Mapping Toll-Like Receptor Signaling Pathway Genes of Zhikong Scallop (Chlamys farreri) with FISH
ZHAO Bosong, ZHAO Liang, LIAO Huan, CHENG Jie, LIAN Shanshan, LI Xuan,HUANG Xiaoting, and BAO Zhenmin
Mapping Toll-Like Receptor Signaling Pathway Genes of Zhikong Scallop () with FISH
ZHAO Bosong, ZHAO Liang, LIAO Huan, CHENG Jie, LIAN Shanshan, LI Xuan,HUANG Xiaoting*, and BAO Zhenmin
,,,266003,
Toll-like receptor (TLR) signaling pathway plays a pivotal role in the innate immune system. Studies on TLR signaling pathway genes in Zhikong scallop () have mainly focused on sequence analysis and expression profiling, no research has been carried out on their localization. The chromosomal position of TLR signaling pathway genes can be valuable for assemblying scallop genome and analysizing gene regulatory networks. In the present study, five key TLR signaling pathway genes (TLR,Myd88,TRAF6,NFκB, andIκB) containing bacterial artificial chromosomes (BACs) were isolated and physically mapped through fluorescencehybridization on five non-homologous chromosome pairs, showing a similar distribution to another five model species. The isolation and mapping of these key immune genes ofwill aid to the research on innate immunity, assignment of interested genes to chromosomes, and integration of physical, linkage and cytogenetic maps of this species.
immunogenetics;; TLR signaling pathway; FISH
1 Introduction
The innate immune system is the first-line defense for all living organisms, and it is almost the only path for invertebrates to cope with the invasion of microorganisms present in the environment (Wang., 2011). Innate immune responses are initiated by germline-encoded pattern-recognition receptors that recognize conserved motifs of pathogens termedpathogen-associated molecules (Meijer., 2004), such as lipopolysaccharides, β-1,3- glucans and peptidoglycans (Janeway, 1989; Ashida., 1998; Hoffmann., 1999). Toll-like receptors (TLRs) are among the most extensively studied pattern-recogni- tion receptors. TLRs act as signal transducers using adaptor proteins (MyD88, TIRAP, TRIF, and TRAM), making the common TLR signaling pathway function. TLR signaling pathway culminates in the activation of a variety of inducible transcriptional factors such as nuclear factor kappa B (NFκB) and interferon-regulatory factor, raising various downstream immunological responses to the invasion of pathogens (Kawai and Akira, 2010).
Toll protein was first reported in(Belvin and Anderson, 1996), and it has now been identified in a wide range of species (Coscia., 2011). Furthermore, the TLR gene family and their associating pathways are evolutionarily conservative from fly to humans (Roach., 2005; Hoffmann and Reichhart, 2002). Recent genomic analysis has detected a rich collection of TLR signaling pathway genes in non-mammalian organisms including marine invertebrates such as(Sasaki., 2009),(Hibino., 2006),(Inamori., 2004) and(Zhang., 2011). The structures, expressions and possible signaling of these genes are well documented. Evidence shows that TLR signaling pathway genes are involved in the innate immune system of marine invertebrates (Coscia., 2011).
Zhikong scallop,Joneset Preston, 1904, is one of the most important maricultured shellfish in northern China. Over the last decade, the population ofis lightened sharply due to various infections. A better understanding of the innate immune system ofwould facilitate the control of infectious diseases. To date, most of the TLR signaling pathway genes have been found in, which includedTLR (Qiu.,2007a),Myd88 (Qiu., 2007b),TRAF6 (Qiu., 2009),NFκB, andIκB (Wang., 2011) with their sequence features characterized clearly. The transcripts of these genes are up-regulated after lipopolysaccharide stimulation and down-regulated once being RNA interferenced (Wang., 2011). A TLR signaling pathway exists in scallop, which may involve in immune signaling and activating downstream response and eliminating invading pathogens (Wang., 2011).
