Shanghai Municipal Education Commission (13YE091), Research Fund for the Doctoral Program of Higher Education of China (20133104120001), and Shanghai Universities First-class Disciplines Project (Fisheries). Y. Chen’s involvement was supported by Shanghai Oversea Teaching Scholar Program and SHOU International Center for Marine Sciences.
Arkhipkin, A., 1993. Age, growth, stock structure and migratory rate of pre-spawning short-finned squidbased on statolith ageing investigations., 16 (4): 313-338.
Arkhipkin, A. I., 2005. Statolith as ‘black boxes’ (life recorders) in squid., 56: 573-583.
Arkhipkin, A. I., Campana, S. E., FitzGerald, J., and Thorrold, S. R., 2004. Spatial and temporal variation in elemental signatures of statoliths from the Patagonian longfin squid ()., 61: 1212-1224.
Argüelles, J., Lorrain, A., Cherel, Y., Graco, M., Tafur, R., Alegre, A., Espinoza, P., Taipe, A., Ayón, P., and Bertrand, A., 2012. Tracking habitat and resource use for the jumbo squid: A stable isotope analysis in the Northern Humboldt Current System., 159 (9): 2105- 2116.
Bettencourt, V., and Guerra, A., 2000. Growth increments and biomineralization process in cephalopod statoliths., 248: 191-205.
Biemann, M. D., and Piatkowski, U., 2001. Amounts and composition of trace elements in the statoliths of loliginid squids: Reflection of environmental conditions?, K: 5.
Campana, S. E., 1999. Chemistry and composition of fish otoliths: Pathways, mechanisms and applications., 188: 263-297.
Chen, X. J., and Zhao, X. H., 2005. Catch distribution of jumbo flying squid and its relationship with SST in the offshore waters of Chile., 27: 173-176 (in Chinese with English abstract).
Chen, X. J., Lu, H. J., Liu, B. L., and Chen, Y., 2011. Age, growth and population structure of jumbo flying squid,, based on statolith microstructure off the Exclusive Economic Zone of Chilean waters., 91 (1): 229- 235.
Chen, X. J., Li, J. H., Liu, B. L., Chen, Y., Li, G., Fang, Z., and Tian, S. Q., 2013. Age, growth and population structure of jumbo flying squid,, off the Costa Rica Dome., 93 (2): 567-573.
Durholtz, M. D., Lipinski, M. R., Przybylowicz, W. J., and Mesjasz-Przybylowicz, J., 1997. Nuclear microprobe mapping of statoliths of Chokka Squidd’Orbigny, 1845., 193: 125-140.
Fowler, A. J., Campana, S. E., Jones, C. M., and Thorrold, S. R., 1995. Experimental assessment of the effect of temperature and salinity on elemental composition of otoliths using solution-based ICPMS., 52: 1421-1430.
Gillanders, B. M., and Kingsford, M. J., 1996. Elements in otolith may elucidate the contribution of estuarine recruitment to sustaining coastal reef populations of a temperate reef fish., 141: 13-20.
Hanlon, R. T., and Messenger, J. B., 1996.. Cambridge University Press, Cambridge, UK, 232pp.
Hu, Z. M., Chen, X. J., Zhou, Y. Q., Qian, W. G., and Liu, B. L., 2011. Forecasting fishing ground ofbased on habitat suitability index off Peru., 5: 67-75 (in Chinese with English abstract).
Ibáñez, C. M., and Cubillos, L. A., 2007. Seasonal variation in the length structure and reproductive condition of the jumbo squid(d’Orbigny, 1835) off central-south Chile., 71: 123-128.
Ikeda, Y., Arai, N., Kidokoro, H., and Sakamoto, W., 2003. Strontium:calcium ratios in statoliths of Japanese common squid(Cephalopoda: Ommastrephidae) as indicators of migratory behavior., 251: 169-179.
Ikeda, Y., Arai, N., and Sakamoto, W., 1995. Preliminary report on the PIXE analysis of the squid statoliths., 5: 159-162.
Ikeda, Y., Arai, N., Sakamoto, W., Kidokoro, H., Mitsuhashi, M., and Yoshida, K., 1999. Preliminary report on PIXE analysis for trace elements ofstatoliths., 65: 161-162.
Ikeda, Y., Arai, N., Sakamoto, W., Kidokoro, H., and Yoshida, K., 1996a. Relationship between statoliths and environmental variables in cephalopods., 6: 339-345.
Ikeda, Y., Arai, N., Sakamoto, W., Nateewathana, A., Murayama, T., Yatsu, A., and Yoshida, K., 1996b. PIXE analysis of trace elements in squid statoliths: Composition between Ommastrephidae and Loliginidae.,6: 537-542.
Ikeda, Y., Arai, N., Sakamoto, W., Kidokoro, H., Yatsu, A., Nateewathana, A., and Yoshida, K., 1997. Comparison on trace elements in squid statoliths of different species’ origin as available key for taxonomic and phylogenetic study., 7: 141-146.
Ikeda, Y., Okazaki, J., Sakurai, Y., and Sakamoto, W., 2002a. Periodic variation in Sr/Ca ratios in statoliths of the Japanese common squidSteenstrup, 1880 (Cepha- lopoda: Ommastrephidae) maintained under constant water temperature.,273: 161-170.
Ikeda, Y., Yatsu, A., Arai, N., and Sakamoto, W., 2002b. Concentration of statolith trace elements in the jumbo flying squid during El Niño and non-El Niño years in the eastern Pacific., 82: 863-866.
Liu, B. L., Chen, X. J., Qian, W. G., Lu, H. J., and Li, S. L., 2010a. Preliminary study on reproductive biology ofin the high sea off Chile., 19: 68-73 (in Chinese with English abstract).
