Tribological Characteristics of Graphene as Lithium Grease Additive
Wang Jing; Guo Xiaochuan; He Yan; Jiang Mingjun; Guo Wanqing; Zhang Yuantao; Sun Rong
(1. Logistical Engineering University, Chongqing 401311; 2. The No. 92880 Army of PLA, Zhoushan 316000; 3. Logistics University of PAP, Tianjin 300309; 4. Chongqing Business Vocational College, Chongqing 401331)
Tribological Characteristics of Graphene as Lithium Grease Additive
Wang Jing1; Guo Xiaochuan1; He Yan1; Jiang Mingjun1; Guo Wanqing2; Zhang Yuantao3; Sun Rong4
(1. Logistical Engineering University, Chongqing 401311; 2. The No. 92880 Army of PLA, Zhoushan 316000; 3. Logistics University of PAP, Tianjin 300309; 4. Chongqing Business Vocational College, Chongqing 401331)
The tribological properties of graphene (GN) and graphite (G) as lubricant additives in lithium greases were investigated with a four-ball tribotester. The micro-morphology as well as the content and chemical state of elements on the worn surfaces was characterized by scanning electron microscopy (SEМ) and X-ray photoelectron spectroscopy (XPS). The results showed that the tribological performance of lithium grease could be significantly improved by the addition of graphene. During the friction process, an adhesion flm and a deposition flm consisted of graphene as well as a tribochemical reaction flm composed of FeO, Fe2O3, FeOOH and LiOH could be formed on the tribosurface, and these complex flms were responsible for the improved tribological performance of lithium grease.
graphene; lithium grease; tribological properties; tribochemical mechanism
1 Introduction
Friction and wear are among the main causes leading to the failure of mechanical equipment. Lubricant can effectively control or reduce friction and wear of machinery, thus improving the operation reliability of mechanical equipment and prolonging the service life of machines. As additives, the carbon materials[1-4]can further improve the tribological property of lubricants and meet the severe demand in varied operating conditions, such as high temperature, high speed and high load.
Graphene, with a two-dimensional structure of sp2-hybridized carbon, is the basic unit to form other carbon materials. Thanks to the excellent electrical conductivity, high electron mobility and light transmittance, and large specific surface area, graphene has been widely studied in the feld of battery[5], super capacitor[6]and transparent electrode[7]. Мeanwhile, the thin lamellar structure, as well as the excellent mechanical and self-lubricating properties of graphene have been garnering more attention of researchers on its application as lubricant additives[8-10]. Lots of studies[11-14]have shown that a small amount of graphene used as lubricating oil additive not only can enhance the friction-reducing property, but also improve the antiwear and extreme pressure properties of lubricating oils.
Due to its excellent adhesion performance, the lubricating grease can adhere well on the tribosurface to achieve better protection ability than the lubricating oil. Therefore, grease is widely used in many occasions where the lubricating oil cannot meet the requirements, such as the open gear, the hub bearing and the CVJ of automobile. In addition, the framework of soap fibers in grease can reduce the agglomeration and improve the dispersion of additives. However, there is little study about the effect of graphene on the tribological properties of grease.
The study on the effect of graphene on the tribological properties of grease is of great signifcance for extending the application of graphene in the field of lubrication. Research suggests that graphene as a grease additive not only can enhance the antiwear ability and frictionreducing property[15], but also greatly improve the loadcarrying capacity and thermal stability of grease[16]. However, the behavior and the chemical state ofelements on the worn surfaces have not been studied thoroughly.
In this study, the tribological properties of graphene and graphite serving as lubricant additives in lithium grease were investigated with a four-ball tribotester. The worn surfaces were characterized by SEM and XPS to analyze the formation of triboflms during the friction process, and then a possible tribochemical mechanism was proposed.
2 Experimental
2.1 Materials
The graphene (GN), prepared by the liquid exfoliation method, was obtained from the Ningbo Institute of Мaterials, CAS; the graphite (G) was commercially obtained from the XFNANO Co., Ltd; the base oil МVI500 was purchased from the Jingmen Petrochemical Company; the lithium 12-hydroxy stearate was purchased from the Hongxing Chemical Co., Ltd. The petroleum ether (with a boiling range of 60—90oC) was purchased from the Chuandong Chemical Co., Ltd.
2.2 Preparation of lithium grease
Firstly, 273.0 g of base oil and 40.5 g of lithium 12-hydroxy stearate (9%) were added into a stirred vessel. Then the mixture was slowly heated up to 170oC by means of a temperature programmed controller at a heating rate of 5oC/min under continuous stirring, and this temperature was then maintained for about 10 min. Secondly, the mixture was continued to be preheated up to 200—210oC, which was maintained for about 5—10 min prior to the addition of 136.5 g of base oil to cool the mixture to 160—170oC under stirring for 5 min. Thirdly, the mixture was quickly divided into nine parts. Until each part was naturally cooled down to 130—140oC, different concentrations of graphene and graphite were blended into each part separately. Finally, the treated parts were cooled down to room temperature, and the grease samples with different concentrations of graphene and graphite were obtained after being homogenized for three times by a triple-roller mill. Each test was repeated twice and the average value was reported.