In recent years, research on TLR signaling pathway genes of scallop has mainly focused on gene expression (Wang., 2011; Qiu., 2007a, b; Qiu., 2009). Physically mapping these genes is still unmentioned, although such mapping can help determine whether there are gene clusters in TLR signaling pathway, and how these genes arearranged on chromosomes. In species with complete genome sequence information, it is relatively easy to identify the physical location of genes through comparing sequences against a reference genome (Lorenzi., 2010). To scallop, however, the whole genome sequence is not available. Thus it is necessary to map genes with other methods such as fluorescencehybridization (FISH). Recently, three bacterial artificial chromosome (BAC) libraries ofhave been constructed based on different restriction enzymes (Zhao., 2013), providing researchers a convenience of physically mapping related genes on the chromosomes of.
In the present study, BAC clones containing five TLR signaling pathway genes (TLR,Myd88,TRAF6,NFκB andIκB) were screened from BAC libraries of. The five genes were mapped tochromosomes through FISH. It provided the first physical mapping of TLR signaling pathway genes in mollusk, aiding to better understanding this pathway and chromosomal assignment of gene sequences.
2 Materials and Methods
2.1 BAC Library Screening
PrimersforTLR,Myd88,TRAF6 andIκB were designed from their homologous cDNAs (Table 1) while those ofNFκB were the published by Wang. (2011). Positive BAC clones were screened by four-di- mensional, two-step PCR from theIII-BAC (BH) andI-BAC (BB) libraries of(Zhao., 2013). The PCR products of gene fragments were reconfirmed by sequencing (Zhao., 2012).

Table 1 The primer sequences used for FISHing toll-like receptor signaling pathway genes
2.2 Preparation of Probe and C0t-1 DNA
BAC DNA was isolated from 20mL of overnight culture using a standard laboratory method (Sambrook., 1989). Approximately 1μg of BAC DNA was labeled with nick translation kit (Roche, Basel, Switzerland) with digoxygenin-11-dUTP or biotin-16-dUTP according to the manufacturer’s instructions. Labeled probes were stored at −20℃.0-1 DNA and enriched repetitive DNA sequences were prepared according to the procedure described early (Hu., 2011).
2.3 Chromosome Preparation
Chromosomes were prepared from trochophore larvae ofwith the method described by Zhang. (2008). Trochophore larvae were treated with 0.01% colchicine for 2h and then exposed to 0.075molL−1of KCl for 30min. Thereafter, the larvae were fixed three times, 15 min each, in Carnoy’s solution (methanol: glacial acetic acid, 3:1). The larvae were dissociated in 50% acetic acid, then dropped onto hot-wet slides and air dried.
2.4 FISH Analysis
Chromosome slides were pretreated with 1.6% pepsin at 37℃ for 30min and washed in 2x saline sodium citrate (SSC) for 5min. Specimens were denatured in a mixture containing 70% formamide and 2x SSC at 75℃ for 2min, followed by immediate dehydration in an ice-cold ethanol gradient (70%, 90%, and 100%; 5min each) and air-drying. One microgram of labeled probe was mixed in a hybridization buffer of 50% deionized formamide and 2x SSC, plus 50ngμL−1 C0-1 DNA. For hybridization, the probe mixture was denatured at 75℃ for 5min and preannealed at 37℃ for 30min. Thereafter, each slide was covered with 20μL of probe mixture and incubated for 16h at 37℃ in a humid box.
For double-color FISH, probes labeled with digoxigenin and biotin were mixed and incubated at 37℃. A series of washes was followed: 50% formamide and 2x SSC, 42℃, 5min; 1x SSC, 42℃, 5min; and 2x SSC at room temperature, 5min. The probes were detected using anti-digoxigenin-rhodamine or/and fluorescein avidin D Cell Sorter Grade. Chromosomes were counterstained with 4’,6-diamidino-2-phenylindole or propidium iodide. Slides were viewed under an Eclipse-600 epifluorescence microscope equipped with a CCD camera. Pictures were merged and edited using LUCIA Cytogenetics and Photoshop CS3.
For karyotype analysis, chromosomes were paired according to their morphology from 20 good metaphases. Short and long arms were measured to calculate the relative length and centrometric index in accordance with Levan. (1964).