Liu, B. L., Chen, X. J., Lu, H. J., Chen, Y., and Qian, W. G., 2010b. Fishery biology of the jumbo squidoff Exclusive Economic Zone of Chilean waters.,74: 687-695.
Ma, J., 2010. Statolith microstructure and microchemistry of the neon flying squid,, in the Northwest Pacific Ocean. Master thesis. Shanghai Ocean University, 82pp (in Chinese).
Nigmatullin, Ch. M., Nesis, K. N., and Arkhipkin, A. I., 2001. A review of the biology of the jumbo squid(Cephalopoda: Ommastrephidae)., 54: 9- 19.
Patterson, H. M., Thorrold, S. R., and Shenker, J. M., 1999. Analysis of otolith chemistry in Nassau grouper () from the Bahamas and Belize using soulution-based ICP-MS.,18: 171-178.
Qian, W. G., Chen, X. J., Zheng, B., and Liu, B. L., 2008. Study on the resource density distribution ofand marine environment in the high sea waters off Chile., 17: 98-103 (in Chinese with English abstract).
Rocha, F., and Vega, M., 2003. Overview of cephalopod fisheries in Chilean waters., 60: 151-159.
Rodhouse, P. G., Robinson, K., Gajdatsy, S. B., Daly, H. I., and Ashmore, M. J. S., 1994. Growth, age structure and environmental history in the cephalopod(Teu- thoidea: Ommastrephidae) at the Atlantic Polar Frontal Zone and on the Patagonian Shelf Edge., 6: 259-267.
Swart, P. K., Elderfield, H., and Greaves, M. J., 2002. A high-resolution calibration of Sr/Ca thermometry using the Caribbean coral., 3: 1-11.
Swearer, S. E., Forrester, G. E., Steele, M. A., Brooks, A. J., and Lea, D. W., 2003. Spatio-temporal and interspecific variation in otolith trace-elemental fingerprints in a temperature estuarine fish assemblage., 56: 1111-1123.
Thorrold, S. R., Jones, G. P., Hellberg, M. E., Burton, R. S., Swearer, S. E., Neigel, J. E., Morgan, S. G., and Warner, R. R., 2002. Quantifying larval retention and connectivity in marine populations with artificial and natural marks.70: 291-308.
Thorrold, S. R., and Shuttleworth, S., 2000.analysis of trace elements and isotope ratios in fish otoliths using laser ablation sector field inductively coupled plasma mass spectrometry., 57: 1232-1242.
Warner, R. R., Hamilton, S. L., Sheehy, M. S., Zeidberg, L. D., Brady, B. C., and Caselle, J. E., 2009. Geographic variation in natal and early larval trace-elemental signatures in the statoliths of the market squid(formerly)., 379: 109-121.
Yan, J., Xu, Q. H., Chen, X. J., Li, G., and Liu, B. L., 2012. Primary studies on the population genetic structure of
in the high seas of eastern Pacific Ocean., 35 (11): 1617-1623.
Yatsu, A., Mochioka, N., Morishita, K., and Toh, H., 1998. Strontium/calcium ratios in statoliths of the neon flying squid,(Cephalopoda), in the North Pacific Ocean., 131: 275-282.
Zacherl, D. C., Paradis, G. D., and Lea, D. W., 2003. Barium and strontium uptake into larval protoconchs and statoliths of the marine neogastropod., 67: 4091-4099.
Zumholz, K.,2005. The influence of environmental factors on the micro-chemical composition of cephalopod statoliths. PhD thesis. University of Kiel, Germany, 86pp.
Zumholz, K., Hansteen, T. H., Klügel, A., and Piatkowski, U., 2006. Food effects on statolith compositon of the common cuttlefish ()., 150: 237-244.
Zumholz, K., Hansteen, T. H., Piatkowski, U., and Croot, P. L., 2007a. Influence of temperature and salinity on the trace element incorporation into statoliths of the common cuttlefish ()., 151: 1321-1330.
Zumholz, K., Klügel, A., Hansteen, T. H., and Piatkowski, U., 2007b. Statolith microchemistry traces environmental history of the boreoatlantic armhook squid.ine, 333: 195-204.
Zúñiga, M. J., Cubillos, L. A., and Ibáñez, C.,2008. A regular pattern of periodicity in the monthly catch of jumbo squid () along the Chilean coast (2002–2005)., 34: 91-99.
(Edited by Qiu Yantao)
DOI 10.1007/s11802-015-2620-2
ISSN 1672-5182, 2015 14 (6): 1059-1067
© Ocean University of China, Science Press and Spring-Verlag Berlin Heidelberg 2015
(March 12, 2014; revised April 21, 2014; accepted August 20, 2015)
* Corresponding author. Tel: 0086-21-61900306 E-mail: xjchen@shou.edu.cn
 Journal of Ocean University of China2015年6期
Journal of Ocean University of China2015年6期
- Journal of Ocean University of China的其它文章
- Inversion Study on Pollutant Discharges in the Bohai Sea withthe Adjoint Method
- Different Responses of Sea Surface Temperature in the North Pacific to Greenhouse Gas and Aerosol Forcing
- The Change Features of the West Boundary Bifurcation Line of the North Equatorial Current in the Pacific
- Wave Pressure Acting on V-Shaped Floating Breakwater in Random Seas
- Dynamic Response of a Riser Under Excitation of Internal Waves
- An Effective Method of UV-Oxidation of Dissolved Organic Carbon in Natural Waters for Radiocarbon Analysis by Accelerator Mass Spectrometry