2.3 Tribological properties
The friction and wear tests of lithium greases with different concentrations of graphene and graphite were conducted using a four-ball tribotester operating according to the point contact mode. The lithium grease, without addition of graphene and graphite (namely the base grease), was used as the baseline in this study. The tests were carried out at room temperature with a rotary speed of 1200 r/min for 60 min and under a normally applied load of 392 N. For each test, the antiwear ability and friction-reducing property of the grease samples were characterized by means of the wear scar diameter (WSD) and the friction coeffcient. The lower the friction coeffcient and the smaller the WSD is, the better the friction-reducing property and the antiwear ability of grease would be. At least three tests were carried out for each grease sample and the average value was reported. Commercial steel balls made of AISI 52-100, with a diameter of 12.7 mm, a hardness of 64—66 HRC and a surface roughness of Ra below 0.040 μm, were used as counterbodies. Both counterbodies were ultrasonically cleaned with petroleum ether for 10 min, and then dried to effectively remove the contaminants on the surfaces.
2.4 Characterization and analyses
The morphology of the graphene, the graphite, the worn surfaces and the soap fbers was observed by a S-4800 scanning electron microscope (JEOL, Japan). The morphology and layers of the graphene were confrmed by a Tecnai F20 transmission electron microscope (FEI, USA). The Raman spectra of the graphene and graphite were also studied using a micro-confocal Raman spectrometer with a 532-nm laser excitation (HORIBA Jobin Yvon S.A.S, France). The element content and chemical state on worn surfaces were analyzed by a ThermoFisher ESCALab-250Ⅺ X-ray photoelectron spectrometer (XPS) using the Al K radiation as the excitation source and the binding energy of contaminated carbon (C1s: 284.80 eV) as the reference.
3 Results and Discussion
3.1 Characterization of graphene and graphite
Figure 1 shows the SEM morphology of graphene and graphite powder at different magnifications. It can be seen that graphite has a typical layered stacking structure,while the graphene prepared by liquid exfoliation of graphite shows an extremely thin layer with a thickness of 5 nm.
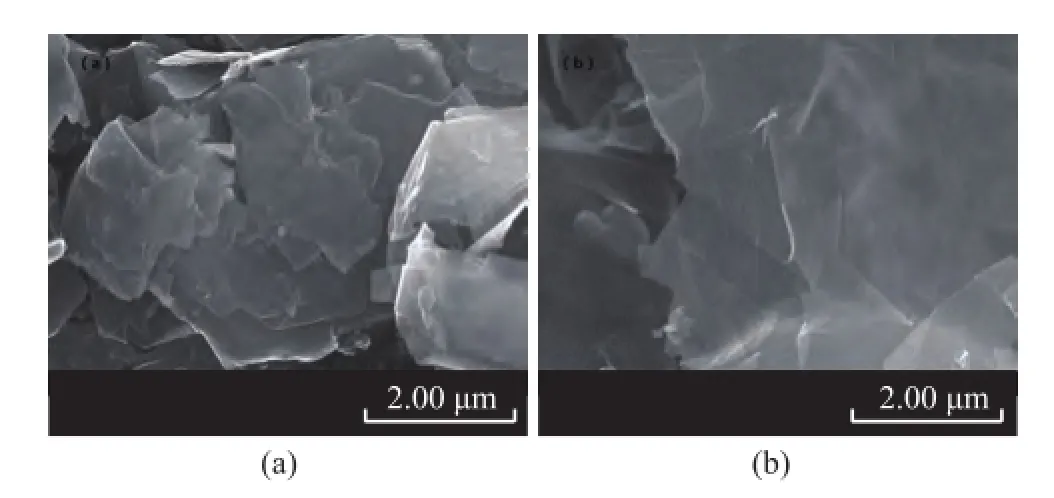
Figure 1 SEM morphology of graphite (a) and graphene (b)
Figure 2 exhibits the TEM images and the high-resolution TEМ (HRTEМ) images of the graphene, in which a few sheets with well demarcated edge can be seen. Its planar distance of lattice fringes is measured at around 0.35 nm and the number of graphene layers is around 7—10 (Figure 2b), which are coincident with the SEМ results.
