Infuence of Different Hydrocarbon Molecules on Physical Properties of Mineral Base Oils
Wang Weijun; Long Jun; He Yifeng; Sun Hongwei; Tian Songbai; Zheng Hui
(SINOPEC Research Institute of Petroleum Processing, Beijing 100083)
Infuence of Different Hydrocarbon Molecules on Physical Properties of Mineral Base Oils
Wang Weijun; Long Jun; He Yifeng; Sun Hongwei; Tian Songbai; Zheng Hui
(SINOPEC Research Institute of Petroleum Processing, Beijing 100083)
Different kinds of base oils with different viscosity were analyzed in this paper, including eight mineral base oils, alkylnaphthalene and three synthetic PAO oils. The infuence of different hydrocarbon molecules on physical properties of mineral base oils was investigated, such as density (d), kinematic viscosity (KV), viscosity index (VI), etc. Possible reasons for some inconsistent phenomena in data processing were also theoretically analyzed in detail. The refractive indexes (RI), d and molecular weight (M) decrease linearly with the increase of paraffnic content other than KV, which declines exponentially. There are no clear relationships between physical properties of base oils and naphthenic content, while polycyclic alkanes show a strong correlation with M and KV. The infuence of aromatics on physical properties of base oils is just the opposite of paraffn’s. VI of the base oils with low aromatics content increases linearly as their paraffnic contents rise when their carbon numbers are approximately equal. However, base oils with high aromatics content follow an utterly different rule, in which VI declines dramatically linearly with the increase in polycyclic aromatic content, which is the essential reason why naphthenic base oils all have terrible viscosity-temperature characteristics while paraffnic base oils usually do not.
base oil; hydrocarbon molecule; physical property
1 Introduction
Lubricating oil is widely applied in industrial production and human life. The major component of lubricant is base oil, which always has a decisive infuence on most properties of lubricant due to its very high mass fraction (85%—99%). Base oils are usually classifed into several types, including mineral base oils, synthetic oils, biooils and semi-synthetic oils. Because of their low cost, facile and fne performance of mineral base oils, they are currently the most popular base oils the market share of which is maintaining at no less than 90% for a long period of time[1].
Among the physical properties of base oils, the most important one is the viscosity which should be high enough to provide proper lubricating flms and also avoid excessive friction loss[2], and it is often a main factor in classifying base oils into various groups according to the American Petroleum Institute[3]in the actual industry production of lubricating oil. Viscosity index is an arbitrary number that reflects the temperaturedependence of viscosity, and the higher the VI, the less the viscosity of oils is affected by temperature. Being different from molecular weight which is usually used to measure the percentage of heavy compounds in base oils, density is linearly correlated to the contents of carbon, oxygen, and sulfur, and can roughly reflect the balance between paraffnic and cyclic compounds of hydrocarbon mixtures. The refractive index is a optical characteristic of base oils, which generally shows a strong correlation to density for the same oil[2,4].
Compared with synthetic oils that commonly have pure components and stable properties, mineral base oils are extremely complex in their chemical composition. They commonly contain tens of thousands of individual hydrocarbons, which are classified into four groups according to the hydrocarbon group composition, e.g. alkanes, aromatics, resins and asphaltenes[5]. In fact, resins and asphaltenes are both complex mixtures that consist of polycyclic aromatic hydrocarbons, sulphur- ornitrogen-containing heterocyclic compounds, mechanical impurities, etc. Therefore, we can classify these hydrocarbons into paraffins, naphthenes, aromatics and non-hydrocarbon compounds on the scale of hydrocarbon molecules[6]. In general, aromatics are always regarded as the most detrimental components of base oils, and so many technologies have been applied to remove them in commercial production, including catalytic hydrocracking and hydrofnishing[7-8]. Through these refnery processes, nearly all the heteroatomic molecules can be removed by saturation except a few percentages of the most thermodynamically stable aromatic species[8]. So base oils exhibit a superior performance properties, but can cause another serious problem, namely: the solubility limitations related with certain additives[8]. Additionally, in most cases, the lower the aromatic contents of base oils are, the better the oil performance is. But there are also a lot of special situations that require high aromatic base oils, especially for operating under low-temperature conditions[6]. It is therefore necessary to understand the variation in the properties of base oils on the basis of their chemical composition.
As a matter of fact, the differences in physical properties and chemical characteristics of mineral base oil are fully determined by their composition. As a key technique with a potential for understanding the carbon and hydrogen distribution of base oils in terms of their average structural parameters, such asCP,CN,CA, etc., (whereCPis further classified intoCipandCnpwhileCNincludesCN,CH3andCN,others[9,15]), which can be related to the bulk properties of base oils, the nuclear magnetic resonance spectroscopy (13C and1H NMR) has not only been widely used to study chemical composition and structure of mineral base oils[8-17], but also has been used for monitoring the degree of refning[8,18-19]. Other analytical methods also have been applied to determine the chemical composition of base oil or assessing the severity of hydrocracking, including the mass spectrometry[7], the gas chromatography-mass spectrometry (GC-MS)[20]and the Fourier transform infrared spectroscopy (FTIR)[21], etc.
It is known that there are two factors that can affect the physical or chemical characteristics of base oils, viz. the content and the chemical structure of hydrocarbons. The approach in this paper is different from many previous studies which have mainly determined the chemical composition of base oils by NМR. Although NМR can well analyze some chemical structures of base oils, these parameters are just the average data calculated from carbon and hydrogen distribution. Therefore, it becomes more diffcult or even impossible to understand the role of each hydrocarbon molecule.
In our studies, we chose the high resolution mass spectrometric method[22–25]to study the relationships between physical properties and different hydrocarbons of eight mineral base oils. Possible reasons for some inconsistent phenomena in data processing were also theoretically analyzed in detail. It will be useful and meaningful if we understand the correlation between the chemical structure, composition and properties of base oils at the hydrocarbon molecular level.
2 Experimental
2.1 Base oils
Different kinds of mineral base oils with different viscosity values were selected for this work, including two naphthenic base oils NS100 and T400 supplied by the Nynas Petroleum (Shanghai) Co., Ltd., four paraffnic base oils including the #2 instrument oil, 400SN, 650SN and 150BS, and two base oils 150N and 500N originated from gas oil hydrocracking.
Мeanwhile, in order to ensure that the research results had wider applicability, some synthetic base oils were also selected as references. As a comparison among pure paraffns, three poly-alphaolefns with different viscosity values, viz. PAO-4, PAO-10, and PAO-40, were analyzed in comparison with eight mineral base oils together, while the alkylnaphthalene sample was completely composed of aromatics.
2.2 Physical properties
The kinematic viscosity (KV) is measured at 40oCand 100oC according to the Chinese standard GB/T 265—1988 using a CANNON CAV®2000, the fully automatic digital kinematic viscometer. Viscosity indexes (VI) are calculated from KV values as specifed in the Chinese standards GB/T 1995—1998, which is equivalent to ASTМ D2270—1993. Мolecular weight (M) is determined according to the Chinese Petroleum Industry Standard SH/T 0583—2004,which is equivalent to ASTM D2503—1992. Density values (d) at 20oC are measured following the Chinese standard GB/T 13377—2010, which is approximately equivalent to ISO 3838: 2004, МOD. Refractive index values (RI) at 20oC are obtained according to the Chinese Petroleum Industry Standard SH/T 0724—2002, which is approximately equivalent to ASTM D1218—1999. The test results are shown in Table 1.