3 Results
3.1 BAC Library Screening
BAC libraries were screened by four-dimensional, two-step PCR ofTLR,Myd88,TRAF6,NFκB andIκB. BAC clones yielded clear single DNA fragments and expected sizes were selected for further use. After PCR screening, all the five genes were found to be represented by at least one BAC clones each (Table 2).

Table 2 Positive bacterial artificial chromosome (BAC) clones containing Toll-like receptor signaling pathway genes identified from scallop BAC libraries through PCR screening
Note:*BAC clones selected for FISH.
3.2 FISH Mapping
For each gene, one representative clone was selected randomly for FISH (Table 2). FISH signals for individual positive BAC clones were analyzed in 20 metaphase chromosome spreads. All the five BAC clones were mapped to the corresponding chromosomes of. TheTLR-containing clone BB87B9 was hybridized to the telomeric region of the short arm on a pair of subtelocentric chromosomes (Fig.1a), and theNFκB-contain- ing clone BH802F5 was mapped to a similar position on a pair of submetacentric chromosomes (Fig.1b). Probes derived from clones BB26G9 containingMyd88 (Fig.1c) and BH409H8 containingTRAF6 (Fig.1d) showed signals in the centromeric region of the long arm on a pair of submetacentric or subtelocentric chromosomes, respectively. TheIκB-containing clone BB275F7 (Fig.1e) was mapped to the central section of the long arm in a pair of submetacentric chromosomes.
In order to test whether all the screened BAC clones were located on the same pair of chromosomes, the representative clone was co-hybridized with each of the other clones through double-color FISH. For example (Fig.2), we co-hybridized BB26G9 with each of the other fiveMyd88-containing clones,, BH89A3, BH254D8, BH794G3, BH925B2, and BB253F6. Co-localization of BB26G9 with each of the five clones was confirmed using probes capable of generating merged signals in each case. We concluded that all the sixMyd88-containing clones were located at the same site in the genome. Similar conclusions were drawn from the study on the remaining four genes.
After karyotyping, the means and standard deviations of the relative length and centromeric index were calculated for chromosome pairs with signals (Table 3). TheIκB-containing chromosomes has a smaller relative length while the remaining four chromosomes with signals were considerably larger than the largest metacentric chromosome. The results indicated that clone BB275F7 containingIκB was localized to a different pair of chromosomes from the other clones, BB87B9, BB26G9, BH409H8, and BH802F5.
Co-hybridization was necessary to estimate whether the latter four BAC clones were located on different pairs of chromosomes separately. However, signals of probes derived from each clone were weakened when all these 4 clones were co-hybridized in one experiment. Thus, two BAC clones were assigned to similar chromosomes to confirm their chromosomal assignments by double-color FISH. Clone BB87B9 containingTLRwas labeled with biotin and BH409H8containingTRAF6 with digoxigenin. Results showed that the two probes were localized to two different subtelocentric chromosome pairs (Fig.3a).
Then, the other two BAC clones BB26G9 (digoxigenin) and BH802F5 (biotin) were co-hybridized, and signals were observed on two non-homologous submetacentric chromosome pairs (Fig.3b). The locations of the four BACs obtained from double-color FISH were consistent with the results of one-color FISH. All these available data indicated that the five BAC clones, which containedTLR,Myd88,TRAF6,NFκB andIκB, respectively, were located in five non-homologous chromosome pairs of.

Fig.1 FISH mapping of bacterial artificial chromosome clones containing CfTLR(a),CfNFκB(b), CfIκB (c), CfMyd88(d), and CfTRAF6 (e) from Chlamys farreri. Inset at top right for each probe corresponds to one chromosomal location showing the labeled chromosome adjacent to the largest metacentric chromosome. Scale bars=5μm.

Fig.2 Double-color FISH showing 6 CfMyd88-containing bacterial artificial chromosome clones co-localized on the Chlamys farreri genome. Red, green, and blue channels were recorded separately and then merged. Red signals indicate localization of clone BB26G9 first mapped using single-color FISH, and green signals indicate clones BH89A3, BH254D8, BH794G3, BH925B2, and BB253F6. Signals are indicated by arrows in merged images.