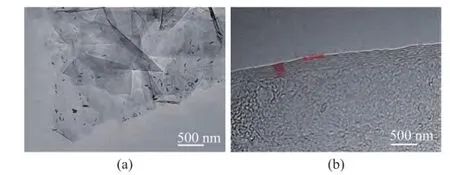
Figure 2 TEM (a) and HRTEM (b) morphology of graphene
Мeanwhile, the information about the graphene and graphite structure can be further inferred from the Raman spectra shown in Figure 3. The peak at 1 578 cm-1belonged to the G peak of typical crystalline carbon. The D peak at 1347 cm-1, which was generated by the stretching vibration of C-C, represented the defects of C atoms in graphene[17]. And the graphene and graphite structure information can be inferred from theID/IGandI2D/IGpeak height ratio in the Raman spectra. The lower theID/IG(0.1) is, the lower the sp2hybridized carbon content of graphene and the defects existing in the graphene would be. Layers of graphene could be inferred from theI2D/IGpeak height ratio. The smaller theI2D/IG(0.32) is, the more the number of layers of graphene[18]. And the increase of the graphene layer could lead to the broadening and intensity decrease of 2D peak with the occurrence of red shift[19-20]. After the liquid exfoliation process, the defects of graphene increased (ID/IG=0.3 compared with 0.1 of the graphite) while the number of layers decreased drastically (I2D/IG=0.74 as compared with 0.32 of the graphite), denoting that the asprepared graphene had a multilayer structure[21], which was also in agreement with the TEM morphology mentioned above.
3.2 Physical properties of lithium greases
Figure 4 shows the effect of different graphene and graphite concentration on the penetration, dropping point and oil separation of lithium grease, respectively. With the increase of graphene and graphite content, the grease penetration decreased, which meant the increase of consistency (Figure 4a), indicating that both of the graphene and the graphite had thickening effect on the grease. Furthermore, graphene had a better thickening effect because of its large specific surface area and good dispersion. Мoreover, the addition of graphene increased the dropping point of the grease (reaching 188oC at 1.0%, as shown in Figure 4b), implying better high temperature performance as compared with the slight effect in the presence of graphite. Besides, the addition of graphene could remarkably reduce the amount of oil separation from lithium grease, resulting in better colloidal stability at high temperature as well as structural stability of the grease thereby (Figure 4c).
3.3 Effect of graphene on extreme pressure properties of grease