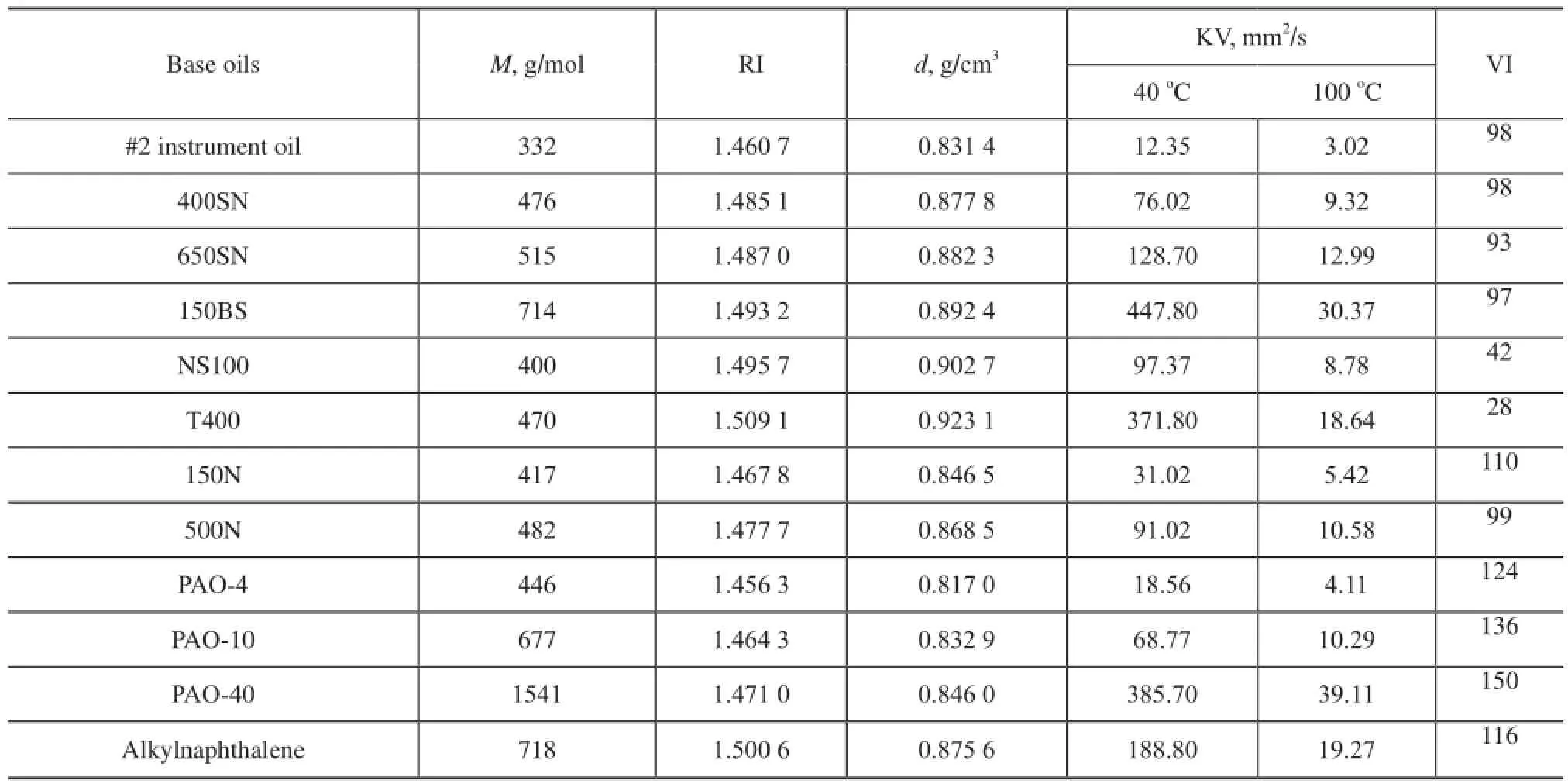
Table 1 Physical properties of base oils
2.3 Hydrocarbon group composition and carbon distribution
The contents of paraffins, naphthenes, aromatics and aromatic sulfur heterocycles in base oils were determined by high resolution mass spectrometry using an Agilent 6890 GC/5973N МS according to the Chinese Petroleum Industry Standard SH/T 0659—1998, which was approximately equivalent to ASTМ D2786 and D3239. The hydrocarbon group composition of eight mineral base oils was further classifed into several common classes on the basis of different ring number for cyclic compounds. The result is shown in Table 2.
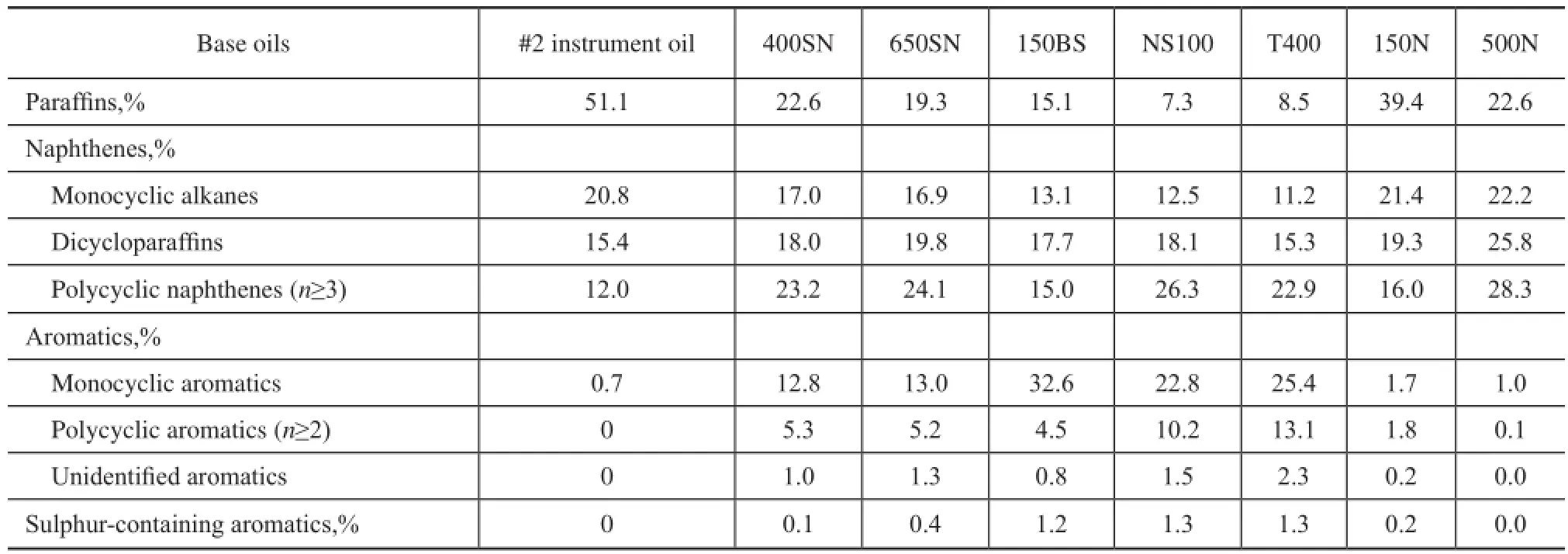
Table 2 Hydrocarbon group composition of mineral base oils
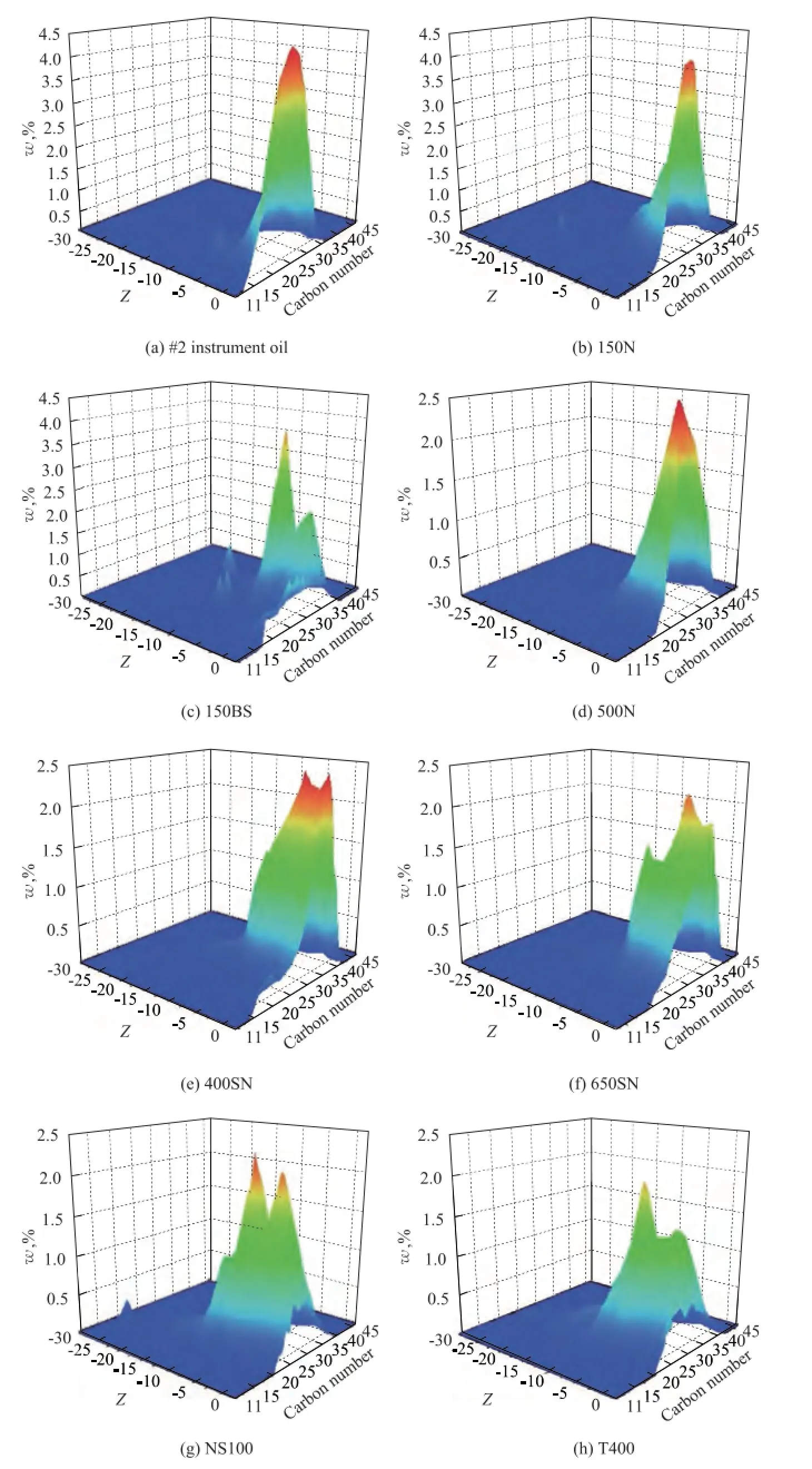
Figure 1 Hydrocarbon carbon number distribution of mineral base oilsZ: means hydrogen defciency values (If the molecular formula of a hydrocarbon is CcHhSsNnOo, thenZ=h– 2c.)
Hydrocarbon carbon number distribution of base oils was measured using gas chromatography feld ionization time-of-fight high-resolution mass spectrometry (GC-FI TOF MS) technology provided by the Research Institute of Petroleum Processing, Sinopec, as shown in Figure 1, which was a new analytical method combining the time-of-fight high-resolution mass spectrometry with GC online separation and field ionization together. And it can be used to analyze the carbon number and compoundtype distribution of hydrocarbons, and sulfur- and nitrogen-containing hydrocarbon compounds in many petroleum products. These data were used to calculate the weighted average carbon number of every hydrocarbon and approximately estimate the hydrocarbon group distribution of eight mineral base oil,CP,CN,CA, as shown in Table 3 and Table 4.