Table 3 Measurements (X±S.D) and classification of Toll-like receptor pathway genes containing chromosomes and the largest metacentric chromosome from metaphases of Chlamys farreri
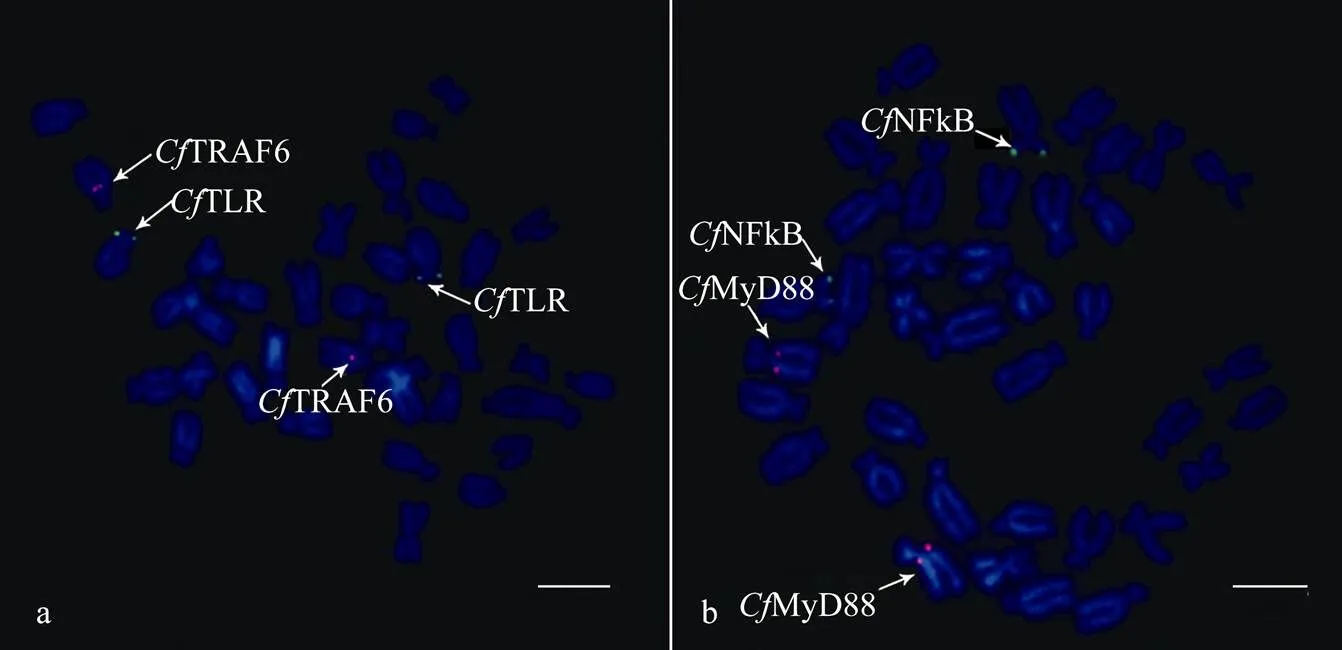
Fig.3 Co-hybridization of Toll-like receptor pathway genes. (a) Bacterial artificial chromosome clones containing CfTLR and CfTRAF6; and (b) clones containing CfNFκB and CfMyd88. Scale bars=5μm.
4 Discussion
An understanding of immune components that underpine host resistance to pathogens is a key step towards elucidating immune mechanisms in scallop. A large number of immune components are known in scallop (Su., 2004; Gao., 2007; Wang., 2007; Yu., 2007; Zhang., 2007). Most of these components have been characterized and analyzed regarding gene function, but few have been physically mapped to chromosomes. In the present study, we used FISH to map five immune genes functioning inTLR signaling pathway in order to study their chromosomal locations. The results showed that each gene occupied a single position on a chromosome pair.
