面—肩—肱型肌营养不良症分子学机制研究进展
林晓丹 何君洁 陈万全 王柠 王志强
·专题综述·
面—肩—肱型肌营养不良症分子学机制研究进展
林晓丹 何君洁 陈万全 王柠 王志强
面⁃肩⁃肱型肌营养不良症(FSHD)呈常染色体显性遗传,以面肌、肩胛带肌和上臂肌群肌无力和肌萎缩发病,逐渐累及躯干肌群和下肢肌群,临床异质性较高,预后相对较好。临床分型包括FSHD1型和FSHD2型,前者与4q35区域D4Z4串联重复序列缺失有关,其上游简单序列长度多态性和下游特殊等位序列4qA/4qB具有选择致病性。4q35区域DNA低甲基化启动表观遗传效应,使D4Z4串联重复序列内DUX4基因去抑制致异常表达,导致多种肌细胞损害效应。后者由DNA甲基化调控基因——SMCHD1基因突变所致。支持面⁃肩⁃肱型肌营养不良症是毒性功能获得性疾病学说,为其治疗研究提供重要靶点。
营养不良,面肩肱型; 基因; 突变; 综述
面⁃肩⁃肱型肌营养不良症[FSHD,在线人类孟德尔遗传数据库(OMIM)编号:158900]于1882年由法国神经病学家Louis Landouzy和Joseph Dejerine首次报告,亦称为Landouzy⁃Dejerine型肌营养不良症[1],是继Duchenne型肌营养不良症(DMD)和强直性肌营养不良症(DM)后临床最常见类型,呈常染色体显性遗传,发病率约为1/2万,20岁时外显率达95%,30%患者为新发突变[2]。通常于青少年期发病,主要表现为对称性或不对称性肌无力和肌萎缩,累及面肌、肩胛带肌和上臂肌群,呈现猫脸、鱼嘴、翼状肩胛、游离肩、衣架肩等典型外观,逐渐向下进展累及躯干肌群和下肢肌群。面⁃肩⁃肱型肌营养不良症患者存在高度家系间和家系内临床异质性,包括无症状携带者、仅面部轻微受累者和四肢瘫痪者,病程进展缓慢,预后相对较好,一般不直接影响寿命,约20%患者最终依靠轮椅[3]。除骨骼肌受累外,部分患者出现神经性耳聋和视网膜毛细血管扩张,少数严重患者还出现癫发作、智力障碍等症状[4⁃5]。面⁃肩⁃肱型肌营养不良症发病机制有别于其他单基因遗传病,不遵循传统致病性突变导致编码蛋白变异的经典模式,成为神经肌肉病领域的研究难点。本文拟对近年来面⁃肩⁃肱型肌营养不良症相关分子学机制研究进展进行概述。
一、分子病理学与分子诊断
面⁃肩⁃肱型肌营养不良症的致病基因定位于第4号染色体长臂亚端粒区(4q35),是首个发现的由大卫星重复序列缺失导致的神经系统遗传性疾病,与该区域多态性EcoRⅠ片段内长度为3.30×103bp的D4Z4串联重复序列(DRs)缺失直接相关:正常人群D4Z4基因拷贝数为11~100个;面⁃肩⁃肱型肌营养不良症患者减少至1~10个,EcoRⅠ片段长度缩短至 < 38 × 103bp[6]。面⁃肩⁃肱型肌营养不良症不仅基因突变类型独特,而且存在2个少见的分子遗传学现象:(1)10q26区域存在与4q35区域高度同源的多态性EcoRⅠ片段,此片段长度亦<38×103bp,但无致病性,二者之间存在高频易位现象,应注意鉴别[7⁃8]。(2)4q35 区域 D4Z4 串联重复序列与上游 3 ×103bp处特异性简单序列长度多态性(SSLP)和下游10×103bp处特殊等位序列4qA/4qB存在密切连锁关系,D4Z4基因缺失与特异性SSLP⁃4qA基因型共存而致病[9⁃10](图 1)。研究显示,我国面⁃肩⁃肱型肌营养不良症的主要基因型是4A161PAS[11]。因此,大片段致病基因的分离和鉴定是基因检测和分子学机制研究的基础。采用脉冲场凝胶电泳(PFGE)联合多重Southern blotting法,是目前国际指南推荐的分子诊断技术[12]。约5%面⁃肩⁃肱型肌营养不良症患者存在相应临床表型,未见4q35区域D4Z4串联重复序列缩短,称为面⁃肩⁃肱型肌营养不良症2型(FSHD2 型)[13]。2012 年,Lemmers等[14]采用全外显子测序(WES)证实FSHD2型致病基因为SMCHD1基因,该基因突变与导致DUX4基因表达的4qA等位基因共同作用而致病,称为双遗传模式。SMCHD1基因单倍体剂量不足机制表明,SMCHD1基因突变导致编码蛋白表达下调,使D4Z4串联重复序列甲基化降低,进而通过与面⁃肩⁃肱型肌营养不良症1型(FSHD1型)相同的表观遗传学机制而发挥作用,因此,不同亚型面⁃肩⁃肱型肌营养不良症具有相似的分子通路。