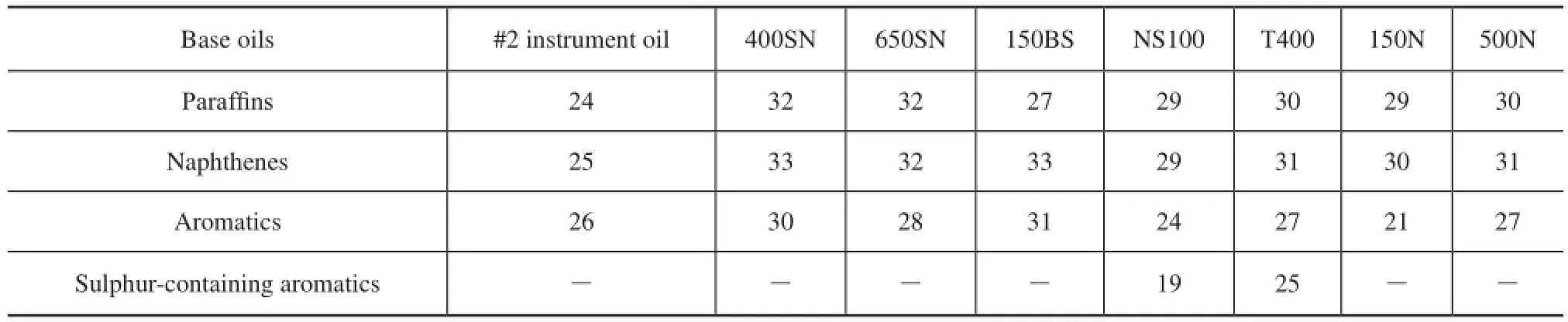
Table 3 Weighted average carbon number of hydrocarbons
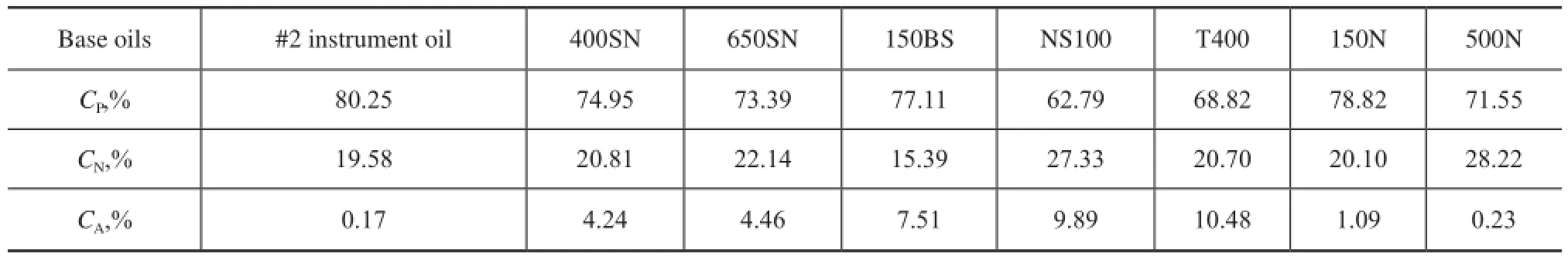
Table 4 Hydrocarbon group distribution of mineral base oils
3 Results and Discussion
3.1 Relationships between physical properties of base oils
The physical properties of base oils are related with their chemical composition, but at the same time, there are also some relationships among themselves. Similarly withd, a property refecting the oil weight per unit volume, RI is also a very common basic characteristic of base oils, which reflects the optical property of base oils. Figure 2 shows thatdis linearly correlated to RI of most base oils (R2of 0.99) except alkylnaphthalene, and Haus, et al.[4]also gave a similar conclusion. As the influence of hydrocarbon composition ondand RI is highly consistent, we can just studydwhen discussing both indicators as shown below.
Besides d and RI, KV is an important physical property of base oils as well. It can be seen from the data listed in Table 1 that KV increases with the increase ofd, RI andM. Although the correlation between KV,dandMis not very ideal as evidenced by further study, it is very clear that the overall trend is approximately linear. Incomparison withd,Mshows a stronger correlation with KV of most base oils except two special oils 150BS and T400. Мeanwhile, the viscosity at different temperature also follows different laws, and KV100is obviously better than KV40, as shown in Figure 3.
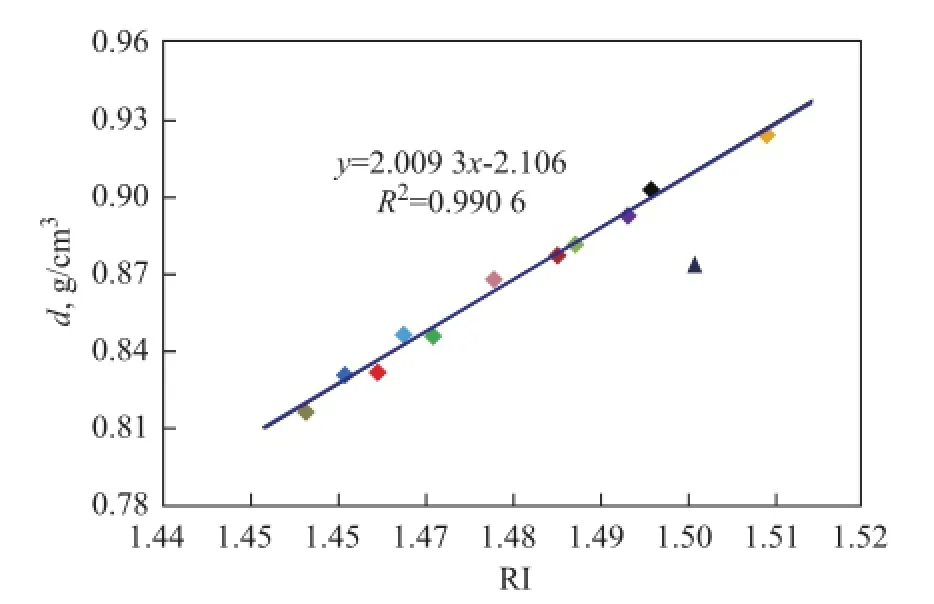
Figure 2 Relationship between RI and d of base oils (Different base oils with different colors in Figure 2 are expressed as follows:brown—PAO-4, blue—#2 instrument oil, red—PAO-10, pale blue—150N, green—PAO-40, pink—500N, dark red—400SN, light green—650SN, purple—150BS, black—NS100, navy blue—Alkylnaphthalene, yellow—T400. And the square means of two coordinate parameters are relevant, while the triangle means that they have nothing to do with each other. Each color and shape at the following pictures have the same meaning as Figure 2)
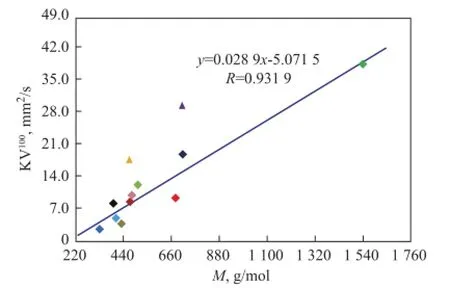
Figure 3 Correlation of KV100and M of base oils
3.2 Influence of paraffins on physical properties of mineral base oils
As the important compounds of mineral base oils, paraffns are commonly divided into two types,n-alkanes and isoparaffns.
Generally speaking, the oxidation stability and viscositytemperature characteristics ofn-alkanes are better than isoparaffins, but their high pour point also leads to wax precipitation more easily when ambient temperature is low enough. In consequence,n-alkanes are not the ideal components of base oils, and there is just a low content of paraffns remaining after solvent dewaxing process in practical production. On the contrary, isoparaffns usually have excellent low temperature fluidity since their pour point decreases dramatically with the increase of the degree of branched chains[26]. Furthermore, isoparaffins are the most ideal compounds of base oils that can beneft from their excellent additive susceptibility, especially for those with less branches and longer side chains[6].
RI and d are strongly correlated with the hydrocarbon composition of mineral base oils. As shown in Figure 4,dof base oils decrease linearly with the increase of paraffnic content, and the same applies to RI. As we all know thatdincreases commonly as the contents of cyclic compounds of hydrocarbon mixtures rise other than paraffns which have the exactly opposite effects ond. The reason why different hydrocarbons have different density values is that the C-C bond length of aromatics is the shortest when their carbon number is approximately equal as compared to naphthenes and paraffns[27], and a shorter C-C bond length means a more compact structure of the hydrocarbon.
Under normal circumstances,Mof a substance with highdis large too, but it is not true in terms of some basic characteristics of mineral base oils. It can be seen from Figure 5 thatMof low aromatic base oils (<20%) shows an excellent linear dependence on paraffns, while three high aromatic base oils (>35%) obviously do not follow this rule. And 150BS demonstrates a positive deviation value, in other word, it is bigger than the expected value, but NS100 and T400 show negative deviation values that are lower than the expected value.
