Integration of phospholipid-drug complex into self-nanoemulsifying drug delivery system to facilitate oral delivery of paclitaxel
Dwei Ding,Bingjun Sun,Weiping Cui,Qin Chen,Xuno Zhng,Hotin Zhng,Zhonggui He,Jin Sun,Cong Luo,*
aWuya College of Innovation,Shenyang Pharmaceutical University,Shenyang 110016,China
b Cancer Hospital of China Medical University,Liaoning Cancer Hospital&Institute,Shenyang 110042,China c School of Life Science and Biopharmaceutics,Shenyang Pharmaceutical University,Shenyang 110016,China
Keywords:PTX PLDC SNEDDS PLDC-SNEDDS Oral delivery
ABSTRACT Self-nanoemulsifying drug delivery system(SNEDDS)has emerged as a promising platform to improve oral absorption of drugs with poor solubility and low permeability.However,large polarity molecules with insufficient lipid solubility, such as paclitaxel (PTX), would suffer from inferior formulation of SNEDDS due to poor compatibility.Herein,phospholipid-drug complex (PLDC) and SNEDDS were integrated into one system to facilitate oral delivery of PTX.First,PTX was formulated into PLDC in response to its inferior physicochemical properties.Then,the prepared PLDC was further formulated into SNEDDS by integrating these two drug delivery technologies into one system(PLDC-SNEDDS).After PLDC-SNEDDS dispersed in aqueous medium,nanoemulsion was formed immediately with an average particle size of ~30 nm.Furthermore,the nanomulsion of PLDC-SNEDDS showed good colloidal stability in both HCl solution (0.1 mol/l,pH 1.0) and phosphate buffer solution (PBS,pH 6.8).In vivo,PTX-PLDC-SNEDDS showed distinct advantages in terms of oral absorption efficiency,with a 3.42-fold and 2.13-fold higher bioavailability than PTX-PLDC and PTX solution,respectively.Our results suggest that the integration of PLDC into SNEDDS could be utilized to facilitate the oral delivery of hydrophobic drugs with large polarity.©2018 Shenyang Pharmaceutical University.Published by Elsevier B.V.This is an open access article under the CC BY-NC-ND license.(http://creativecommons.org/licenses/by-nc-nd/4.0/)
1. Introduction
Chemotherapy is one of the most commonly used therapeutic strategies for the clinical treatment of malignant tumors[1].However,despite of many advantages of oral administration,most of chemotherapeutic agents are still administered by intravenous route,resulting in poor patient acceptance,increased financial cost and potential adverse risks associated with injections [2,3].Such a situation should be attributed to low oral bioavailability due to the exist of the multiple barriers involved in the oral delivery of anticancer drugs,including inferior physicochemical properties of drugs and multiple physiological and biochemical barriers within the gastrointestinal(GI) tract [4-7]. Therefore, how to overcome these obstacles is of crucial importance for the intravenous-to-oral switch in cancer chemotherapy.
Paclitaxel (PTX), a typical anti-tubulin agent, has been be widely used for cancer therapy in clinical practice [2].However, the inefficient oral absorption greatly hinders the oral delivery of PTX. As a result, PTX administrated intravenously in clinic, and how to realize oral delivery is still challenging. Multiple obstacles hinder the oral absorption of PTX, such as inferior physicochemical properties (e.g. large polarity molecule and poor aqueous solubility), low intestinal permeability,and P-gp substrate [4,8].To overcome these barriers, many nanoparticle drug delivery systems (nano-DDS) have been developed to facilitate the oral absorption of PTX,including nanoemulsions,liposomes,nano-micelles,and nanoparticles[9-12].Among them,self-nanoemulsifying drug delivery system (SNEDDS),an anhydrous homogeneous mixture of oils, drugs, surfactants, and cosurfactants, emerged as a promising nano-platform and intrigued an interest in nanoemulsions for oral drug delivery [13-15]. Oil-in-Water(O/W) nanoemulsions will be spontaneously formed when SNEDDS exposed to GI fluids[13].The physicochemical properties of drugs encapsulated in nanoemulsions will be significantly changed, and multiple bio-barriers in GI tract will be overcome, leading to improved oral bioavailability[13].
However, large polarity molecules with insufficient lipid solubility,such as taxane drugs,may suffer from inferior formulation of SNEDDS due to the poor compatibility [14]. To address the challenge of insufficient lipid solubility, several strategies have been developed, including chemical modification of drugs with lipid moieties, fatty acid ionic complex,and phospholipid-drug complex(PLDC)[15-17].Among these,PLDC,a complex with the therapeutic agents bounded by the polar portion of phospholipid, demonstrated distinct advantage in the improvement of lipid solubility for polarity drugs[17-21].In addition to the improved lipid solubility,PLDC also exhibits promising ability to improve the oral absorption of drugs[17].Therefore,combination of PLDC and SNEDDS could be a promising strategy in oral drug delivery.We proposed that the lipophilic phospholipid layer of PLDC could significantly increase the liposolubility of PTX,thereby improve its compatibility in SNEDDS formulation.Therefore,integration of PLDC into SNEDDS may provide a new strategy to facilitate oral delivery of PTX.