二、分子遗传学及发病机制
面⁃肩⁃肱型肌营养不良症是典型人类孟德尔遗传性疾病,但4q35区域D4Z4串联重复序列致病性缩短的分子学机制极为复杂,一直是疾病研究的难点,研究者们致力于寻找D4Z4基因上下游和内部可能的效应基因。Gabellini等[15⁃16]发现,面⁃肩⁃肱型肌营养不良症患者4q35区域D4Z4串联重复序列上游120×103bp处FRG1基因呈异常高表达,遂构建转基因小鼠模型,出现类似面⁃肩⁃肱型肌营养不良症临床表型和病理改变,故认为FRG1基因异常表达可以干扰mRNA前体剪切修饰,与骨骼肌生长发育有关。因此,FRG1 基因成为重要候选基因[17⁃18]。此后多项研究显示,该转基因小鼠模型基因结构仅与面⁃肩⁃肱型肌营养不良症患者部分相似,未能验证转基因小鼠模型存在 FRG1 基因异常表达[19⁃20]。2010 年,Snider等[21]发现,4q35 区域 D4Z4 串联重复序列内DUX4基因表达上调,证实该基因在面⁃肩⁃肱型肌营养不良症发病机制中发挥重要作用。DUX4基因包含2个同源序列和2个富含鸟嘌呤⁃胞嘧啶(GC)的重复序列,形成读码框(ORF),其末端连接多聚腺苷酸信号(PAS)以稳定DUX4基因转录和翻译(图1)。研究显示,D4Z4基因富含CpG岛(CpG island),CpG岛主要位于转录调控区附近,是一种重要表观遗传学修饰方式[22]。2013 年,Hartweck 等[23]证实,面⁃肩⁃肱型肌营养不良症的4q35区域D4Z4串联重复序列内存在3个DNA低甲基化区域,即DR1、DR2和DR3区域,尤以DR1区域(位于D4Z4基因5’端)低甲基化程度最显著。2014年,Gaillard等[24]比较面⁃肩⁃肱型肌营养不良症患者与无症状携带者DNA甲基化水平,发现面⁃肩⁃肱型肌营养不良症患者DNA甲基化水平明显降低。DNA甲基化虽未改变基因结构,但可引起局部DNA构象稳定性改变,从而调控基因表达,进一步证实面⁃肩⁃肱型肌营养不良症是一种表观遗传效应的遗传性疾病学说,DNA甲基化检测成为参考诊断指标[24]。
面⁃肩⁃肱型肌营养不良症具有高度临床异质性,可能与以下因素有关:(1)4q35区域D4Z4串联重复序列拷贝数与临床表型呈负相关,EcoRⅠ片段长度缩短越明显、临床表型越严重、外显年龄越早、累及肌群越多[3]。(2)表观遗传效应,DNA 甲基化水平与临床表型呈负相关,存在相同D4Z4串联重复序列拷贝数的患者,DNA甲基化水平越低、临床表型越严重[24]。(3)临床表型的调控基因,研究显示,SMCHD1和DNMT3B基因是FSHD1型的调控基因,SMCHD1或DNMT3B基因突变的FSHD1型患者表现出更严重的临床表型,即SMCHD1和DNMT3B基因可能与面⁃肩⁃肱型肌营养不良症的致病性存在协同作用[25⁃26]。目前公认的面⁃肩⁃肱型肌营养不良症发病机制是,4q35区域D4Z4串联重复序列缺失致DNA甲基化水平降低,在表观遗传效应调控下染色质构象改变失去稳定性,引起DUX4基因在骨骼肌细胞中表达,产生的DUX4蛋白对肌细胞产生多种毒性作用。
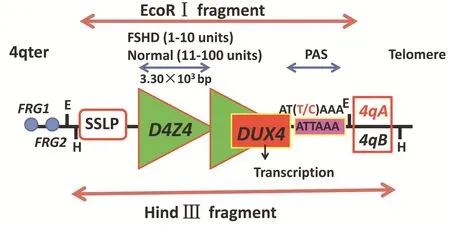
图1 面⁃肩⁃肱型肌营养不良症4q35区域相关基因结构模式图:4q35区域EcoRⅠ片段由数目不等的长度为3.30×103bp的D4Z4串联重复序列组成,其上游简单序列长度多态性和下游特殊等位序列4qA/4qB具有选择致病性,D4Z4串联重复序列内存在DUX4基因,其末端连接多聚腺苷酸信号Figure 1 The structure mode of FSHD related genes in 4q35 region:the fragment of 4q35⁃EcoRⅠ is consisted of a series of D4Z4 repeats which is composed of numbers of 3.30×103bp,its upstream SSLP and downstream specific allele sequence 4qA/4qB have pathogenic selective effect.The DUX4 gene exists inside D4Z4 repeats and connects PAS on the terminal.