Early studies have shown that genes with similar expression patterns tend to cluster more frequently than those with different expression patterns (Liu and Han, 2009; Chen., 2010). Inand human, there is about 43% and 65% of analyzed pathways showing significant physical clustering of genes across the genome, respectively (Lee and Sonnhammer, 2003). Immune genes inare highly concentrated on chromosome 2, clustered in regions of high recombination rates (Wegner, 2008), which may be a fast and effective way to control expression of genes. As to scallop, the lack of whole genome data limits the research of immune genes. Recently, 2 lipopolysaccharide and beta-1,3-glucan binding protein genes and 3 membrane transport genes have been shown clustered in 2 scallop BAC clones (Zhao., 2012), there may exist groups of functionally related genes that are linked, which could cluster in scallop.
For comparison analysis, the distributions of TLR signaling pathway genes in five model species (Table 4) were obtained from the NCBI database (NCBI Map Viewer, http://www.ncbi.nlm.nih.gov/mapview/). In, all the five TLR signaling pathway genes locate on five non-homologous chromosome pairs. However, there are different distribution patterns in the remaining four species. In, there are two TLR components (Tol-1 and IκB-1) co-locate on chromosome 1, 9.2Mb apart. In, cactus and dorsal, which are homologous with IκB and NFκB, respectively, are co-located on the long arm of chromosome 2, 1.1Mb apart, while MyD88 is located on the other arm of chromosome 2. In, NFκB3 spaces out TRAF6 49.5 Mb apart on chromosome 7. In, TRAF6 is located on the short arm of chromosome 11, while NFκB3 is located on the long arm of the same chromosome. In summary, the candidate immune genes TLR, MyD88, TRAF6, IκB and NFκB are distantly linked in the latter four species.
As to, the five immune genes studied located in five non-homologous chromosome pairs, indicating that the TLR pathway may not show significant clustering as in. These TLR signaling pathway genes were significantly more distant than other functionally related genes, such as lipopolysaccharide and beta-1,3- glucan binding protein genes and membrane transport genes. However, the non-clustering of these genes possibly has little effect on the immune response. In, there is no significant difference in gene expression between clustered and non-clustered immune genes (Wegner, 2008). Here, the co-expression of TLR signaling pathway genes inmay not act in a distance-dependent way.

Table 4 Chromosomal localization of Toll-like receptor signal pathway genes in five model organisms
Note: * Gene ID in NCBI GENE database.
FISH is a powerful tool significantly contributing to aquaculture genome research. FISH mapping of multicopy genes and repetitive elements has been frequently reported in scallop. Huang. (2007) mapped ribosomal DNA and (TTAGGG)n telomeric sequence to chromosomes inZhang. (2007) detected histone H3 gene sites by FISH in four scallops,,,, and. All these results have led to research advance on bivalve evolution and facilitated chromosome identification. However, there is a limited range of probes derived from multi-copy genes and repetitive elements. Mapping of large-insert clones will extend the application of FISH. Nine P1 clones were mapped in the eastern oyster,, identifying seven chromosomes (Wang., 2005). In, Zhang. (2008) identified eight of nineteen chromosomes by co-hybridizing eight fosmid clones. In the present study, we anchored five BAC clones with immune genes to five non-homologous chromosome pairs. These results will provide useful probes for chromosome identification of.
Acknowledgements
We thank Xunshan Aquatic Product Group Co., Ltd. (Rongcheng, China) for scallop samples. This research was financially supported by the National Natural Science Foundation of China (31270047), the National High Tech R&D Program (2012AA10A410), the National Basic Research Program of China (2010CB126402), and the National Key Technology R&D Program of China (2011BAD45B01 and 2011BAD13B05).
Ashida, M., and Brey, P. T., 1998. Recent advances on the research of the insect prophenoloxidase cascade. In:Chapman and Hall, London, 135-172.
Belvin, M. P., and Anderson, K. V., 1996. A conserved signaling pathway: The Drosophila toll-dorsal pathway., 12: 393-416.
Chen, W., Meaux, J., and Lercher, M. J., 2010. Co-expression of neighbouring genes in Arabidopsis: Separating chromatin effects from direct interactions., 11: 178.