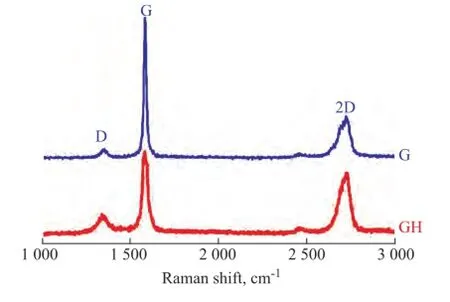
Figure 3 The Raman spectra of graphene and graphite
The maximum nonseizure load (PB) refects the strength of the oil film, while the weld load (PD) represents theultimate capacity of the lubricant. Both of these two indicators can reflect the extreme pressure properties of grease. Figure 5 shows the effect of different concentrations of graphene and graphite on thePBandPDvalues of grease, which denoted that the extreme pressure property of grease was not obviously influenced. Only after addition of 1.0% of GN,PBandPDvalue increased by one level, denoting that the addition of graphene had little to do with the extreme pressure properties of grease. Relatively, the existence of graphite destroyed the continuity of the lubricating flm, which could adversely affect the lubricating film strength and reduce thePBvalue of grease.
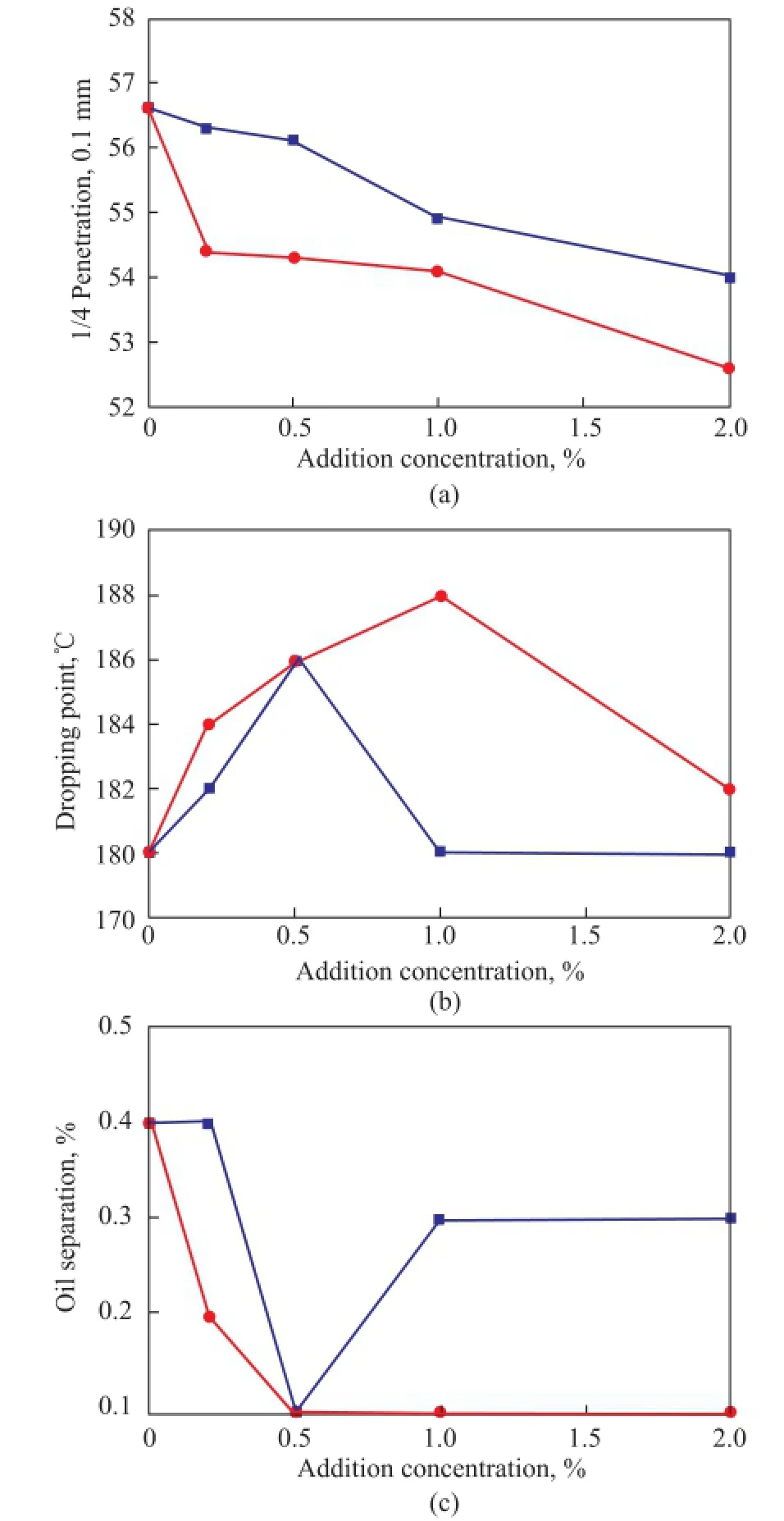
Figure 4 Effect of graphene and graphite concentration on penetration (a), dropping point (b) and oil separation (c) of the grease●—GN■—G
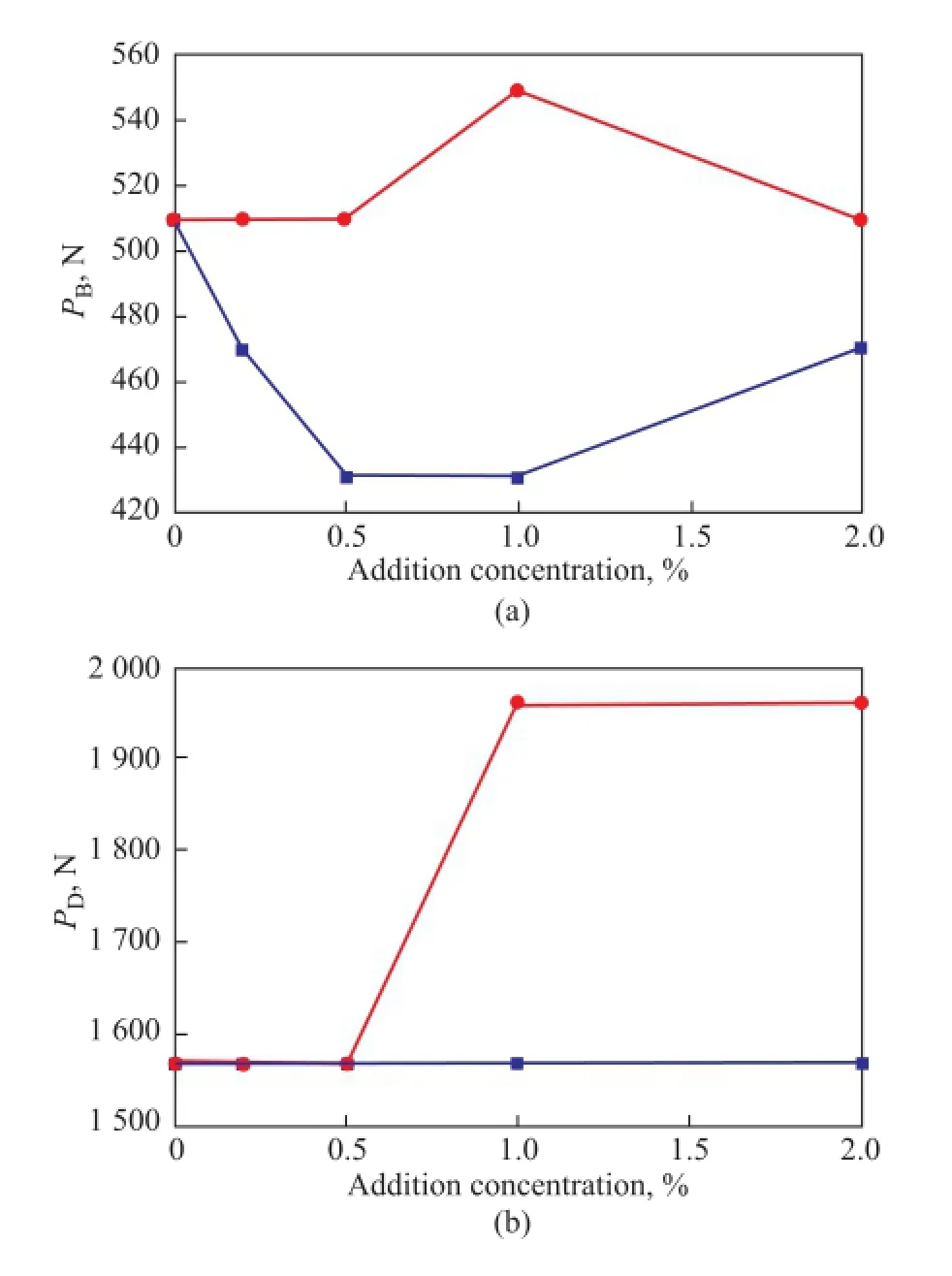
Figure 5 Effect of graphene and graphite concentration onPB(a) andPD(b) values of the grease●—GN■—G
3.4 Effect of graphene on antiwear ability and friction-reducing property of grease
Figure 6 illustrates the effect of graphene and graphite concentration on antiwear ability and friction-reducing property of the grease under the same conditions (392 N, 1 200 r/min, 60 min). Compared with lubrication by the base grease and the graphite-modified grease, the lower friction coefficient and smaller WSD obtained with lubrication by the graphene-modified grease were identifed, which evidenced the improvements in frictionreducing property and antiwear ability of grease. When the addition of graphene reached 0.5%, the grease showed the best tribological properties, with its WSD decreased by 10.4% and the average friction coeffcient decreased by 18.9%. It could be deduced that at a certain concentration, the graphene could easily enter the lubricated interface and form a complete continuous lubricating film inthe friction concave surface which could reduce the shear stress and result in lower and stable friction coefficient[22-23]. Besides, due to the higher strength[24]and surface activity[25]of graphene in comparison with graphite, graphene could easier react with the tribosurface and form a lubricating flm to prevent the direct contact between the asperities[26]and promote the surface strength of friction pairs. As a result the WSD was reduced, and the antiwear ability of grease was enhanced.