三、DUX4蛋白功能研究
DUX4基因表达失调和DUX4蛋白功能异常是面⁃肩⁃肱型肌营养不良症发病的关键环节。DUX4基因是反转录基因,编码2条全长(DUX4⁃fl)和截短(DUX4⁃s)的DUX4蛋白,在人类生殖细胞和早期胚胎干细胞(ESCs)中正常表达,晚近研究显示,DUX4基因在胚胎早期对诱导合子基因组激活(ZGA)起关键调节作用,此后则处于沉默状态[27],但在面⁃肩⁃肱型肌营养不良症患者骨骼肌中呈异常表达。低水平DUX4⁃fl蛋白可以引起下游多种改变,激活一系列去抑制级联反应,导致肌细胞凋亡和萎缩、炎症反应、分化缺陷和氧化应激,但其具体生物学功能尚未完全阐明[28]。目前较为公认的DUX4蛋白在骨骼肌中表达的病理生理学机制包括:(1)细胞凋亡学说,DUX4蛋白可以诱导抑癌基因p53表达,导致细胞凋亡,引起肌肉损害[29]。(2)T淋巴细胞介导的细胞炎症反应学说,DUX4蛋白异常表达可以激活免疫反应,类似吞噬细胞介导的抗肿瘤反应。免疫反应激活CD4+T细胞和CD8+T细胞,发生以T淋巴细胞介导为主的血管周围炎性细胞浸润,引起肌细胞肥大和细胞核聚集,导致肌肉损害[30]。(3)DUX4蛋白表达与长末端重复序列(LTR)的反转录转座子和内源性重复序列的转录激活相关,同时抑制自身免疫对逆转录病毒感染的应答,通过转录激活防御素 DEFB103 抑制肌细胞分化、再生[31]。Jones等[32]和Mitsuhashi等[33]予肌细胞、斑马鱼和蟾蜍注射微量DUX4 mRNA以建立面⁃肩⁃肱型肌营养不良症细胞和动物模型,触发多种级联反应,导致肌细胞凋亡、卫星细胞发育抑制、炎症反应和氧化应激反应障碍等一系列病理生理改变。Ansseau等[34]通过显微注射腺相关病毒载体包装的D4Z4基因,向野生型小鼠C57BL/6导入不同长度的D4Z4串联重复序列,可以观察到与人类高度同源的DUX4基因高表达,但未出现相应临床表型。因此,目前普遍认为DUX4基因异常表达是面⁃肩⁃肱型肌营养不良症的分子学机制,但某些关键途径尚不明确,如面⁃肩⁃肱型肌营养不良症是受DUX4基因异常表达的单因素调控还是多因素联合调控。
四、小结和展望
面⁃肩⁃肱型肌营养不良症目前尚无有效治疗方法,适当的康复训练可以延缓疾病进展。发病机制已趋于明朗,是表观遗传效应导致的毒性功能获得性(toxic gain of function)疾病,其中DUX4基因去抑制致异常表达是关键致病机制,成为今后治疗研究的重要靶点。基因治疗方面,主要通过反义寡核苷酸(ASO)或干扰RNA(RNAi)技术抑制DUX4基因过表达,从而达到改善肌肉损害之目的[35⁃36]。近年来,采用新型基因编辑技术实现多靶点精准基因调控、体外调控DUX4基因表达、寻找抑制DUX4基因的小分子化合物为面⁃肩⁃肱型肌营养不良症的治疗带来新的曙光。
[1]Sorrel⁃Dejerine Y,Fardeau M.Birth and metamorphosis of Landouzy⁃Dejerine progressive atrophic myopathy.Rev Neurol(Paris),1982,138:1041⁃1051.
[2]Lunt PW, Harper PS. Genetic counselling in facioscapulohumeral muscular dystrophy.J Med Genet,1991,28:655⁃664.
[3]Lin F,Wang ZQ,Lin MT,Murong SX,Wang N.New insights into genotype⁃phenotype correlations in Chinese facioscapulohumeral muscular dystrophy: a retrospective analysis of 178 patients.Chin Med J(Engl),2015,128:1707⁃1713.