Coscia, M. R., Giacomelli, S., and Oreste, U., 2011. Toll-like receptors: An overview from invertebrates to vertebrates., 8: 210-226.
Gao, Q., Song, L., Ni, D., Wu, L., Zhang, H., and Chang, Y., 2007. cDNA cloning and mRNA expression of heat shock protein 90 gene in the haemocytes of Zhikong scallop., 147: 704-715.
Hibino, T., Loza-Coll, M., Messier, C., Majeske, A. J., Cohen, A. H., Terwilliger, D. P., Buckley, K. M., Brockton, V., Nair, S. V., Berney, K., Fugmann, S. D., Anderson, M. K., Pancer, Z., Cameron, R. A., Smith, L. C., and Rast, J. P., 2006. The immune gene repertoire encoded in the purple sea urchin genome., 300: 349-365.
Hoffmann, J. A., Kafatos, F. C., Janeway, C. A., and Ezekowitz, R. A., 1999. Phylogenetic perspectives in innate immunity., 284: 1313-1318.
Hoffmann, J. A., and Reichhart, J. M., 2002. Drosophila innate immunity: An evolutionary perspective., 3: 121-126.
Huang, X., Hu, X., Hu, J., Zhang, L., Wang, S., Lu, W., and Bao, Z., 2007. Mapping of ribosomal DNA and (TTAGGG)n telomeric sequence by FISH in., 73: 393-398.
Hu, L., Shang, W., Sun, Y., Wang, S., Ren, X., Huang, X., and Bao, Z., 2011. Comparative cytogenetics analysis of,, andwith0-1DNA by fluorescencehybridization., 2011: 785831.
Inamori, K., Ariki, S., and Kawabata, S., 2004. A Toll-like receptor in horseshoe crabs., 198: 106- 115.
Janeway, J. C., 1989. Approaching the asymptote? Evolution and revolution in immunology.. 54: 1-13.
Kawai, T., and Akira, S., 2010. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors., 11: 373-384.
Lee, J. M., and Sonnhammer, E. L., 2003. Genomic gene clustering analysis of pathways in eukaryotes., 13: 875-882.
Levan, A., Fredga, K., and Sandberg, A. A., 1964. Nomenclature for centrometric position on chromosomes., 52: 201-220.
Liu, X., and Han, B., 2009. Evolutionary conservation of neighboring gene pairs in plants., 437: 71-79.
Lorenzi, L., Molteni, L., and Parma, P., 2010. FISH mapping in cattle (L.) is not yet out of fashion., 51: 497-499.
Meijer, A. H., Gabby Krens, S. F., Medina Rodriguez, I. A., He, S., Bitter, W., Ewa Snaar-Jagalska, B., and Spaink, H. P., 2004. Expression analysis of the Toll-like receptor and TIR domain adaptor families of zebrafish., 40: 773-783.
Qiu, L., Song, L., Xu, W., Ni, D., and Yu, Y., 2007a. Molecular cloning and expression of a Toll receptor gene homologue from Zhikong scallop,., 22: 451-466.
Qiu, L., Song, L., Yu, Y., Xu, W., Ni, D., and Zhang, Q., 2007b. Identification and characterization of a myeloid differentiation factor 88 (MyD88) cDNA from Zhikong scallop., 23: 614-623.
Qiu, L., Song, L., Yu, Y., Zhao, J., Wang, L., and Zhang, Q., 2009. Identification and expression of TRAF6 (TNF receptor-associated factor 6) gene in Zhikong scallop., 26: 359-367.
Roach, J. C., Glusman, G., Rowen, L., Kaur, A., Purcell, M. K., Smith, K. D., Hood, L. E., and Aderem, A., 2005. The evolution of vertebrate Toll-like receptors., 102: 9577-9582.
Sambrook, J., Fritsch, E. F., and Maniatis, T., 1989.. 2nd edition. Cold Spring Harbor Laboratory, New York, 1659pp.
