间变型多形性黄色瘤型星形细胞瘤
邵立伟 王辅林
·临床病理报告·
间变型多形性黄色瘤型星形细胞瘤
邵立伟 王辅林
目的报道1例男性间变型多形性黄色瘤型星形细胞瘤患儿的临床资料,探讨其临床病理学、免疫表型、基因突变特征,以及诊断与鉴别诊断要点。方法与结果男性患儿,11岁,头痛伴左上肢无力15 d。头部CT和MRI显示右侧颞叶和基底节区巨大占位性病变,提示胶质瘤。遂行右侧颞叶和基底节区占位性病变切除术,术中可见肿瘤呈囊实性,实性部分呈灰黄色,质地柔软,血供丰富,无包膜,与周围组织界限清晰。术中冰冻病理学提示低级别胶质瘤,遂分块全切除肿瘤。组织学形态观察,肿瘤细胞呈多形性,由梭形和圆形星形胶质细胞以及单核细胞和多核瘤巨细胞组成,核分裂象罕见;局部可见较成熟的神经元或节细胞分化成分,伴淋巴细胞浸润;部分区域肿瘤细胞呈间变特征,细胞密度增加,异型性明显,以圆形和梭形细胞为主,核分裂象>5个/10高倍视野,血管内皮细胞增生,伴血管周围假“菊形团”样结构,局灶性坏死。免疫组织化学染色,低级别肿瘤细胞胞质表达胶质纤维酸性蛋白(GFAP)和BRAF V600E、胞质和胞核表达S⁃100蛋白、胞膜表达CD34,少数肿瘤细胞胞质表达突触素和非磷酸化神经丝重链SMI⁃32,Ki⁃67抗原标记指数为3%;低级别和高级别肿瘤细胞胞核均表达P53;高级别肿瘤细胞胞质表达GFAP和BRAF V600E,Ki⁃67抗原标记指数为30%。网织纤维染色可见肿瘤细胞周围包绕基底膜样物质。基因检测显示,低级别和高级别肿瘤均存在BRAF V600E杂合突变。结论2016年世界卫生组织中枢神经系统肿瘤分类将间变型多形性黄色瘤型星形细胞瘤定义为核分裂象>5个/10高倍视野,属WHOⅢ级,预后较WHOⅡ级多形性黄色瘤型星形细胞瘤差。鉴别诊断主要包括胶质母细胞瘤、毛细胞型星形细胞瘤和节细胞胶质瘤,尽管上述肿瘤临床表现、组织学形态、免疫表型和基因突变有重叠,但生物学行为、治疗及预后各异。
黄瘤病; 星形细胞瘤; 间变; 免疫组织化学; 病理学
多形性黄色瘤型星形细胞瘤(PXA)是临床罕见的原发性星形细胞肿瘤,占所有星形细胞肿瘤的1%以下,具有特征性临床病理学和影像学特点,好发于儿童和青年,中位发病年龄为22岁,通常位于大脑浅表位置,累及软脑膜和脑组织,约98%发生于幕上,尤以颞叶常见,多数患者存在长期癫病史[1⁃2]。组织病理学以肿瘤细胞多形性为特征,伴单核细胞或多核瘤巨细胞、嗜酸性小体,血管周围可见丰富淋巴细胞浸润,肿瘤组织附着于网状纤维。2016年世界卫生组织(WHO)中枢神经系统肿瘤分类取消“伴间变特征的多形性黄色瘤型星形细胞瘤”命名,将核分裂象≥5个/10高倍视野(HPF)者定义为“间变型多形性黄色瘤型星形细胞瘤”,属WHOⅢ级,伴或不伴坏死[1,3]。近年报道的间变型多形性黄色瘤型星形细胞瘤病例逐渐增多[4⁃11]。本文报道1例发生于颞叶和基底节区的间变型多形性黄色瘤型星形细胞瘤患儿,并复习相关文献,初步探讨其临床病理学特点、免疫表型和BRAF V600E突变形式,并与组织学形态、免疫表型和基因突变相似的肿瘤进行鉴别诊断。
病历摘要
患儿 男性,11岁。主因头痛伴左上肢无力15 d,于2016年1月15日入院。患者15 d前无明显诱因出现头痛伴左上肢无力,右侧肢体正常,无肢体抽搐、意识障碍、大小便失禁。外院头部CT检查显示,右侧颞叶和基底节区巨大囊实性占位性病变伴幕上积水,考虑高级别胶质瘤(图1)。头部MRI显示,右侧颞叶和基底节区巨大囊实性占位性病变伴幕上积水,T1WI可见病变界限相对清晰,呈以低信号为主的混杂信号影,周围水肿带呈低信号;T2WI可见病变呈不均匀高信号;FLAIR成像呈稍高信号;增强扫描病变呈环形和斑片状不均匀明显强化,囊性变区未见明显强化(图2)。为求进一步诊断与治疗,遂至我院就诊,门诊以“右侧颞叶巨大占位性病变”收入院。患者自发病以来,精神良好,睡眠、饮食可,体重无明显变化,大小便正常。
既往史、个人史及家族史 既往身体健康,身高和体重发育正常,智力发育正常,否认手术、外伤和输血史,否认药物和食物过敏史,预防接种史不详。生于山西省,久居本地,无疫区居住史,无疫情、疫水接触史,无牧区、矿山、高氟区、低碘区居住史,无化学性物质、放射物、毒物接触史,无毒品接触史,无吸烟、饮酒史。父母和兄长身体健康,家族中无传染性疾病和遗传性疾病病史。