[4]Padberg GW,Brouwer OF,de Keizer RJ,Dijkman G,Wijmenga C,Grote JJ,Frants RR.On the significance of retinal vascular disease and hearing loss in facioscapulohumeralmuscular dystrophy.Muscle Nerve Suppl,1995,2:S73⁃80.
[5]Grosso S,Mostardini R,Di Bartolo RM,Balestri P,Verrotti A.Epilepsy, speech delay, and mental retardation in facioscapulohumeral muscular dystrophy.Eur J Paediatr Neurol,2011,15:456⁃460.
[6]van Deutekom JC,Wijmenga C,van Tienhoven EA,Gruter AM,Hewitt JE,Padberg GW,van Ommen GJ,Hofker MH,Frants RR.FSHD associated DNA rearrangements are due to deletions of integral copies of a 3.2 kb tandemly repeated unit.Hum Mol Genet,1993,2:2037⁃2042.
[7]van Overveld PG,Lemmers RJ,Deidda G,Sandkuijl L,Padberg GW,Frants RR,van der Maarel SM.Interchromosomal repeat array interactions between chromosomes 4 and 10:a model for subtelomeric plasticity.Hum Mol Genet,2000,9:2879⁃2884.
[8]Wu ZY,Wang ZQ,Murong SX,Wang N.FSHD in Chinese population:characteristics of translocation and genotype⁃phenotype correlation.Neurology,2004,63:581⁃583.
[9]Lemmers RJ,de Kievit P,Sandkuijl L,Padberg GW,van Ommen GJ, Frants RR, van der Maarel SM.Facioscapulohumeral muscular dystrophy is uniquely associated with one of the two variants of the 4q subtelomere.Nat Genet,2002,32:235⁃236.
[10]Wang ZQ,Wang N,van der Maarel S,Murong SX,Wu ZY.Distinguishing the 4qA and 4qB variants is essential for the diagnosisof facioscapulohumeralmusculardystrophyin the Chinese population.Eur J Hum Genet,2011,19:64⁃69.
[11]Lin F,He JJ,Lin XD,Wang DN,Lin HX,Liu XY,Lin MT,WangN,WangZQ.A largecohort studyconfirming that specific haplotype 4A161PAS is exclusively associated with the Chinese FSHD1.Clin Genet,2016,90:558⁃559.