There are two reasons whyMof high aromatic base oils does not show good correlation with the paraffn content. First of all,Mmeasured by vapor pressure osmometry, a colligative property of solution tested according to Chinese Petroleum Industry Standard SH/T 0583, is a number-average molecular weight, which is a quotient of the total mass of system divided by the total number of moles of hydrocarbon mixtures and is more affected by low weight hydrocarbons in a mixture system of base oils. Therefore, it is more seriously diverging from the actual values if more high molecular hydrocarbons exist in base oils. Furthermore, compared with paraffins, the effect of molecular weight is no longer obvious, aromatics have more and more influence on molecular weight for high aromatic base oils, and the influence also depends on aromatic types. It can be seen from Table 3 that the average carbon number of 150BS is 31, while that of NS100 and T400 is just 24 and 27, which are signifcantlyless than the former. But at the same time, the polycyclic aromatic contents of NS100 and T400 are 2—3 times higher than 150BS, as shown in Table 2. It also can be seen clearly from Figure 1 that the hydrocarbon carbon number distribution of 150BS centers on the area with higherZand bigger carbon number, while NS100 and T400 show different circumstance, because the carbon number distribution of these base oils is broader.

Figure 4 Infuence of paraffns ondof mineral base oils
Figure 5 Infuence of paraffns on M of mineral base oils
The infuence of hydrocarbon composition on kinematic viscosity is similar toM, but there are also some differences between them. Based on the study results, the general tendency showing that KV of base oils declines with the increase in paraffin content is exponentially obvious. Мeanwhile, the viscosity values at different temperature also vary between each other, and the correlation of KV100is obviously better than KV40, as shown in Figure 6.
It is well known that the viscosity is a property of solution that is usually used to characterize the friction resistance of inner molecules when the fluid is moving. The viscosity of base oils, which is a complex organic mixture system, is strongly dependent on the size (e.g., molecular weight), shape (e.g., chain branching and inter-chain entanglement) and chemical composition of different constituents, and their interactions[4]. In general, the viscosity increases with the increase of molecular weight, but the chemical structure also is an important influencing factor when the molecular weight is approximately equal. Among these factors the most important one is cyclic structure, the effect of which on viscosity is usually higher than the chain structure. If the rings of two cyclic hydrocarbons are equal, the length ofn-alkyl side chain will become more important, and it is believed commonly that viscosity will increase with the increase in chain length[27]. All in all, the larger the molecule is, the more complex the chemical structure is, and the greater the friction resistance against the relative motion of inner molecules would be, when oil fows and a higher viscosity is the inevitable trend.
We can find from Figure 6 that KV100of 150BS oil is clearly bigger than the expected value. The most important factor is the infuence of its molecular weight, which is much higher than other mineral base oils used in the experiments. The viscosity variation trend of three polyalphaolefin samples, PAO-4, PAO-6, and PAO-40, the molecular weight of which is ascending in sequence, can also indirectly prove the obvious effects of molecular weight on viscosity from another perspective, as shown in Table 1. Besides, the high aromatic content should also contribute to the viscosity of 150BS. It can be seen from the data listed in Table 5 that upon comparing 150BS with NS100 and T400 we can fnd their differences easily. Not only the average carbon number of 150BS is signifcantly bigger than the other two oils, but also the percentage of monocyclic aromatics in total aromatics (such as alkylbenzenes) is higher too. In other words, aromatics in 150BS oil mainly are composed of light aromatics with few rings but longn-alkyl side chains, hence its molecular structures are more complex and viscosity is also higher. On the contrary, aromatics of two naphthenic base oils, NS100 and T400, are heavy aromatics with more rings consisting of less or shortern-alkyl side chains, the molecular structures of which are simple and compact, so their viscosity is naturally low.

Figure 6 Infuence of paraffns on KV100of mineral base oils

Table 5 Percentage of monocyclic aromatics in the total aromatics of mineral base oils
The reasons that make KV100of NS100 show negative deviation are opposite to that of 150BS. One reason is its extremely low molecular weight, which is just higher than #2 instrument oil, and the other one is its high aromatic content, in particular polycyclic aromatics which have less or shortern-alkyl side chains. In comparison with T400 oil, which is a naphthenic base oil, the average carbon number of NS100 is just 24, which is much less than 29 of T400. But the ratio of polycyclic aromatics, the rings of which are greater than or equal to two of NS100, is 33.91%, while that of T400 is 37.75%, which is just higher than the former by no more than 4 percentage points. We can also see from Figure 1 that there are no clear differences between their hydrocarbon carbon number distribution whenZis high, while NS100 tends to distribute in the area with low carbon number but T400 has a wide-ranging distribution obviously asZdeclines. It is very clear that aromatics of NS100 are polycyclic ones albeit with lessn-alkyl side chains, and therefore, its viscosity is also lower.
VI is an arbitrary indicator that refects the temperaturedependence of oil viscosity, which is also a very important property of base oils. In general, VI has been shown to be the highest for paraffins, followed by monocyclic or dicyclic naphthenes and aromatics, while polycyclic aromatics or naphthenes and polar compounds exhibit extremely low values[26]. That is, VI decreases not only with the increase in branching of the paraffins or paraffnic chains attached to naphthenic and aromatic rings for long side-chain paraffins, but also with the increase of rings for rigidly structured hydrocarbons of base oils[28].
In order to study the influence of paraffins on VI, four base oils with nearly the same average carbon number and low aromatic content were selected to correlate with each other, which showed a good linear relationship as shown by Figure 7. Three high aromatic base oils, 150BS, NS100, T400, and #2 instrument oil, the carbon number of which is much less than others, all deviate from the expected values at different degree. In other word, VI of low aromatic base oils increases linearly as paraffnic contents raise when their carbon number is approximately equal, and several published researches[26,29]also gave a similar conclusion. But it is not always true when the aromatic content is high enough or the carbon number is greatly different from each other among base oils. The reasons why this might take place will be discussed in the following section in detail.
Generally, the average chain length (ACL) represents the number of carbon atoms in the normal and isoparaffnic chains and also as a part of side chains attached to naphthenic or aromatic rings. According to many literature reports[8,13,17,30], a decrease in ACL among the paraffnic oils increases the VI. Judging from the chemical composition level, PAO is polymerized from α-olefns, so its molecular weight increase means that its carbon chains become longer. It can be seen from the data in Table 1 that VI of three polyalphaolefins, PAO-4, PAO-10, and PAO-40, improve with the increase of its М, which possibly indicates that the longer the carbon chain, the higher the VI is. This regular scenario is just the opposite of afore-mentioned one, and it might not be always right only in case that the paraffnic content of base oils is high enough, so that other compounds will have less or even no effect on it. In this study, the paraffnic content of #2 instrument oil is as high as 51.1%, but the average carbon number is just 24, and its hydrocarbon carbon number distribution also centers on the area with high Z but low carbon number as it can be seen from Figure 1, and perhaps it is the most important key factor leading to its lower viscosity index.