体格检查 患儿体温36.4℃,脉搏80次/min,呼吸 19 次/min,血压 102/66 mm Hg(1 mm Hg=0.133 kPa);身 高 150 cm,体 重 35 kg,体 重 指 数(BMI)15.60 kg/m2,发育正常,营养良好,面容表情正常,自主体位;神志清楚,语言流利,查体合作,胸腹部检查未见异常。神经系统检查:双侧瞳孔等大、等圆,直径约2.50 mm,对光反射灵敏,眼球各向活动充分;鼻唇沟对称,口角无歪斜,伸舌居中;左上肢肌力4级、余肢体5级,肌张力均正常,无肌萎缩、肌束颤;痛温觉和轻触觉正常,两点辨别觉、图形觉、位置觉和音叉震动觉正常;双侧快复轮替动作、指鼻试验、跟⁃膝⁃胫试验稳准,Romberg征阴性,直线行走试验阴性;病理征阴性,脑膜刺激征阴性。自主神经系统检查:全身皮肤温度和湿度适中,皮肤弹性好,皮肤划痕试验阴性。
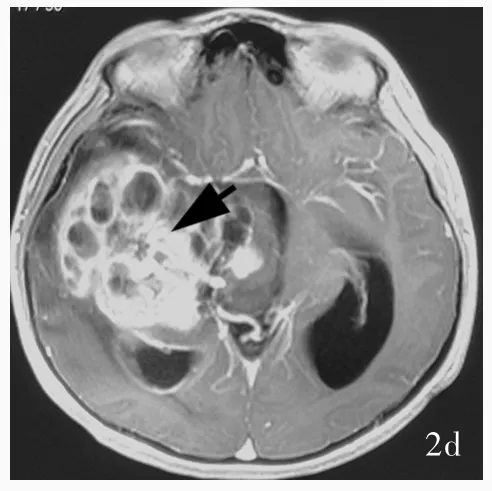

图1 头部CT检查显示,右侧颞叶和基底节区巨大囊实性占位性病变,呈不规则混杂密度影,其内可见多房性低密度囊性变区,周围可见片状低密度水肿带(箭头所示);右侧侧脑室受压,中线结构向左侧偏移 图2 头部MRI检查所见 2a 横断面T1WI显示,病变界限相对清晰,呈以低信号为主的混杂信号影,周围水肿带呈低信号(箭头所示) 2b 横断面T2WI显示,病变呈不均匀高信号(箭头所示) 2c 横断面FLAIR成像显示病变呈稍高信号(箭头所示) 2d 横断面增强T1WI显示,病变呈环形和斑片状不均匀明显强化(箭头所示),囊性变区未见明显强化Figure 1 Cranial CT scan showed a huge space⁃occupying cystic⁃solid lesion in right temporal lobe and basal ganglia(arrow indicates),which was an irregular mixed density with multiple low⁃density cystic changes,surrounded by patchy low⁃density edema.Obvious space⁃occupying effect was found.The right ventricle was compressed and the midline was shifted to left. Figure 2 Cranial MRI findings.Axial T1WI showed the lesion boundary was relatively clear,and mixed signal lesion with predominant hypointense signal were seen(arrow indicates).Periphal edema zone with low⁃signal was seen(Panel 2a).Axial T2WI showed a heterogeneous hyperintense signal lesion(arrow indicates,Panel 2b).Axial FLAIR imaging displayed slight hyperintense signal lesion(arrow indicates,Panel 2c).Axial enhanced T1WI revealed heterogeneous ring or patchy enhancement lesion(arrow indicates),whereas enhancement was not seen in cystic region(Panel 2d).
诊断与治疗经过 实验室检查各项指标均于正常值范围。结合外院影像学检查,临床诊断为胶质瘤。完善术前准备,于2016年1月19日在全身麻醉下行MRI导航下右侧颞叶胶质瘤切除术。术中可见肿瘤位于大脑皮质下1 cm处,呈囊实性,实性部分呈灰黄色,质地柔软,血供丰富,无包膜,与周围组织界限清晰,瘤体主要位于右侧颞叶并突入侧脑室,中线结构向左侧偏移。切取实性部分行快速冷冻病理学检查,术中报告低级别胶质瘤,遂于手术显微镜下分块全切除肿瘤,行组织病理学检查。(1)大体标本观察:术中和术后送检标本为灰白色破碎组织,大小分别为1.50 cm×1.00 cm×0.30 cm和9 cm×5 cm×2 cm,质地柔软,血供丰富,无包膜。