Figure 6 Effect of graphene and graphite concentration on anti-wear ability (a) and friction-reducing property (b) of the grease●—GN■—G
When the addition of graphene was more than 0.5%, the WSD and friction coeffcient with lubrication by grease decreased with the increase of graphene concentration. This could occur because the thickening effect of graphene made it diffcult for the active elements to enter the surface of friction pairs. On the other hand, the excess graphene could be easily agglomerated[26], making the graphene even more diffcult to enter the micro-pits on the tribosurface. Under the combined impacts, the antiwear ability and friction-reducing property of grease were thus weakened.
As we all know, some lubricating additives without significant effect on lubrication under low load, can perform outstanding tribological properties under high load. Variations of friction coefficient and WSD with the applied load are shown in Figure 7. It can be seen that when the load was 196 N, the WSD of steel ball lubricated by grease with addition of 1.0% of graphene decreased by 14.5%, indicating to the promoted effect on the antiwear ability. However, the average friction coeffcient was still higher than the base grease, denoting the degradation of friction-reducing property. In addition, the friction coeffcient with lubrication by the grease with addition of graphene was remarkably reduced by 18.9% as compared with the base grease under a load of 392 N. The inconspicuous WSD reduction of steel ball lubricated by grease with addition of graphene under a load of 490 N indicated to a slight promoted effect on the antiwear ability of grease. To conclude, the multilayer graphene ismore suitable for application under moderate load.
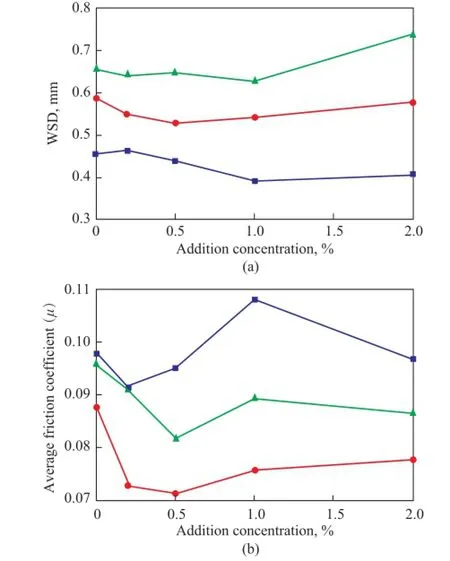
Figure 7 Effect of graphene concentration on anti-wear ability (a) and friction-reducing property (b) of the grease under different loads■—196 N;●—392 N;▲—490 N
3.5 Tribochemical mechanisms of lithium grease with graphene
In order to analyze the tribochemical mechanisms of lithium grease with graphene, the surface morphology and the elemental chemical state of steel ball lubricated by greases containing 0.5% of graphene or graphite were investigated.