Based on this rationale, an integrated hybrid nano-DDS based on PLDC and SNEDDS was developed to improve the oral bioavailability of PTX. First, PLDC was prepared by a solvent evaporation method.Then,the prepared PLDC was further formulated into SNEDDS by integrating these two drug delivery technologies into one system(PLDC-SNEDDS).After formulation optimization,the physicochemical properties and stability of both PLDC and PLDC-SNEDDS were well characterized.Finally,the pharmacokinetics of PTX solution,PLDC and PLDCSNEDDS was evaluated in rats.
2. Materials and methods
2.1. Materials
PTX was obtained from Meilun Biotech Co.Ltd.(Dalian,China).Soybean phospholipid was obtained from Shanghai Advanced Vehicle Technology Co.,Ltd.(Shanghai,China).Oleic acid and ethyl oleate were obtained from Tianjin Hengxing Chemical Reagent Co., Ltd. (Tianjin, China). Glycerin, Cremophor EL35 (hydrogenated castor oil) and Labrafil M 1944CS (oleoyl macrogolglycerides) were obtained from BASF Corporation(Germany). Heparin sodium was obtained from Tianjin Bio-Chemical Pharmaceutical Factory. (Tianjin, China). All other chemicals and solvents utilized in this study were analytical HPLC grade.
2.2. Preparation and characterization of PLDC
The mixture of soybean phospholipid and PTX (1:1,mol/mol)were placed in a laboratory flask and dissolved in anhydrous alcohol (10 mg/ml, total concentration). The reaction sustained with stirring for 6 h at 60°C. Then, anhydrous alcohol was evaporated under reduced pressure. The residues were gathered and placed in a vacuum drying oven overnight,then phospholipid-PTX complex (PTX-PLDC) was obtained.The optimal formulation was determined by the investigation into the reaction solvents, reaction temperature, reaction time, and total reaction concentration. The prepared PLDC of PTX was well characterized by infrared (IR) spectra,differential scanning calorimetry(DSC),X-Ray Diffraction(XRD).
2.3. Preparation and characterization of PLDC-SNEDDS
Labrafil M 1944CS was selected as oil phase for PLDC-SNEDDS,with Cremophor EL35 and glycerin as surfactants,and cosurfactants, respectively. Briefly, Labrafil M 1944CS, Cremophor EL35, and glycerin (6:5:1, w/w/w) were mixed together and heated at 40°C to prepare blank SNEDDS. PTX loaded PLDCSNEDDS (PTX-PLDC-SNEDDS, 1.6% of PTX loaded) was prepared by dissolving a certain amount of PTX-PLDC in the prepared blank-SNEDDS at 37°C. Then deionized water (10 ml)was added in PTX-PLDC-SNEDDS(100 μl)under stirring to obtain PTX-PLDC-SNEDDS colloidal dispersion.The particle size and polydispersity index(PDI)of the colloidal dispersion were determined by a Malvern ZetaSizer (Nano ZS, Malvern. U.K).And the morphology of PTX-PLDC-SNEDDS was observed by transmission electron microscopy(TEM).
2.4. Colloidal stability of PLDC-SNEDDS colloidal dispersions
For oral drug delivery,it is necessary to evaluate the colloidal stability of the prepared PTX-PLDC-SNEDDS in GI tract with various pH values.Therefore,in this section,the colloidal stability of the prepared PTX-PLDC-SNEDDS was studied by determining the mean diameter and PDI of the colloidal dispersion after incubated in HCl solution(0.1 mol/l,pH 1.0)and phosphate buffer solution(PBS,pH 6.8).In addition,the longterm stability of the colloidal dispersion of PTX-PLDC-SNEDDS were stored at room temperature for three months.The particle size and PDI was determined at timed intervals by dynamic light scattering(DLS).