经体积分数为10%中性甲醛溶液固定、常规脱水、石蜡包埋、4 μm连续切片,分别行HE染色、免疫组织化学染色和网状纤维染色。(2)HE染色:肿瘤组织可见两种组织学形态,术中送检组织和部分术后送检组织肿瘤细胞呈多形性,由梭形和圆形星形胶质细胞以及单核细胞和多核瘤巨细胞组成,核分裂象罕见,可见较成熟的神经元或节细胞分化成分,伴淋巴细胞浸润,局灶性泡沫样细胞聚集,未见血管内皮细胞增生和坏死灶,病变累及软脑膜,呈现低级别肿瘤(WHOⅡ级)组织学形态改变(图3,4)。部分术后送检组织肿瘤细胞密度增加、异型性明显,以圆形和梭形星形胶质细胞成分为主,不典型核分裂象>5个/10高倍视野,可见血管内皮细胞增生,伴血管周围假“菊形团”样结构,局灶性坏死,呈高级别肿瘤(WHOⅢ级)组织学形态改变(图5)。(3)免疫组织化学染色:采用SP二步法,检测用试剂盒购自北京中杉金桥生物技术有限公司,检测用抗体包括突触素(Syn,1∶100),胶质纤维酸性蛋白(GFAP,1∶400),R132H⁃突变的异柠檬酸脱氢酶1(IDH1,1∶200),P53(1∶75),S⁃100 蛋白(S⁃100,1∶2000),CD34(1∶100),CD68(1∶100),神经微丝蛋白(NF,1∶100),非磷酸化神经丝重链 SMI⁃32(1∶7500),上皮膜抗原(EMA,工作液),BRAF V600E(工作液)和Ki⁃67抗原,均购自北京中杉金桥生物技术有限公司。结果显示,低级别肿瘤细胞胞质表达 GFAP(图 6a)和 BRAF V600E(图 6b)、胞质和胞核表达S⁃100、胞膜表达 CD34(图6c),少数肿瘤细胞胞质表达 Syn(图 6d)和 SMI⁃32(图 6e),Ki⁃67 抗原标记指数为3%(图6f),不表达NF和EMA;高级别和低级别肿瘤细胞胞核均表达P53(图7a);高级别肿瘤细胞胞质表达GFAP(图7b)和BRAF V600E(图7c),不表达 R132H⁃突变的 IDH1和 CD34,Ki⁃67抗原标记指数为30%(图7d)。(4)特殊染色:网织纤维染色显示肿瘤细胞周围包绕基底膜样物质。(5)基因检测:采用聚合酶链反应(PCR)扩增BRAF V600E位点,再行Sanger测序,结果显示,低级别和高级别肿瘤均存在BRAF V600E杂合突变(图8)。最终病理诊断为:(右侧颞叶和基底节区)间变型多形性黄色瘤型星形细胞瘤(WHOⅢ级)。术后未辅助放射治疗和药物化疗。患者共住院22 d,出院时病情平稳,恢复良好。出院后9个月失访。

图3 术中送检标本组织学形态光学显微镜观察所见 HE染色 3a 肿瘤细胞呈多形性,由梭形细胞以及单核细胞和多核瘤巨细胞组成 ×100 3b 可见单核细胞和多核瘤巨细胞,肿瘤细胞异型性明显 ×200Figure 3 Optical microscopy findings of histological patterns of sample of intra⁃operation HE staining Pleomorphic histological appearance of tumor was characterized by spindled cells being intermingled with mononucleated or multinucleated giant cells(Panel 3a). ×100 Nuclear pleomorphism and bizarre were seen in mononucleated or multinucleated tumor giant cells(Panel 3b). ×200
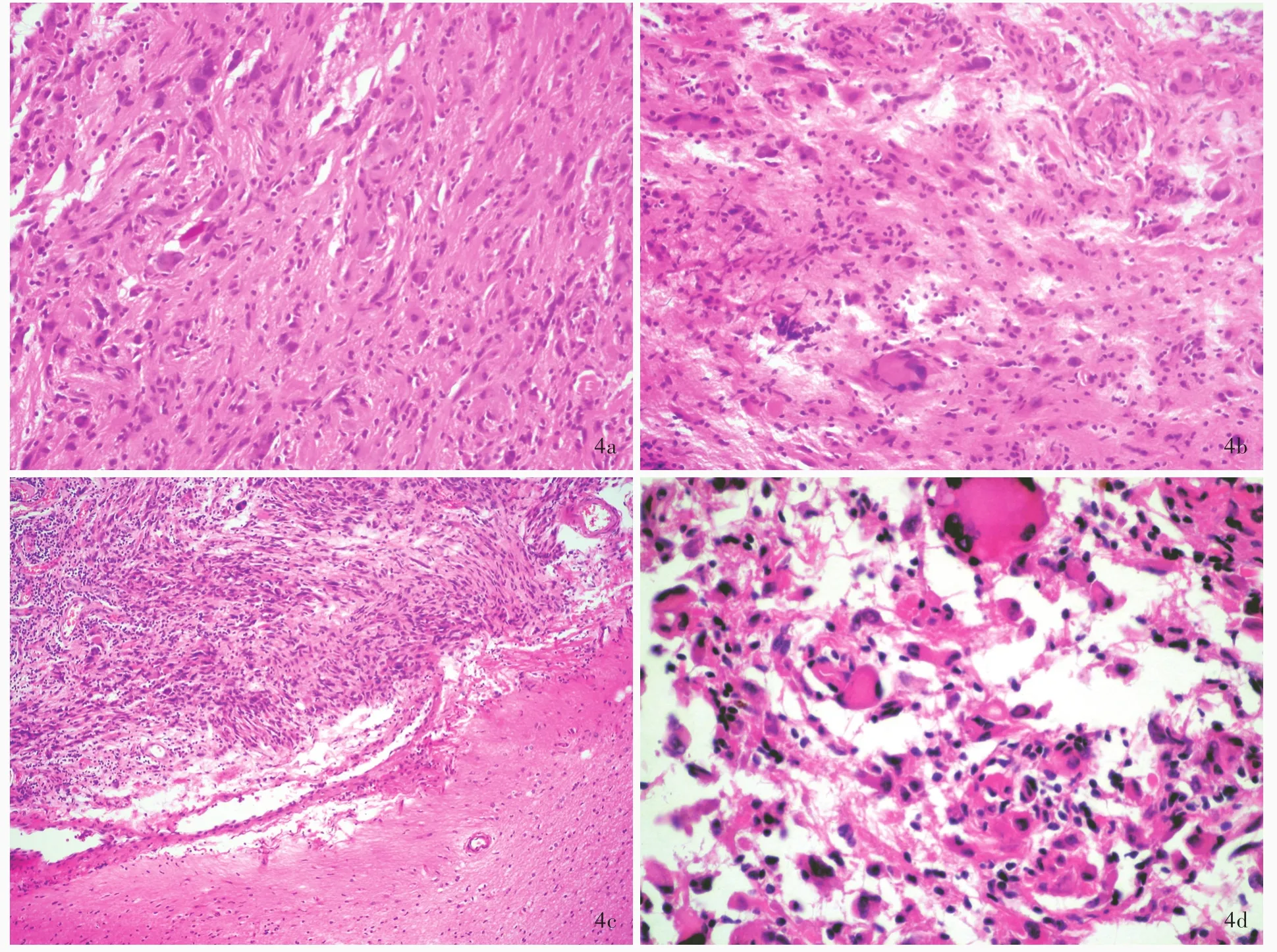
图4 部分术后送检标本(低级别肿瘤)组织学形态光学显微镜观察所见 HE染色 4a 肿瘤组织由梭形细胞以及单核细胞和多核瘤巨细胞组成 ×200 4b 多核瘤巨细胞胞核呈“马蹄”样排列 ×200 4c 肿瘤位于脑表浅位置,侵及软脑膜,与大脑皮质分界清晰 ×100 4d 部分区域可见较多单核细胞和多核瘤巨细胞聚集 ×400Figure 4 Optical microscopy findings of histological patterns of sample(low grade tumor)of surgical excision HE staining Tumor was composed of spindled cells,mononucleated