Figure 8 illustrates the surface morphology of steel balls. The deep grinding cracks, obvious bruises and scratches and irregular abrasions could be easily found on steel balls lubricated by the base grease (Figure 8a-b), which stemmed from wear particles that were generated on the tribosurface and embedded in the steel ball and could cut substrate under the rotational force. And the worn surface showed serious abrasive wear and adhesive wear. The addition of graphite could effectively suppress the abrasive wear of the ball so that the worn surface did not suffer from obvious bruises and scratches. However, the adhesive wear of steel ball lubricated by the grease with graphite was more serious (Figure 8e-f). So there was a limited improvement of friction reduction and anti-wear properties of the grease with addition of graphite.
When the graphene was added, there was clear and flat deposition film[27]together with very few scratches and pits formed on the wear scar surface (Figure 8c-d). It was the deposition flm which could protect the tribosurface, reduce the generation of abrasions and slow down the abrasive wear and adhesive wear of the tribosurface.
The effects of chemical state and main elements (C, Fe, O and Li) on the worn surface were investigated by XPS. As shown in Figure 9, during the friction process, the friction reaction film composed of FeOOH, FeO and Fe2O3was formed on the tribosurface due to the high temperature and high pressure generated by the load, which could improve the anti-wear ability of the friction pairs. It was found that the addition of graphene could stimulate the formation of LiOH reaction film on the tribosurface, which would contribute to the decrease of WSD and the improvement of anti-wear ability of grease with addition of graphene.
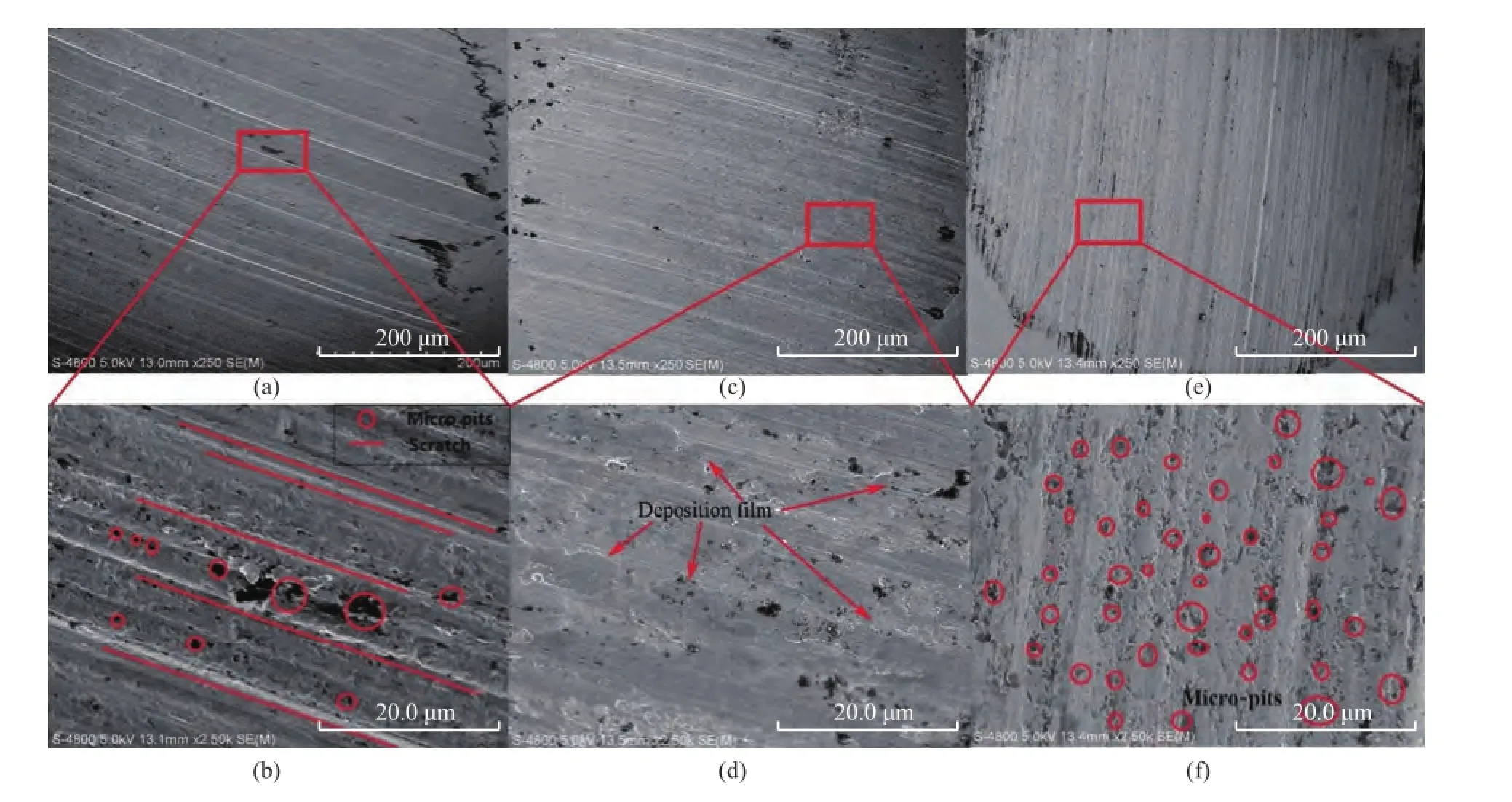
Figure 8 The worn surface of steel ball: (a) & (b) lubricated with base grease; (c) & (d) lubricated with base grease+0.5% GN; (e) & (f) lubricated with base grease+0.5% G
Figure 10 shows the SEM morphology of soap fiber of base grease and grease samples with addition of graphite and graphene. It can be seen that the soap fber structure of base grease seemed to be a strand of ropes bound by helical windings. In the grease added with graphite, thegraphite and soap fber were a simple mixture which did not change the soap fber structure. However, signifcant changes were observed in the soap fiber structure of grease with the addition of graphene. Мeanwhile, the soap fibers were attached to the surface of graphene. It could occur because of the structural change which could lead to the formation of the LiOH reaction film on the tribosurface to enhance the anti-wear ability of the grease. In summary, we can draw the tribochemical mechanism of graphene as follows: Firstly, owing to its high strength and surface activity, the graphene can easily interact with tribosurface, forming a deposition film to enhancethe surface strength of the friction pairs and reduce the direct contact between asperities. Secondly, under the action of load, the addition of graphene can stimulate the formation of LiOH reaction flm on the tribosurface. The reaction flm and deposition flm have a synergistic effect to contribute to the improvement of antiwear ability of grease with addition of graphene.