Sasaki, N., Ogasawara, M., Sekiguchi, T., Kusumoto, S., and Satake, H., 2009. Toll-like receptors of the ascidian,: Prototypes with hybrid functionalities of vertebrate Toll-like receptors., 284: 27336-27343.
Su, J., Song, L., Xu, W., Wu, L., Li, H., and Xiang, J., 2004. cDNA cloning and mRNA expression of the lipopolysaccharide- and beta-1,3-glucan-binding protein gene from scallop., 239: 69-80.
Wang, H., Song, L., Li, C., Zhao, J., Zhang, H., Ni, D., and Xu, W., 2007, Cloning and characterization of a novel C-type lectin from Zhikong scallop., 44: 722-731.
Wang, M., Yang, J., Zhou, Z., Qiu, L., Wang, L., Zhang, H., Gao, Y., Wang, X., Zhang, L., Zhao, J., and Song, L., 2011. A primitive Toll-like receptor signaling pathway in mollusk Zhikong scallop., 35: 511-520.
Wang, Y., Xu, Z., Pierce, J. C., and Guo, X., 2005. Characterization of Eastern oyster (Gmelin) chromosomes by fluorescence in situ hybridization with bacterio- phage P1 clones., 7: 207-214.
Wegner, K. M., 2008. Clustering ofimmune genes in interplay with recombination rate., 3: e2835.
Yu, Y., Qiu, L., Song, L., Zhao, J., Ni, D., Zhang, Y., and Xu, W., 2007. Molecular cloning and characterization of a putative lipopolysaccharide-induced TNF-a factor (LITAF) gene homo- logue from Zhikong scallop., 23: 419-429.
Zhang, H., Song, L., Li, C., Zhao, J., Wang, H., Gao, Q., and Xu, W., 2007. Molecular cloning and characterization of a thioester-containing protein from Zhikong scallop., 44: 3492-3500.
Zhang, L., Bao, Z., Wang, S., Huang, X., and Hu, J., 2007. Chromosome rearrangements in Pectinidae (Bivalvia: Pteriomorphia) implied based on chromosomal localization of histone H3 gene in four scallops., 130: 193-198.
Zhang, L., Bao, Z., Wang, S., Hu, X., and Hu, J., 2008. FISH mapping and identification of Zhikong scallop () chromosomes., 10: 151-157.
Zhang, L., Li, L., and Zhang, G., 2011. AToll-like receptor and comparative analysis of TLR pathway in invertebrates., 30: 653-660.
Zhao, B., Cheng, J., Chen, L., Yu, N., Huang, X., and Bao, Z., 2013. Construction of three bacterial artificial chromosome (BAC) libraries for Zhikong scallop ()., 43: 57-63.
Zhao, C., Zhang, T., Zhang, X., Hu, S., and Xiang, J., 2012. Sequencing and analysis of four BAC clones containing innate immune genes from the Zhikong scallop ()., 502: 9-15.
(Edited by Qiu Yantao)
DOI 10.1007/s11802-015-2643-8
ISSN 1672-5182, 2015 14 (6): 1075-1081
© Ocean University of China, Science Press and Spring-Verlag Berlin Heidelberg 2015
(April 4, 2014; revised August 21, 2014; accepted June 20, 2015)
* Corresponding author. Tel: 0086-532-82031802 E-mail: xthuang@ouc.edu.cn
 Journal of Ocean University of China2015年6期
Journal of Ocean University of China2015年6期
- Journal of Ocean University of China的其它文章
- Inversion Study on Pollutant Discharges in the Bohai Sea withthe Adjoint Method
- Different Responses of Sea Surface Temperature in the North Pacific to Greenhouse Gas and Aerosol Forcing
- The Change Features of the West Boundary Bifurcation Line of the North Equatorial Current in the Pacific
- Wave Pressure Acting on V-Shaped Floating Breakwater in Random Seas
- Dynamic Response of a Riser Under Excitation of Internal Waves
- An Effective Method of UV-Oxidation of Dissolved Organic Carbon in Natural Waters for Radiocarbon Analysis by Accelerator Mass Spectrometry