[12]Tawil R,Kissel JT,Heatwole C,Pandya S,Gronseth G,Benatar M;Guideline Development,Dissemination,and Implementation Subcommittee of the American Academy of Neurology;Practice Issues Review Panel of the American Association of Neuromuscular&Electrodiagnostic Medicine.Evidence⁃based guideline summary:evaluation,diagnosis,and management of facioscapulohumeral muscular dystrophy,report of the guideline development,dissemination,and implementation subcommittee of the American Academy of Neurology and the practice issues review panel of the American Association of Neuromuscular&Electrodiagnostic Medicine.Neurology,2015,85:357⁃364.
[13]de Greef JC,Lemmers RJ,Camaño P,Day JW,Sacconi S,Dunand M,van Engelen BG,Kiuru⁃Enari S,Padberg GW,Rosa AL,Desnuelle C,Spuler S,Tarnopolsky M,Venance SL,Frants RR,van der MaarelSM,TawilR.Clinicalfeatures of facioscapulohumeral muscular dystrophy 2.Neurology,2010,75:1548⁃1554.
[14]Lemmers RJ,Tawil R,Petek LM,Balog J,Block GJ,Santen GW,Amell AM,van der Vliet PJ,Almomani R,Straasheijm KR,Krom YD,Klooster R,Sun Y,den Dunnen JT,Helmer Q,Donlin⁃Smith CM,Padberg GW,van Engelen BG,de Greef JC,Aartsma⁃Rus AM,Frants RR,de Visser M,Desnuelle C,Sacconi S,Filippova GN,Bakker B,Bamshad MJ,Tapscott SJ,Miller DG,van der Maarel SM.Digenic inheritance of an SMCHD1mutationandanFSHD⁃permissiveD4Z4allele causes facioscapulohumeralmusculardystrophy type2.Nat Genet,2012,44:1370⁃1374.
[15]Gabellini D,Green MR,Tupler R.Inappropriate gene activation in FSHD:a repressor complex binds a chromosomal repeat deleted in dystrophic muscle.Cell,2002,110:339⁃348.
[16]Gabellini D,D'Antona G,Moggio M,Prelle A,Zecca C,Adami R,Angeletti B,Ciscato P,Pellegrino MA,Bottinelli R,Green MR,TuplerR.Facioscapulohumeralmusculardystrophyin mice overexpressing FRG1.Nature,2006,439:973⁃977.
[17]Wuebbles RD,Hanel ML,Jones PL.FSHD region gene 1(FRG1) is crucial for angiogenesis linking FRG1 to facioscapulohumeral muscular dystrophy⁃associated vasculopathy.Dis Model Mech,2009,2:267⁃274.
[18]Pistoni M,Shiue L,Cline MS,Bortolanza S,Neguembor MV,Xynos A,Ares M Jr,Gabellini D.Rbfox1 downregulation and altered calpain 3 splicing by FRG1 in a mouse model of facioscapulohumeral muscular dystrophy(FSHD).PLoS Genet,2013,9:E1003186.
[19]KloosterR,Straasheijm K,Shah B,Sowden J,FrantsR,Thornton C,TawilR,van derMaarelS.Comprehensive expression analysis of FSHD candidate genes at the mRNA and protein level.Eur J Hum Genet,2009,17:1615⁃1624.
[20]Masny PS,Chan OY,de Greef JC,Bengtsson U,Ehrlich M,Tawil R,Lock LF,Hewitt JE,Stocksdale J,Martin JH,van der Maarel SM,Winokur ST.Analysis of allele⁃specific RNA transcription in FSHD by RNA⁃DNA FISH in single myonuclei.Eur J Hum Genet,2010,18:448⁃456.
[21]Snider L,Geng LN,Lemmers RJ,Kyba M,Ware CB,Nelson AM,Tawil R,Filippova GN,van der Maarel SM,Tapscott SJ,Miller DG. Facioscapulohumeral dystrophy: incomplete suppression of a retrotransposed gene.PLoS Genet,2010,6:E1001181.