and multinucleated tumor giant cells(Panel 4a). ×200 Nuclei of multinucleated tumor giant cell were arranged in a horseshoe⁃like pattern at edge of the cell(Panel 4b). × 200 Tumor cells invaded pia mater(leptomeningeal)and clearly delineated from the underlying cerebral cortex(Panel 4c). ×100 Prominent mononucleated or multinucleated tumor giant cells were seen in part of the region(Panel 4d). ×400
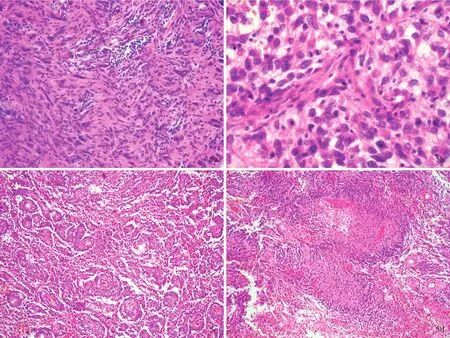
图5 部分术后送检标本(高级别肿瘤)组织学形态光学显微镜观察所见 HE染色 5a 可见典型多形性黄色瘤型星形细胞瘤成分 ×200 5b 肿瘤细胞呈相对一致的圆形或卵圆形,可见不典型核分裂象 ×400 5c 肿瘤细胞围绕血管周围排列,可见血管周围假“菊形团”样结构 ×100 5d 可见局灶性坏死,肿瘤细胞呈“栅栏”样排列 ×100Figure 5 Optical microscopy findings of histological patterns of sample(high grade tumor)of surgical excision HE staining Typical histologic feature of PXA,WHO gradeⅡwas seen(Panel 5a). ×200 Atypical mitotic activity was seen in the round or oval shape tumor cells(Panel 5b). ×400 Tumor cells were arranged around the vascular as perivascular pseudorosettes(Panel 5c). ×100 Focal necrosis was seen and tumor cells were arranged in column(Panel 5d). ×100
讨 论
Kepes等[2]于1979年首次报告并命名多形性黄色瘤型星形细胞瘤,是临床罕见的原发性星形细胞肿瘤,具有特征性组织病理学特点。肿瘤细胞呈梭形细胞、单核细胞和多核瘤巨细胞的多形性特点,伴胞体宽大的多核瘤巨细胞,胞质表达GFAP,肿瘤细胞周围包绕致密网织纤维,是一种特殊类型的星形细胞肿瘤,故命名为“多形性黄色瘤型星形细胞瘤”。多形性黄色瘤型星形细胞瘤仅占颅内肿瘤的0.09%,占所有星形细胞肿瘤的<1%[1],好发于儿童和青年,亦有老年患者的报道[12],无性别和种族差异。
多形性黄色瘤型星形细胞瘤组织病理学特点主要表现为[13]:(1)肿瘤细胞呈多形性,即梭形细胞、肥胖细胞以及单核细胞和多核瘤巨细胞多种成分,胞核和核仁大小不一,常见核内包涵体(INIs)。(2)可见黄色瘤型星形细胞,即脂质聚集于肿瘤细胞内,融合并充满或占据大部分胞质,其星形胶质细胞特性经GFAP染色证实。(3)可见网织纤维,即肿瘤细胞周围包绕的基底膜样物质,经网织纤维染色证实。此外,还可见嗜酸性小体,间质或血管周围淋巴细胞和浆细胞浸润,后者形成血管周围淋巴“袖套”或局灶性聚集。
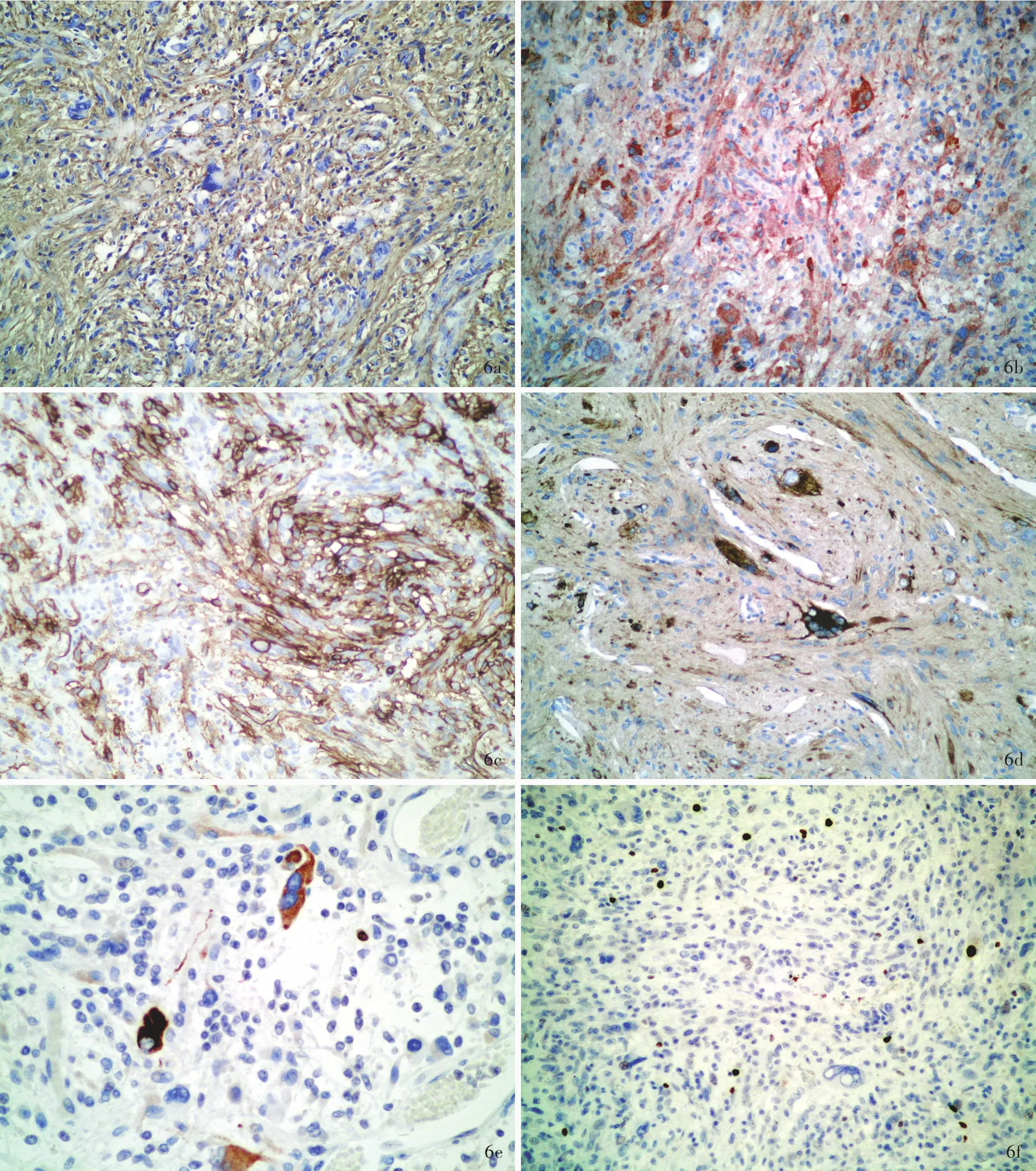
图6 低级别肿瘤光学显微镜观察所见 免疫组织化学染色(SP二步法) 6a 肿瘤细胞胞质表达GFAP ×200 6b 肿瘤细胞胞质表达BRAF V600E ×200 6c 肿瘤细胞胞膜表达CD34 ×200 6d 少数肿瘤细胞胞质表达Syn ×200 6e 个别神经元样细胞胞质表达非磷酸化神经丝重链SMI⁃32 ×400 6f Ki⁃67抗原标记指数约3% ×100Figure 6 Optical microscopy findings of low grade tumor Immunohistochemical staining(SP) The cytoplasm of tumor cells