3.3 Infuence of naphthenes on physical properties of mineral base oils
Usually, notwithstanding the oxidation stability and viscosity-temperature characteristics are worse than paraffins, naphthenes are still an important kind of hydrocarbons in base oils, in particular the monocyclicalkanes which have no or less branched side chains, mainly owing to its low pour point and good dissolving capacity for polar compounds[6].

Figure 7 Infuence of paraffns on VI of mineral base oils
There is not a clear correlation between physical properties of base oils and the total content of naphthenes, and, by and large,Mand KV increase with the rise of naphthenic contents, whiled, RI and VI are disordered clearly.
Since naphthenic rings are substituted by longn-alkyl side chains, many properties of monocyclic alkanes are quite similar to those of paraffns. As the content of monocycloparaffns increases,Mis obviously disordered, and the overall trend of KV is declining, while d and RI, which are all sensitive to chemical composition of base oils, fall linearly, although the correlation is not very well understood.
The chemical structure of dicycloparaffns is a transition state from paraffins to naphthenes, so there is no clear relationship between their content and physical properties, such asd, RI and VI. Being different from monocycloparaffins in nature, М and KV of dicycloparaffins increase with an increasing content of dicycloparaffns.
Polycyclic naphthenes, the ring number of which is greater than or equal to three, have a great effect on the physical properties. In general, with the increase in the content of polycyclic naphthenes,d, RI and VI also increase correspondingly.Mincreases linearly while KV of base oils decreases exponentially with the exception of three high aromatic base oils, as shown in Figure 8 and Figure 9. Мoreover, the correlation between the viscosity values at different temperature and the polycyclic naphthenes is also different from paraffins, and KV40is much better than KV100for studying the oil property.
We can clearly know from the data listed in Table 6 that not only the polycyclic naphthenes content in 150BS is less, which is just higher than #2 instrument oil, but its total naphthenes content is the lowest among eight base oils. Therefore, naphthenes have a limited influence on KV and М of base oils, and perhaps the high aromatic content, especially the content of light aromatics with less aromatic rings and longn-alkyl side chains, has even a greater impact, which has been discussed in detail previously.
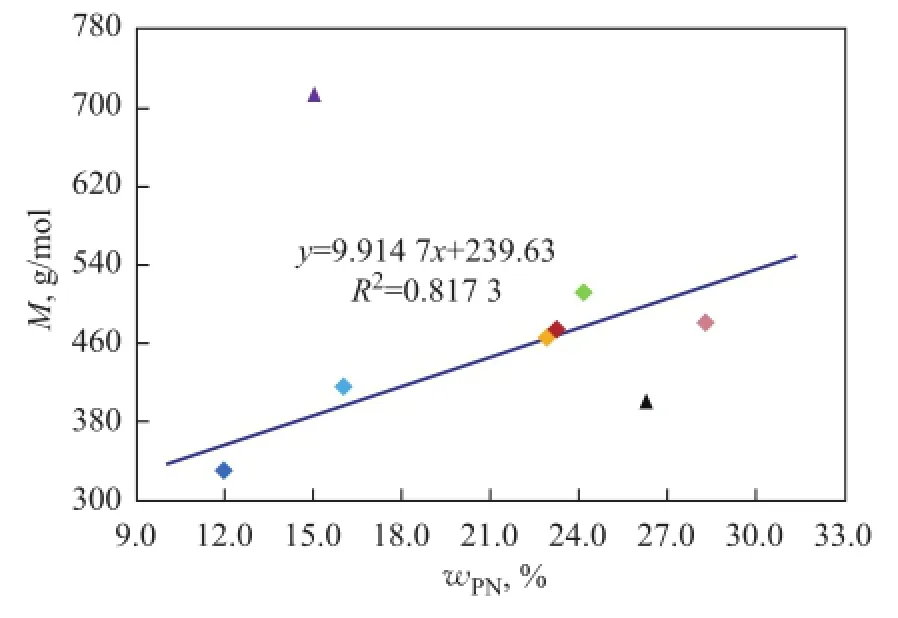
Figure 8 Infuence of polycyclic naphthenes on M of mineral base oils

Figure 9 Infuence of polycyclic naphthenes on KV40of mineral base oils

Figure 1 0Infuence of aromatics on d of mineral base oils
NS100 and T400 oils are all naphthenic base oils, and there are few differences in their naphthenic hydrocarbons distribution. The data in Table 6 show that in comparison with NS100, the content of polycyclic alkanes of T400 is slightly lower, but the percentage of polycyclic alkanes in total naphthenes of T400 is approximately equal to that of NS100. We can know from Table 3 that their average carbon number ofnaphthenic hydrocarbons is close between each other. While KV40of T400 is much high than NS100 as shown in Figure 9, this also indicates that the influence of naphthenic hydrocarbons is much less than aromatics. Therefore, we should pay attention to the different roles of hydrocarbons in mineral base oils.
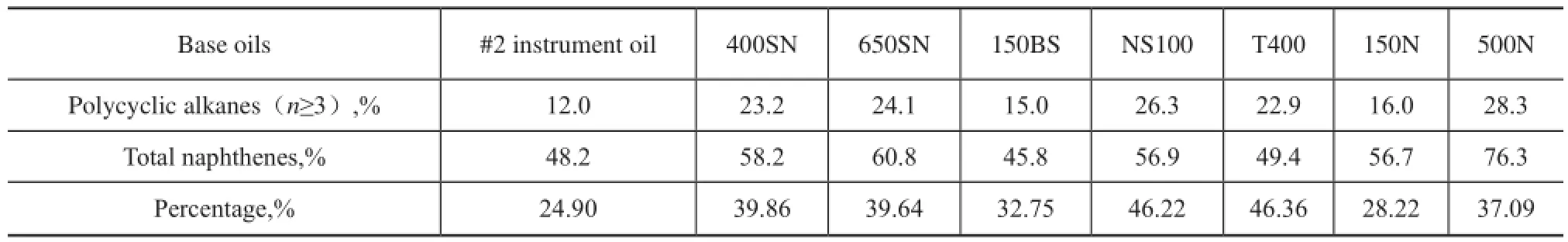
Table 6 Percentage of polycyclic alkanes in the total naphthenes of mineral base oils
3.4 Infuence of aromatics on physical properties of mineral base oils
With much worse antioxidation capabilities and viscositytemperature properties than saturated hydrocarbons, aromatics are often regarded as the most detrimental compounds of mineral base oils, especially the polycyclic aromatics. But the existence of low molecular weight aromatics not only can effectively retard the deep oxidation of saturated hydrocarbons but also improve the capacity for dissolving polar compounds and the low temperature performance of base oils[6]. In this sense, it is signifcantly benefcial to the properties of oils to contain a small amount of light aromatics in base oils. Total aromatics have a significant influence on the physical properties of base oils. RI,d, KV andMincrease dramatically in different degree with an increasing content of aromatics, while the VI values decline.
Since the density of paraffinic constituents is generally lower than that of aromatics,dof mineral base oils increases linearly with an increasing aromatic content, and the same situation applies to RI, as shown in Figure 10. When the aromatic content is less than 1%, the aromatics have little or no influence on the physical properties of both #2 instrument oil and 500N, therefore, we can neglect their effects upon discussing in the following section.