[22]de Greef JC,Wohlgemuth M,Chan OA,Hansson KB,Smeets D,Frants RR,Weemaes CM,Padberg GW,van der Maarel SM.Hypomethylation isrestricted to the D4Z4 repeatarrayin phenotypic FSHD.Neurology,2007,69:1018⁃1026.
[23]Hartweck LM,Anderson LJ,Lemmers RJ,Dandapat A,Toso EA,Dalton JC,Tawil R,Day JW,van der Maarel SM,Kyba M.A focaldomain ofextremedemethylation within D4Z4 in FSHD2.Neurology,2013,80:392⁃399.
[24]Gaillard MC,Roche S,Dion C,Tasmadjian A,Bouget G,Salort⁃Campana E,Vovan C,Chaix C,Broucqsault N,Morere J,Puppo F,Bartoli M,Levy N,Bernard R,Attarian S,Nguyen K,Magdinier F.Differential DNA methylation of the D4Z4 repeat in patients with FSHD and asymptomatic carriers.Neurology,2014,83:733⁃742.
[25]Sacconi S,Lemmers RJ,Balog J,van der Vliet PJ,Lahaut P,van Nieuwenhuizen MP,Straasheijm KR,Debipersad RD,Vos⁃Versteeg M,Salviati L,Casarin A,Pegoraro E,Tawil R,Bakker E,Tapscott SJ,Desnuelle C,van der Maarel SM.The FSHD2 gene SMCHD1 is a modifier of disease severity in families affected by FSHD1.Am J Hum Genet,2013,93:744⁃751.
[26]van den Boogaard ML,Lemmers RJ,Balog J,Wohlgemuth M,Auranen M,Mitsuhashi S,van der Vliet PJ,Straasheijm KR,van den Akker RF,Kriek M,Laurense⁃Bik ME,Raz V,van Ostaijen⁃Ten Dam MM,Hansson KB,van der Kooi EL,Kiuru⁃Enari S,Udd B,van Tol MJ,Nishino I,Tawil R,Tapscott SJ,van Engelen BG,van der Maarel SM.Mutations in DNMT3B modify epigenetic repression ofthe D4Z4 repeatand the penetrance of facioscapulohumeral dystrophy.Am J Hum Genet,2016,98:1020⁃1029.
[27]De Iaco A,Planet E,Coluccio A,Verp S,Duc J,Trono D.DUX⁃family transcription factors regulate zygotic genome activation in placental mammals.Nat Genet,2017,49:941⁃945.
[28]de la Kethulle de Ryhove L,Ansseau E,Nachtegael C,Pieters K,Vanderplanck C,Geens M,Sermon K,Wilton SD,Coppée F,Lagneaux L,Belayew A.The role of D4Z4⁃encoded proteins in the osteogenic differentiation ofmesenchymalstromalcells isolated from bone marrow.Stem Cells Dev,2015,24:2674⁃2686.
[29]Wallace LM,Garwick SE,Mei W,Belayew A,Coppee F,LadnerKJ,Guttridge D,Yang J,HarperSQ.DUX4,a candidate gene forfacioscapulohumeralmusculardystrophy,causes p53⁃dependent myopathy in vivo.Ann Neurol,2011,69:540⁃552.
[30]Arahata K,Ishihara T,Fukunaga H,Orimo S,Lee JH,Goto K,Nonaka I. Inflammatory response in facioscapulohumeral muscular dystrophy(FSHD):immunocytochemical and genetic analyses.Muscle Nerve Suppl,1995,2:S56⁃66.
[31]Young JM,Whiddon JL,Yao Z,Kasinathan B,Snider L,Geng LN,Balog J,Tawil R,van der Maarel SM,Tapscott SJ.DUX4 binding to retroelements creates promoters that are active in FSHD muscle and testis.PLoS Genet,2013,9:E1003947.