was positive for GFAP(Panel 6a)and BRAF V600E(Panel 6b). ×200 Tumor cells membrane was positire for CD34(Panel 6c). ×200 The cytoplasm of a few tumor cells was positive for Syn(Panel 6d). × 200 The cytoplasm of individual neuron⁃like cells was positive for SMI⁃32(Panel 6e). × 400 Ki⁃67 labeling index was about 3%(Panel 6f). × 100
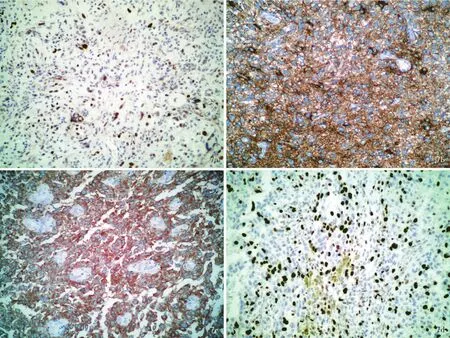
图7 高级别肿瘤光学显微镜观察所见 免疫组织化学染色(SP二步法) 7a 肿瘤细胞胞核表达P53 ×100 7b 肿瘤细胞胞质表达GFAP ×200 7c 肿瘤细胞胞质表达BRAF V600E ×200 7d Ki⁃67抗原标记指数约30% ×100Figure 7 Optical microscopy findings of high grade tumor Immunohistochemical staining(SP) Tumor cells nucleus was positive for P53(Panel 7a). ×100 The cytoplasm of tumor cell was positive for GFAP(Panel 7b)and BRAF V600E(Panel 7c). ×200 Ki⁃67 labeling index was about 30%(Panel 7d). ×100
在2007年WHO中枢神经系统肿瘤分类中,多形性黄色瘤型星形细胞瘤属WHOⅡ级,伴明显核分裂象或坏死者命名为“伴间变特征的多形性黄色瘤型星形细胞瘤”[14]。2016年WHO中枢神经系统肿瘤分类取消该命名,将间变型多形性黄色瘤型星形细胞瘤(WHOⅢ级)列为一种明确类型,其诊断标准为:核分裂象≥5个/10高倍视野,可伴坏死[13]。间变型多形性黄色瘤型星形细胞瘤病变部位、临床表现和影像学特点均与WHOⅡ级的多形性黄色瘤型星形细胞瘤相似[13]。组织病理学典型特征是核分裂活跃,呈局灶性或弥漫性分布。常见的坏死灶与高核分裂活性密切相关。微血管增生不明显,常与核分裂活性和坏死相关。与WHOⅡ级的多形性黄色瘤型星形细胞瘤相比,间变型多形性黄色瘤型星形细胞瘤细胞多形性不明显,突出表现为弥漫性和浸润性生长模式[13]。有文献报道,间变型多形性黄色瘤型星形细胞瘤可见小细胞、纤维样、上皮样/横纹肌样成分[15⁃17]。
多形性黄色瘤型星形细胞瘤细胞表达GFAP和S⁃100[1];存在明显的神经元分化特征,表达相应的神经元标志物[18],如 Syn、嗜铬素 A(CgA)、NF、β⁃微管蛋白(β⁃tubulin)和微管相关蛋白⁃2(MAP⁃2);部分表达P53。大多数多形性黄色瘤型星形细胞瘤核分裂象罕见或缺如,Ki⁃67 抗原标记指数 < 2%[1]且随着肿瘤恶性程度的增加而升高,间变型多形性黄色瘤型星形细胞瘤达10%甚至20%[5]。多形性黄色瘤型星形细胞瘤细胞表达CD34,阳性率达84%,但间变型多形性黄色瘤型星形细胞瘤CD34阳性率下降,仅为44%,可资鉴别[19]。
有50%~78%的WHOⅡ级多形性黄色瘤型星形细胞瘤存在BRAF基因突变,尤以BRAF V600E突变最为常见,故肿瘤细胞表达BRAF V600E,免疫组织化学染色检测BRAF V600E敏感性和特异性均较高[20]。研究显示,约75%WHOⅡ级多形性黄色瘤型星形细胞瘤存在BRAF V600E突变,WHOⅢ级间变型多形性黄色瘤型星形细胞瘤BRAF V600E突变率较低(47.4%)[10],且儿童与成人无明显差异[3]。BRAF V600E突变亦见于其他原发性中枢神经系统肿瘤,特别是胶质母细胞瘤(GBM)、毛细胞型星形细胞瘤(PA)和节细胞胶质瘤(GG)[21]。

图8 Sanger测序显示,BRAF基因存在第15外显子杂合突变c.1799A>TFigure 8 The 1799 basic group of 15th exon of BRAF occurred heterozygosis mutation:A>T.