Based on the analysis mentioned above, the compact chemical structure of rings plays a key role in the high density of aromatics, making the molecular volume calculated with each atom much smaller. In Figure 10, 150BS oil shows a clearly negative deviation, which is mainly ascribed to its high monocyclic aromatic content. It can be seen from the data depicted in Table 2 that the total aromatics content of 150BS is as high as NS100, but itsCAvalue is less than the latter by about 2 percentage points (Table 4), which means that the proportion of aromatic rings is much less, and as a result, its density is low too.

Figure 1 1Infuence of aromatics on KV40of mineral base oils
Similar to naphthenes, KV of base oils increases exponentially with an increasing total aromatic content, and the correlation of KV40is much better than KV100, as shown in Figure 11. It can be seen from Figure 11 that 650SN, NS100 and 150BS oils all deviate from the expected values in different degree. Based on the data listed in Figure 6, Figure 9 and Table 5, we can fnd that the content and type of aromatics in 650SN are similar to those of 400SN, therefore, the differences in viscosity between them are mainly caused by saturated hydrocarbons other than aromatics, in particular naphthenes. As shown in Figure 1, the hydrocarbon carbon number distribution of 650SN is also very similar to 400SN except for the high Z area, in which the carbon number distribution in 400SN is more concentrated. The reasons why 150BS shows a positive deviation and NS100 displays a negative deviation have been discussed above in detail, so we will not describe them hereby again.
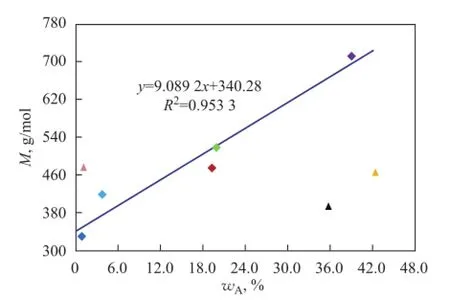
Figure 1 2Infuence of aromatics on M of mineral base oils
TheMof base oil increases with an increasing total aromatic content, as shown in Figure 12. Being different from paraffins and naphthenes,Mof NS100 and T400 is greatly deviated from the expected values other than 150BS,Mof which is always much higher than other oils.
The possible reasons whyMof two naphthenic base oils are much lower than the expected values in the case of high contents of aromatic hydrocarbons, in particular polycyclic aromatics, while other oils do not have lowerMvalue will theoretically analyzed in detail as shown below.
Firstly, as we all know, the relative atomic mass of hydrogen is very small as compared to carbon, so that hydrogen has a little contribution to the weight of a molecule. Since the percentage of carbon in molecules is constantly rising with the increase of index of hydrogen deficiency of oil hydrocarbons, the molecular weight of aromatics is generally much higher than that of saturated hydrocarbons, but it is not always right just only in the case when the carbon number of aromatics and saturated hydrocarbons is approximately equal. Therefore, the carbon number of hydrocarbons other than their types is the most important factor that can decide the molecular weight of base oil. It can be seen from Table 3 and Table 5 that, although the aromatic content of NS100 and T400 is as high as that of 150BS, their average carbon number is much lower than 150BS and even lower than 400SN and 650SN, the total aromatics of which are much less. And in fact, the М values of NS100 and T400 are not only much less than 150BS but also slightly lower than 400SN and 650SN, which is in agreement with their average carbon number trend.
Furthermore, another important reason for low molecular weight of NS100 and T400 might be caused by the test method, which has been discussed previously in detail. Polycyclic aromatics of naphthenic base oils are heavier aromatic compounds as compared with the monocyclic aromatics, which are often regarded as light compounds, so the higher the heavier aromatic content is, the worse the colligative property of oils is, and more seriously it would diverge from the actual value.
Being different from monocyclic alkanes, monocyclic aromatics withn-alkyl side chains have nothing in common with paraffns, and their infuence on properties of base oils is similar to that of total aromatics, although with worse correlation than the latter.
Like total aromatics, the infuence of polycyclic aromatics with their rings being equal to or more than two on the properties of mineral base oils is great too, especially on VI, which shows a very perfect linear correlation with the polycyclic aromatic content, as shown in Figure 13.

Figure 1 3Infuence of polycyclic aromatics on VI of mineral base oils
We can find from Figure 7 that VI of low aromatic mineral base oils shows a good linear correlation with the paraffnic content, and 150BS slightly diverges from the expected value, while the VI values of NS100 and T400 are seriously lower than other oils. Now, as it can be seen clearly from Figure 13, VI of base oils declines dramatically from a linear relationship with the increase of polycyclic aromatics. Based on the analysis mentioned above, it is the high polycyclic aromatic content that makes VI of both NS100 and T400 much lower than 150BS, which also has high total aromatics content but less polycyclic aromatic content, and this is the essential reason why naphthenic base oils all have terribleviscosity-temperature characteristics while paraffnic base oils usually do not have in practice.
In addition, the large CPvalue of 150BS, which reaches up to 77.11%, also contributes to its high VI, since a published research[4]reported that VI of base oils showed a good positive linear correlation with CPvalues. We can see clearly from Table 2 and Table 4 that 150BS has less paraffnic content but much more total aromatic content and high CPvalue as compared with 400SN and 650SN, which are all paraffinic base oils with lower aromatic contents and CPvalues but higher paraffinic contents. Besides, we should also notice that polycyclic aromatics content of 150BS is slightly lower than that of 400SN and 650SN, although its total aromatic content is much higher than other paraffinic base oils. As it has been discussed above, the high CPvalue and paraffinic content can be benefcial to VI of a base oil, but aromatics are obviously unfavorable, especially polycyclic aromatics. Eventually, the result that balances different factors’ effects is that VI of 150BS should be approximately equal to 400SN and even greater than 650SN.
This conclusion also can be verifed by the high viscosity index of alkylnaphthalene, which is comprised of pure aromatic compounds and selected as a comparison in experiments, indicating that it is significantly beneficial and not harmful to the properties of oils, if base oils contain a slight amount of light aromatics, such as alkyl benzenes, benzocycloparaffns, etc.
4 Conclusions
The physical properties of base oils are strongly related to their chemical composition. RI, d and M of mineral base oils decrease linearly with an increasing paraffnic content other than KV, which declines exponentially. There is no clear relationship between physical properties of base oils and naphthenes, while polycyclic alkanes show strong correlation with M and KV. The influence of aromatics on oil properties is just the opposite of paraffins. In addition, the average carbon number of hydrocarbons other than types is the most important factor that can decide M of base oil. VI of low aromatic mineral base oils increases linearly with a rising paraffinic content, when their carbon number is approximately equal. However, high aromatic base oils follow an utterly different rule, in which VI deviates dramatically from a linear relationship with the increase in polycyclic aromatic content, which is the essential reason why naphthenic base oils all have detrimental viscosity-temperature characteristics while paraffnic base oils usually do not have in practice.