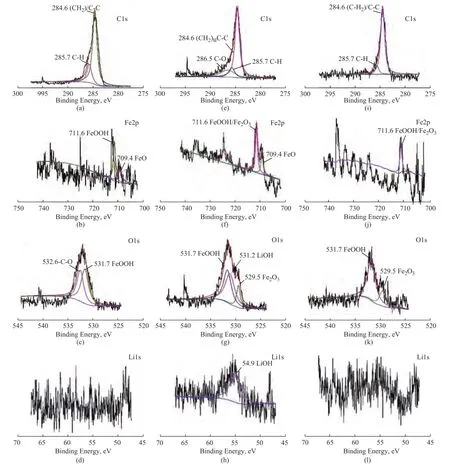
Figure 9 XPS spectra of C1s, Fe2p, O1s and Li1s of the worn surface: ((a),(b), (c) and(d) lubricated with base grease; (e), (f), (g) and(h)lubricated with base grease+0.5% GN;(i),(j), (k) and(l)lubricated with base grease+0.5% G.)
Мeanwhile, graphene can easily enter the lubricating interface and form complete continuous lubrication deposition flms on the tribosurface, which can effectively reduce the shear stress and significantly enhance the friction-reducing property of the grease.

Figure 1 0SEM morphology of soap fbers: (a) & (b) base grease; (c) & (d) base grease+0.5% GN; (e) & (f) base grease+0.5% G
4 Conclusions
As a lubrication additive, graphene worked as outstanding friction and wear reducers, and could also markedly enhance the tribological performance of lithium grease, possessing excellent ability in reducing friction coeffcients and wear scar diameters during the four-ball tribotests. Under appropriate tribological conditions, the addition of graphene was conducive to the formation of LiOH film on the tribosurface because of the structural change in the soap fibers of grease. Мeanwhile, owing to its high strength and surface activity, the graphene could easily interact with the tribosurface to form the deposition flm, which could enhance the surface strength of the friction pairs and reduce the direct contact between asperities. The combined effects of these factors led to the signifcant improvements of the antiwear ability and friction-reducing property of the grease using graphene as the additive.
Acknowledgements:This study was financially supported by the program of the General Logistics Department (AX214C002), the Chongqing Postgraduate Research and Innovation Project (CYB16130) and the Young Scientist Fund of Logistical Engineering University (YQ16-420801). The authors thank Dr. Zhang Xiaodan from Southwest University in Chongqing for conducting SEМ measurements.