应注意与胶质母细胞瘤两种亚型巨细胞型胶质母细胞瘤和上皮样胶质母细胞瘤、毛细胞型星形细胞瘤及节细胞胶质瘤相鉴别。(1)巨细胞型胶质母细胞瘤:肿瘤组织以大量异形多核瘤巨细胞、丰富网状纤维和淋巴细胞浸润为特征,亦可见血管周围假“菊形团”样结构,与多形性黄色瘤型星形细胞瘤组织学形态相重叠,应予以鉴别[22]。多形性黄色瘤型星形细胞瘤核分裂象罕见,未见坏死,Ki⁃67抗原标记指数<2%,易区分。间变型多形性黄色瘤型星形细胞瘤和巨细胞型胶质母细胞瘤均表现为核分裂活跃,特别是当前者出现广泛坏死时,难以区分。Reifenberger等[19]研究显示,多形性黄色瘤型星形细胞瘤表达CD34,而巨细胞型胶质母细胞瘤不表达,可资鉴别。此外,与多形性黄色瘤型星形细胞瘤不同,巨细胞型胶质母细胞瘤几乎不表达神经元标志物[NF、神经元核抗原(NeuN)、Syn等],可资鉴别[22]。(2)上皮样胶质母细胞瘤:好发于儿童和青年,肿瘤细胞可见多核瘤巨细胞、脂肪化、促纤维增生反应,约 50%患者存在 BRAF V600E 突变[23⁃24]。二者不同之处在于,间变型多形性黄色瘤型星形细胞瘤缺乏肿瘤细胞形态一致性特点,可见嗜酸性小体[25⁃26]和局灶性典型低级别多形性黄色瘤型星形细胞瘤成分[13]。(3)毛细胞型星形细胞瘤:约9%患者存在BRAF V600E突变,主要见于幕上毛细胞型星形细胞瘤患者[27],免疫组织化学染色BRAF V600E阳性。二者鉴别诊断要点在于,毛细胞型星形细胞瘤的致密纤维区域由特征性细长突起的双极肿瘤细胞组成,含丰富Rosenthal纤维;稀疏区可见微囊结构和规则的假少突胶质细胞结构[28],并伴透明样变性的厚壁血管和海绵状血管瘤样等退行性变成分。(4)节细胞胶质瘤:系发育异常的神经元和肿瘤性胶质细胞组成的神经元及混合性神经元⁃胶质肿瘤,约70%以上的WHOⅠ级节细胞胶质瘤位于颞叶,临床症状主要为局限性癫发作[29]。肿瘤性神经元的大小和分布差异较大,可见双核神经元、钙化、促纤维增生反应、淋巴细胞浸润和嗜酸性小体;梭形细胞成分与弥漫性星形细胞瘤、毛细胞型星形细胞瘤或多形性黄色瘤型星形细胞瘤等组织学形态相似。肿瘤性神经元成分表达神经元标志物,如MAP⁃2、NF、CgA、Syn和CD34;肿瘤性胶质细胞表达GFAP[29⁃31]。有 20% ~ 60%的节 细 胞 胶质 瘤 存 在BRAF V600E 突变[27,32⁃33]。节细胞胶质瘤细胞成分缺乏多形性黄色瘤型星形细胞瘤的细胞多形性特点,即梭形细胞及单核细胞和多核瘤巨细胞的混合成分,以及特征性富含脂质的多核瘤巨细胞,可资鉴别。
多形性黄色瘤型星形细胞瘤有相对良性的临床病程。一项纳入74例多形性黄色瘤型星形细胞瘤患者的研究显示,5年无进展生存率和总生存率分别为70.9%和90.4%;手术切除范围是最具意义的肿瘤复发预测因素[3]。间变型多形性黄色瘤型星形细胞瘤患者预后较差、生存期较短,5年总生存率显著低于多形性黄色瘤型星形细胞瘤患者(55.6%对89.4%),且伴坏死的患者5年总生存率低于不伴坏死的患者(42.2%对90.2%)。儿童和成人的5年无进展生存率(67.99%对62.4%)和总生存率无显著差异(87.4%对76.3%);BRAF V600E突变的预后意义不明[3]。多形性黄色瘤型星形细胞瘤患者应进行长期随访。
[1]Giannini C,Scheithauer BW,Burger PC,Brat DJ,Wollan PC,Lach B,O'Neill BP.Pleomorphic xanthoastrocytoma:what do we really know about it?Cancer,1999,85:2033⁃2045.
[2]Kepes JJ, Rubinstein LJ, Eng LF. Pleomorphic xanthoastrocytoma:a distinctive meningocerebralglioma of young subjects with relatively favorable prognosis.A study of 12 cases.Cancer,1979,44:1839⁃1852.
[3]Ida CM,Rodriguez FJ,Burger PC,Caron AA,Jenkins SM,Spears GM,Aranguren DL,Lachance DH,GianniniC.Pleomorphic xanthoastrocytoma:natural history and long⁃term follow⁃up.Brain Pathol,2015,25:575⁃586.