[32]JonesTI,Chen JC,Rahimov F,Homma S,Arashiro P,Beermann ML,King OD,Miller JB,Kunkel LM,Emerson CP Jr,WagnerKR,Jones PL.Facioscapulohumeralmuscular dystrophy familystudiesofDUX4 expression:evidence for disease modifiers and a quantitative model of pathogenesis.Hum Mol Genet,2012,21:4419⁃4430.
[33]MitsuhashiH,Mitsuhashi S,Lynn⁃JonesT,KawaharaG,Kunkel LM.Expression of DUX4 in zebrafish development recapitulates facioscapulohumeral muscular dystrophy.Hum Mol Genet,2013,22:568⁃577.
[34]Ansseau E,Domire JS,Wallace LM,Eidahl JO,Guckes SM,GiesigeCR,Pyne NK,Belayew A,HarperSQ.Aberrant splicing in transgenes containing introns,exons,and V5 epitopes:lessonsfrom developing an FSHD mouse model expressing a D4Z4 repeat with flanking genomic sequences.PLoS One,2015,10:E0118813.
[35]Shadle SC,Zhong JW,Campbell AE,Conerly ML,Jagannathan S,Wong CJ,Morello TD,van der Maarel SM,Tapscott SJ.DUX4⁃induced dsRNA and MYC mRNA stabilization activate apoptotic pathways in human cell models of facioscapulohumeral dystrophy.PLoS Genet,2017,13:E1006658.
[36]Ansseau E,Vanderplanck C,Wauters A,Harper SQ,Coppée F,Belayew A.Antisense oligonucleotides used to target the DUX4 mRNA as therapeutic approaches in facios scapulo humeral muscular dystrophy(FSHD).Genes(Basel),2017,8:93.
Progress in researchon molecularmechanism of facioscapulohumeralmuscular dystrophy
LIN Xiao⁃dan,HE Jun⁃jie,CHEN Wan⁃jin,WANG Ning,WANG Zhi⁃qiang
Department of Neurology,the First Affiliated Hospital of Fujian Medical University,Fuzhou 350005,Fujian,China
Corresponding author:WANG Zhi⁃qiang(Email:fmuwzq@fjmu.edu.cn)
Facioscapulohumeralmusculardystrophy (FSHD),characterized by symmetric or asymmetric muscular weakness of the initial onset of facial,shoulder⁃girdle and upper arm muscles,and descending to limb muscles,is a classical autosomal dominant myopathy with high clinical diversity and relatively good prognosis.FSHD is catigorized into two types,FSHD1 and FSDH2.Previous studies have demonstrated that 95%patients with FSHD1 were associated with a contraction of D4Z4 microsatellite repeats on chromosome 4q35,which was pathogenic in the genetic backgrounds,including a special sequence of simple sequence length polymorphism(SSLP)proximal to the D4Z4 repeats and the 4qA/4qB polymorphism distal to the repeats.In recent years,several reports have confirmed that 4q35 locus leads to DNA hypomethylation and inner DUX4 gene transcription by epigenetic effect.The abnormal expression of DUX4 further activates several genes,which inhibit myogenesis,sensitize cells to oxidative stress and induce muscle atrophy.And not only that,FSHD2 is formed by another methylation regulation gene——SMCHD1 mutations.More and more evidences supported that toxic gain of function mechanism plays an important role in the occurrence of FSHD.The DUX4 gene becomes an important target for treatment study in the future.
Muscular dystrophy,facioscapulohumeral; Genes; Mutation; Review
This study was supported by Key Project of the National Natural Science Foundation of China(No.U1505222),the National Natural Science Foundation of China(No.81671237),Science and Technology Plan Project of Fujian Province,China(No.2016Y9010),and Natural Science Foundation of Fujian Province,China(No.2017J01196).
10.3969/j.issn.1672⁃6731.2017.08.004
国家自然科学基金重点资助项目(项目编号:U1505222);国家自然科学基金资助项目(项目编号:81671237);福建省科技计划项目(项目编号:2016Y9010);福建省自然科学基金资助项目(项目编号:2017J01196)
350005福州,福建医科大学附属第一医院神经内科
王志强(Email:fmuwzq@fjmu.edu.cn)
2017⁃06⁃05)