[1] An J X, Xing C, Lv L. The current status and development trend for supply and demand of base oils at home and abroad [J]. Petroleum Products Application Research, 2015, (1): 11-19
[2] Haus F, Boissel O, Junter G A. Мultiple regression modelling of mineral base oil biodegradability based on their physical properties and overall chemical composition [J]. Chemosphere, 2003, 50 (7): 939-948
[3] API Publication 1509. Engine Oil Licensing and Certifcation System, 12thed., Appendix E. American Petroleum Institute, 1993
[4] Haus F, Geman J, Junter G A. Primary biodegradability of mineral base oils in relation to their chemical and physical characteristics [J]. Chemosphere, 2001, 45 (6): 983-990
[5] Basu B, Singh М P, Kapur G S, et al. Prediction of biodegradability of mineral base oils from chemical composition using artificial neural networks [J]. Tribology International, 1998, 31 (4): 159-168
[6] Wang W J, Sun H W, Tian S B. Influence of base oil molecular composition on the performance of lubricating greases [A]. Proceedings of the Eighteenth Session of Technical Communication Meeting of Lubricating Grease in China [C]. 2015: 140-148
[7] Pillon L Z. Use of mass spectrometry to study the effect of hydrocracking on the composition of base stocks [J]. Petroleum Science & Technology, 2002, 20 (1/2): 223-232
[8] Sharma B K, Adhvaryu A, Pereza J М, et al. Effects of hydroprocessing on structure and properties of base oils using NМR [J]. Fuel Processing Technology, 2008, 89: 984-991
[9] Sastry М I S, Chopra A, Sarpal A S, et al. Carbon type analysis of hydro-treated and conventional lube-oil base stocks by IR spectroscopy [J]. Fuel, 1996, 75 (12): 1471-1475
[10] Adhvaryu A, Sharma Y K, Singh I D, et al. Studies on the oxidative behavior of base oils and their chromatographic fractions [J]. Fuel, 1999, 78: 1293-1302
[11] Sharma B K, Stipanovic A J. Pressure viscosity coeffcient of lubricant base oils as estimated by nuclear magnetic resonance spectroscopy [J]. Industrial & Engineering Chemistry Research, 2002, 41 (19): 4889-4898
[12] Barman B N. Behavioral differences between group I and group II base oils during thermo-oxidative degradation [J]. Tribology International, 2002, 35: 15-26
[13] Adhvaryu A, Erhan S Z, Sahoo S K, et al. Thermo-oxidative stability studies on some new generation API group II and III base oils [J]. Fuel, 2002, 81: 785-791
[14] Sharma B K, Stipanovic A J. Pulsed field gradient NМR spectroscopy: Applications in determining the pressure viscosity coeffcient and low-temperature fow properties of lubricant base oils [J]. Industrial & Engineering Chemistry Research, 2003, 42 (7): 1522-1529
[15] Sharma B K, Adhvaryu A, Sahoo S K, et al. Infuence of chemical structures on low-temperature rheology, oxidative stability, and physical properties of group II and III base oils [J]. Energy & Fuels, 2004, 18 (4): 952-959
[16] Wang Q, Ling H, Shen B X, et al. Evaluation of hydroisomerization products as lube base oils based on carbon number distribution and hydrocarbon type analysis [J]. Fuel Processing Technology, 2006, 87: 1063-1070
[17] Rajendiran A, Krishnasamy K, Kabilan S, et al. Thermal, spectral, oxidation stability and antioxidant behavior on Group II base oils [J]. Fuel, 2014, 137: 122-134
[18] Sarpal A S, Kapur G S, Мukherjee S, et al. Characterization by13CNMR spectroscopy of base oils produced by different processes [J]. Fuel, 1997, 76 (7): 931-937
[19] Singh I D, Aloopwan М K S, Chaudhary G S, et al. Structural changes during hydrogenation of lube distillates: NМR studies [J]. Fuel, 1992, 71 (11): 1335-1337
[20] Keller М A, Saba C S. Oxidative stability and degradation mechanism of a cyclotriphosphazene lubricant [J]. Analytical Chemistry, 1996, 68 (19): 3489-3492
[21] Dong J, Van de Voort F R, Ismail A A, et al. Rapid determination of the carboxylic acid contribution to the total acid number of lubricants by Fourier transform infrared spectroscopy [J]. Lubrication Engineering, 2000, 56 (6): 12-20
[22] Zhu X Y, Liu Z L, Xu Y Q, et al. Detailed analysis of diesel by GC feld ionization time-of-fight high-resolution mass spectrometry [J]. Acta Petrolei Sinica (Petroleum Processing Section), 2010, 26 (2): 277-282 (in Chinese)
[23] Wang N X, Liu Z L, Zhu X Y, et al. Study on hydrocarbon compositions of paraffns in hydrocracking tail oil by mass spectrometry [J]. Petroleum Processing and Petrochemicals, 2014, 45 (5): 94-100 (in Chinese)
[24] Jiang J J, Liu Y R, Liu Z L, et al. Paraffins species distribution analysis in petroleum middle fractions by GC field ionization time-of-flight mass spectrometry [J]. Chinese Journal of Analytical Chemistry, 2016, 44 (3): 416-422 (in Chinese)
[25] Liu Y R, Jiang J J, Liu Z L, et al. Identification of isoparaffn components in petroleum middle fractions [J]. Chinese Journal of Chromatography, 2016, 34 (2): 215-221 (in Chinese)
[26] Gao S B, Liu H B, Wang X М, et al. Effect of Base Oil Composition on Lubricant Performance [J]. Chemical Technology Мarket, 2010, 33 (9): 36-39 (in Chinese)
[27] Xu C М, Yang Z H. Petroleum Refnery Engineering [M].4th Ed. Beijing: Petroleum Industry Press, 2009: 61, 66, 71 (in Chinese)
[28] Hu S W, Guo Q Z, Xia G F, et al. Influence of Composition on Properties of Lube Base Oils Produced by Hydroisomerization Dewaxing Process [J]. Acta Petrolei Sinica (Petroleum Processing Section), 2015, 31 (4): 831-835 (in Chinese)
[29] Chen W Y, Zou K, Wang X W, et al. Research of properties and composition of several base oils by hydrocracking process [J]. Petroleum Processing and Petrochemicals, 2014, 45 (10): 94-98 (in Chinese)
[30] Zhang Z, Wen H X, Zhang М, et al. Studies on the relation between the structural characteristics of base oils and its properties (I) -The effect of saturate structures on its properties [J]. Lubrication Engineering, 2002, (6): 35-37
Received date: 2016-09-05; Accepted date: 2016-10-26.
Professor Sun Hongwei, Tel.: +86-10-8236-8297. E-mail: sunhw.ripp@sinopec.com.
- 中国炼油与石油化工的其它文章
- Tribological Characteristics of Graphene as Lithium Grease Additive
- Regeneration of Simulated Deactivated Hollow Titanium Silicate Zeolite by Secondary Crystallization in the TPAOH Solution
- Commercial Application of Novel Heavy Oil Catalytic Cracking Catalyst HSC
- A FCC Catalyst Prepared by in situ Technique Based on Application of Filter Residue and Kaolin
- Mesoporous Ti-Mo Mixed Oxides Catalyzed Transformation of Carbohydrates into 5-Hydroxymethylfurfural
- Study on Rheological Characteristics of a Grease Used in High Speed Bearing