[4]Rutkowski MJ,Oh T,Niflioglu GG,Safaee M,Tihan T,Parsa AT.Pleomorphic xanthoastrocytoma with anaplastic features:retrospective case series.World Neurosurg,2016,95:368⁃374.
[5]Patibandla MR,Nayak M,Purohit AK,Thotakura AK,Uppin M,Challa S.Pleomorphic xanthoastrocytoma with anaplastic features:a rare case reportand review ofliterature with reference to current management.Asian J Neurosurg,2016,11:319.
[6]Choudry UK,Khan SA,Qureshi A,Bari E.Primary anaplastic pleomorphic xanthoastrocytoma in adults:case report and review of literature.Int J Surg Case Rep,2016,27:183⁃188.
[7]Zhi C,Hao ZF,Mei KY,Ouyang XM,Weng JL,Zhong L,Tang T,LiLN.Pleomorphic xanthoastrocytoma with anaplastic features.Zhongguo Xian Dai Shen Jing Ji Bing Za Zhi,2015,15:655⁃660[.郅程,郝卓芳,梅开勇,欧阳小明,翁洁玲,钟玲,唐甜,李丽娜.伴间变特征的多形性黄色瘤型星形细胞瘤.中国现代神经疾病杂志,2015,15:655⁃660.]
[8]Sun CY,Yu SZ.Pleomorphic xanthoastrocytoma with anaplastic features:one case report and review of literature.Zhongguo Xian Dai Shen Jing Ji Bing Za Zhi,2014,14:1091⁃1095[.孙翠云,于士柱.伴间变特征的多形性黄色瘤型星形细胞瘤.中国现代神经疾病杂志,2014,14:1091⁃1095.]
[9]Niamathullah S,Sivaselvam S,Ghosh M,Ghosh S.Pleomorphic xanthoastrocytoma with anaplastic features:a case report.Indian J Pathol Microbiol,2014,57:101⁃104.
[10]Schmidt Y,Kleinschmidt⁃DeMasters BK,Aisner DL,Lillehei KO,Damek D.Anaplastic PXA in adults:case series with clinicopathologic and molecular features.J Neurooncol,2013,111:59⁃69.
[11]Montano N,Papacci F,Cioni B,Gaudino S,Della Pepa GM,Conforti G,Di Bonaventura R,Novello M,Lauriola L,Meglio M.Primary multicentric anaplastic pleomorphic xanthoastrocytoma with atypical features.J Clin Neurosci,2013,20:1605⁃1608.
[12]Perkins SM,Mitra N,Fei W,Shinohara ET.Patterns of care and outcomes of patients with pleomorphic xanthoastrocytoma:a SEER analysis.J Neurooncol,2012,110:99⁃104.
[13]Komri T.The 2016 WHO classification of tumours of the central nervous system:the major points of revision.Neurol Med Chir(Tokyo),2017,57:301⁃311.
[14]Hirose T,Ishizawa K,Sugiyama K,Kageji T,Ueki K,Kannuki S.Pleomorphic xanthoastrocytoma:a comparative pathological study between conventional and anaplastic types.Histopathology,2008,52:183⁃193.
[15]Kepes JJ.Pleomorphic xanthoastrocytoma:the birth ofa diagnosis and a concept.Brain Pathol,1993,3:269⁃274.
[16]Jeong JY,Suh YL,Hong SW.Atypical teratoid/rhabdoid tumor arising in pleomorphic xanthoastrocytoma: a case report.Neuropathology,2014,34:398⁃405.
[17]Chacko G,Chacko AG,Dunham CP,Judkins AR,Biegel JA,Perry A.Atypical teratoid/rhabdoid tumor arising in the setting of a pleomorphic xanthoastrocytoma.J Neurooncol,2007,84:217⁃222.
[18]Giannini C,Scheithauer BW,Lopes MB,Hirose T,Kros JM,VandenBerg SR. Immunophenotype of pleomorphic xanthoastrocytoma.Am J Surg Pathol,2002,26:479⁃485.
[19]Reifenberger G, Kaulich K, Wiestler OD, Blumcke I.Expression of the CD34 antigen in pleomorphic xanthoastrocytomas.Acta Neuropathol,2003,105:358⁃364.
[20]Ida CM,Vrana JA,Rodriguez FJ,Jentoft ME,Caron AA,Jenkins SM,GianniniC.Immunohistochemistry is highly sensitive and specific for detection of BRAF V600E mutation in pleomorphic xanthoastrocytoma.Acta NeuropatholCommun,2013,1:20.
[21]Myung JK,Cho H,Park CK,Kim SK,Lee SH,Park SH.Analysis of the BRAF(V600E)mutation in central nervous system tumors.Transl Oncol,2012,5:430⁃436.
[22]Martinez⁃Diaz H,Kleinschmidt⁃DeMasters BK,Powell SZ,Yachnis AT. Giant cell glioblastoma and pleomorphic xanthoastrocytoma show different immunohistochemical profiles for neuronal antigens and p53 but share reactivity for classⅢbeta⁃tubulin.Arch Pathol Lab Med,2003,127:1187⁃1191.
[23]TanakaS,Nakada M,NobusawaS,SuzukiSO,SabitH,Miyashita K,Hayashi Y.Epithelioid glioblastoma arising from pleomorphic xanthoastrocytoma with the BRAF V600E mutation.Brain Tumor Pathol,2014,31:172⁃176.
[24]Kleinschmidt⁃DeMasters BK,Aisner DL,Foreman NK.BRAF VE1 immunoreactivity patterns in epithelioid glioblastomas positive for BRAF V600E mutation.Am J Surg Pathol,2015,39:528⁃540.
[25]Kleinschmidt⁃DeMasters BK,Alassiri AH,Birks DK,Newell KL,Moore W,LilleheiKO.Epithelioid versus rhabdoid glioblastomas are distinguished by monosomy 22 and immunohistochemical expression of INI⁃1 but not claudin 6.Am J Surg Pathol,2010,34:341⁃354.