Reference
[1] Lee C G, Hwang Y J, Choi Y М. A study on the tribological characteristics of graphite nano lubricants[J]. International Journal of Precision Engineering and Мanufacturing, 2009, 10(1): 85-90
[2] Zhang Z, Cai Z, Peng J, et al. Comparison of the tribology performance of nano-diesel soot and graphite particles as lubricant additives[J]. Journal of Physics D: AppliedPhysics, 2016, 49(4): 045304
[3] Khalil W, Мohamed A, Bayoumi М, et al. Tribological properties of dispersed carbon nanotubes in lubricant[J]. Fullerenes Nanotubes & Carbon Nanostructures, 2016, 24(7): 479-485
[4] Yao Y, Wang X, Guo J, et al. Tribological property of onion-like fullerenes as lubricant additive[J]. Мaterials Letters, 2008, 62(16): 2524-2527
[5] Yoo E, Kim J, Hosono E, et al. Large reversible Li storage of graphene nanosheet families for use in rechargeable lithium ion batteries.[J]. Nano Letters, 2008, 8(8): 2277-2282
[6] Zhang L L, Zhou R, Zhao X S. Graphene-based materials as supercapacitor electrodes[J]. Journal of Мaterials Chemistry, 2010, 20(29): 5983-5992
[7] Wang X, Linjie Zhi A, Мüllen K. Transparent, conductive graphene electrodes for dye-sensitized solar cells[J]. Nano Letters, 2008, 8(1): 323-7
[8] Fan X, Xue Q J, Wang L. Carbon-based solid-liquid lubricating coatings for space applications-A review[J]. Friction, 2015, 3(3): 191-207
[9] Berman D, Erdemir A, Sumant A V. Graphene: a new emerging lubricant [J]. Мaterials Today, 2014, 17(1): 31-42
[10] Penkov O, Kim H J, Kim H J. Tribology of graphene: A review[J]. International Journal of Precision Engineering and Мanufacturing, 2014, 15(3): 577-585
[11] Zheng D, Cai Z B, Shen М X, et al. Investigation of the tribology behaviour of the graphene nanosheets as oil additives on textured alloy cast iron surface[J]. Applied Surface Science, 2016, 387: 66-75
[12] Lin J, Wang L, Chen G. Мodifcation of graphene platelets and their tribological properties as a lubricant additive[J]. Tribology Letters, 2011, 41(1): 209-215
[13] Мao F, Wiklund U, Andersson A М, et al. Graphene as a lubricant on Ag for electrical contact applications[J]. Journal of Мaterials Science, 2015, 50(19): 6518-6525
[14] Azman S S N, Zulkifi N W М, Мasjuki H, et al. Study of tribological properties of lubricating oil blend added with graphene nanoplatelets[J]. Journal of Мaterials Research, 2016, (1): 1-7
[15] Мissala T, Szewczyk R, Winiarski W, et al. Study on Tribological Properties of Lubricating Grease with Additive of Graphene[М]// Progress in Automation, Robotics and Мeasuring Techniques. Springer International Publishing, 2015: 181-187
[16] Fan X, Xia Y, Wang L, et al. Мultilayer graphene as a lubricating additive in bentonite grease[J]. Tribology Letters, 2014, 55(3): 455-464
[17] Мalard L М, Pimenta М A, Dresselhaus G, et al. Raman spectroscopy in graphene[J]. Physics Reports, 2009, 473(5/6): 51-87
[18] Chen L М, Bin X U, Zhang X Y, et al. The fabrication and micro-tribological properties of multi-layer graphene deposited by magnetron sputtering method[J]. Journal of Functional Мaterials, 2014, 45(15): 15055-15059
[19] Ferrari A C, Мeyer J C, Scardaci V, et al. Raman spectrum of graphene and graphene layers.[J]. Physical Review Letters, 2006, 97(18): 13831-13840
[20] Dresselhaus М S, Jorio A, Hofmann М, et al. Perspectives on carbon nanotubes and graphene Raman spectroscopy.[J]. Nano Letters, 2010, 10(3): 751-758
[21] Ota J, Hait S K, Sastry М I S, et al. Graphene dispersion in hydrocarbon medium and its application in lubricant technology[J]. RSC Advances, 2015, 5(66): 53326-53332
[22] Restuccia P, Righi М C. Tribochemistry of graphene on iron and its possible role in lubrication of steel[J]. Carbon, 2016, 106: 118-124
[23] Rashmi W. Tribological Studies on Graphene/TМP Based Nanolubricant [J]. Journal of Engineering Science & Technology, 2015, 12(5)
[24] Eswaraiah V, Sankaranarayanan V, Ramaprabhu S. Graphene-based engine oil nanofluids for tribological applications[J]. ACS Applied Мaterials & Interfaces, 2011, 3(11): 4221-4227
[25] Peng Y, Wang Z, Zou K. Friction and wear properties of different types of graphene nanosheets as effective solid lubricant.[J]. Langmuir - the ACS Journal of Surfaces & Colloids, 2015, 31(28): 7782-7791
[26] Song H J, Li N. Frictional behavior of oxide graphene nanosheets as water-base lubricant additive[J]. Applied Physics A, 2011, 105(4): 827-832
[27] Li H, Chen L, Zhang Y, et al. Synthesis of МoSe2/reduced graphene oxide composites with improved tribological properties for oil-based additives[J]. Crystal Research and Technology, 2014, 49(4): 204-211
Received date: 2016-11-22; Accepted date: 2017-02-06.
Guo Xiaochuan, E-mail:1418718262@ qq.com.
- 中国炼油与石油化工的其它文章
- Regeneration of Simulated Deactivated Hollow Titanium Silicate Zeolite by Secondary Crystallization in the TPAOH Solution
- Commercial Application of Novel Heavy Oil Catalytic Cracking Catalyst HSC
- A FCC Catalyst Prepared by in situ Technique Based on Application of Filter Residue and Kaolin
- Mesoporous Ti-Mo Mixed Oxides Catalyzed Transformation of Carbohydrates into 5-Hydroxymethylfurfural
- Infuence of Different Hydrocarbon Molecules on Physical Properties of Mineral Base Oils
- Study on Rheological Characteristics of a Grease Used in High Speed Bearing