[26]Aisner DL,Newell KL,Pollack AG,Kleinschmidt⁃Demasters BK,Steinberg GK,Smyth LT,Vogel H.Composite pleomorphic xanthoastrocytoma⁃epithelioid glioneuronal tumor with BRAF V600E mutation:report of three cases.Clin Neuropathol,2014,33:112⁃121.
[27]Schindler G,Capper D,Meyer J,Janzarik W,Omran H,Herold⁃Mende C,Schmieder K,Wesseling P,Mawrin C,Hasselblatt M,Louis DN,Korshunov A,Pfister S,Hartmann C,Paulus W,Reifenberger G,von Deimling A.Analysis of BRAF V600E mutation in 1320 nervous system tumors reveals high mutation frequencies in pleomorphic xanthoastrocytoma,ganglioglioma and extra⁃cerebellar pilocytic astrocytoma.Acta Neuropathol,2011,121:397⁃405.
[28]Colin C,Padovani L,Chappé C,Mercurio S,Scavarda D,Loundou A,Frassineti F,André N,Bouvier C,Korshunov A,Lena G,Figarella⁃Branger D.Outcome analysis of childhood pilocytic astrocytomas:a retrospective study of 148 cases at a single institution.Neuropathol Appl Neurobiol,2013,39:693⁃705.
[29]WolfHK,MüllerMB,SpänleM,ZentnerJ,Schramm J,Wiestler OD.Ganglioglioma:a detailed histopathological and immunohistochemical analysis of 61 cases.Acta Neuropathol,1994,88:166⁃173.
[30]Hirose T,Scheithauer BW,Lopes MB,Gerber HA,Altermatt HJ,VandenBerg SR.Ganglioglioma:an ultrastructuraland immunohistochemical study.Cancer,1997,79:989⁃1003.
[31]Blümcke I,Giencke K,Wardelmann E,Beyenburg S,Kral T,Sarioglu N,Pietsch T,WolfHK,Schramm J,ElgerCE,Wiestler OD.The CD34 epitope is expressed in neoplastic and malformative lesions associated with chronic,focal epilepsies.Acta Neuropathol,1999,97:481⁃490.
[32]Chappé C,Padovani L,Scavarda D,Forest F,Nanni⁃Metellus I,Loundou A,Mercurio S,Fina F,Lena G,Colin C,Figarella⁃Branger D.Dysembryoplastic neuroepithelial tumors share with pleomorphic xanthoastrocytomas and gangliogliomas BRAF(V600E)mutation and expression.Brain Pathol,2013,23:574⁃583.
[33]Murray JC,Donahue DJ,Malik SI,Dzurik YB,Braly EZ,Dougherty MJ, Eaton KW, Biegel JA. Temporal lobe pleomorphic xanthoastrocytoma and acquired BRAF mutation in an adolescent with the constitutional 22q11.2 deletion syndrome.J Neurooncol,2011,102:509⁃514.
Anaplastic pleomorphic xanthoastrocytoma
SHAO Li⁃wei,WANG Fu⁃lin
Department of Pathology,Chinese PLA General Hospital,Beijing 100853,China
Corresponding author:WANG Fu⁃lin(Email:wfl301@yeah.net)
ObjectiveTo investigate the clinicopathological features,immune phenotype and gene mutation characteristics,and diagnosis or differential diagnosis of anaplastic pleomorphic xanthoastrocytoma(PXA).Methods and ResultsA 11 ⁃year⁃old male patient presented with more than half month of headache,and left upper limb weakness.Cranial CT and MRI revealed a large space⁃occupying lesion in the right parietal lobe and basal ganglia which was suggested as glioma.During operation the tumor was examined.It was a cystic⁃solid lesion.The solid part was soft and greyish yellow with rich blood supply and without membrane,and the boundary was clear.Intraoperative freezing pathologic examination showed the tumor was a low grade glioma.The right parietal glioma was completely removed piece by piece under the microscopy.Histologically,the tumor cells were polymorphism,including spindle or round astrocytes,monocytes and multinuclear tumor giant cells.Mitoses were rarely seen.Differentiation of mature neuronal cells or ganglion cells with lymphocyte infiltration were seen in focal region.In some regions,tumor cells were anaplastic,and cellularity were increased.Atypical round or spindle cells were seen,and atypical mitoses>5/10 high power field(HPF)were found.Microvascular proliferation,perivascular pseudorosettes and localized necrosis were also evident. Immunohistochemically,tumor cells were positive for glial fibrillary acidic protein(GFAP),BRAF V600E,S⁃100 protein(S⁃100),CD34,synaptophysin(Syn),non⁃phosphorylation neurofilament heauy chain SMI⁃32 and P53,but negative for isocitrate dehydrogenase 1(IDH1),neurofilament protein(NF)and epithelial membrane antigen(EMA).Ki⁃67 labeling index was about 3%in low grade tumor cells,while Ki⁃67 labeling index was about 30%in high grade tumor cells.In reticular fibre tissue staining,a lot of reticular fibre tissue were seen. BRAF V600E heterozygous mutation c.1799A>T was detected by Sanger sequencing.Conclusions Anaplastic PXA in gradeⅢis defined as mitoses>5/10 HPF in World Health Organization(WHO)classification of tumors of the central neuvous system,2016.Its prognosis is worse than gradeⅡ tumor.The differential diagnosis from glioblastoma(GBM),pilocytic astrocytoma(PA)and ganglioglioma(GG)should be kept in mind,because all of them having some overlaps in clinicopathological presentations,imaging manifestation,immunophenotype features and genetic mutation,but quite different in their biological behavior,treatment and prognosis.
Xanthomatosis; Astrocytoma; Anaplasia; Immunohistochemistry; Pathology
10.3969/j.issn.1672⁃6731.2017.08.011
100853北京,解放军总医院病理科
王辅林(Email:wfl301@yeah.net)
2017⁃06⁃27)