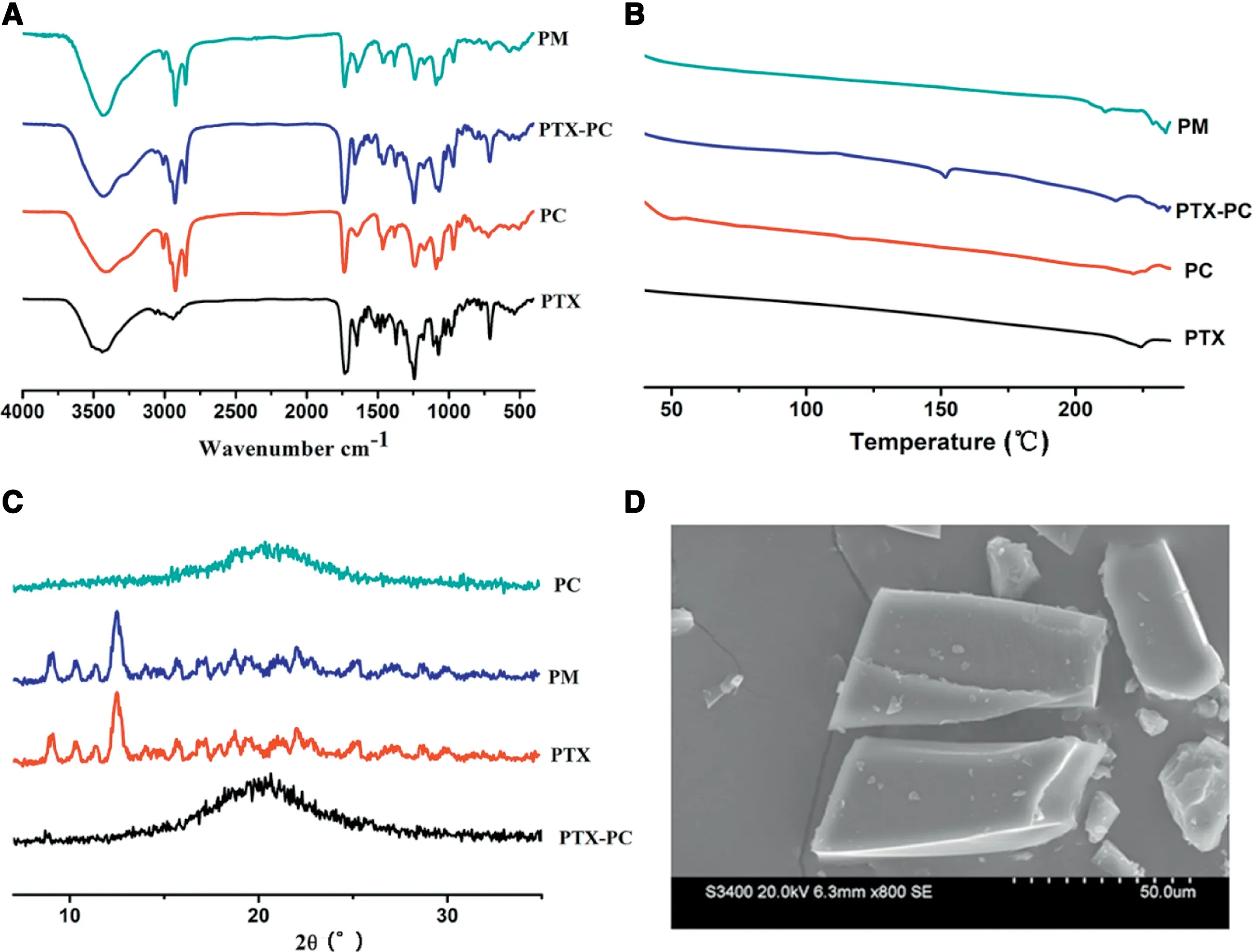
Fig 1-Characterizations of formulations:(A)IR spectra;(B)DSC curves;(C)XRD diffractogram and(D)SEM.(PM:physical mixture;PC:phospholipid complex).
2.5. Animals
Sprague-Dawley (SD) rats (200-250 g) were provided by the Laboratory Animal Center of Shenyang Pharmaceutical University. The animal experiments in the present study were carried out according to the Guidelines for Care and Use of Laboratory Animals approved by the Institutional Animal Ethical Care Committee (IAEC) of Shenyang Pharmaceutical University.
2.6. Pharmacokinetics study in rats
SD rats were randomly divided into 4 groups. A single dose of 5 mg/kg PTX solution was administrated intravenously and a single dose of 30 mg/kg PTX solution, PTX-PLDC and PTXPLDC-SNEDDS were orally administered.After administration,0.3 ml blood samples were collected and centrifuged at timed intervals, and the obtained plasma fraction was stored at-20°C until analysis.The concentration of PTX in plasma was analyzed by a validated UPLC-MS-MS method on an ACQUITY UPLC system (Waters Co., Ltd., Milford, MA, USA). The maximum plasma concentration(Cmax)and the peak-reaching time(Tmax) were directly read from the concentration-time curve.The area under curve (AUC) and the half-life (T1/2) were calculated using DAS 2.0 software. The absolute bioavailability and relative bioavailability (Frel)of the formulations were calculated,respectively.
2.7. Statistical analysis
All acquired data were shown as means±standard deviation(SD).Student’s t-test was used to evaluate any significant differences in the experiments,and P <0.05 was considered statistically significant.
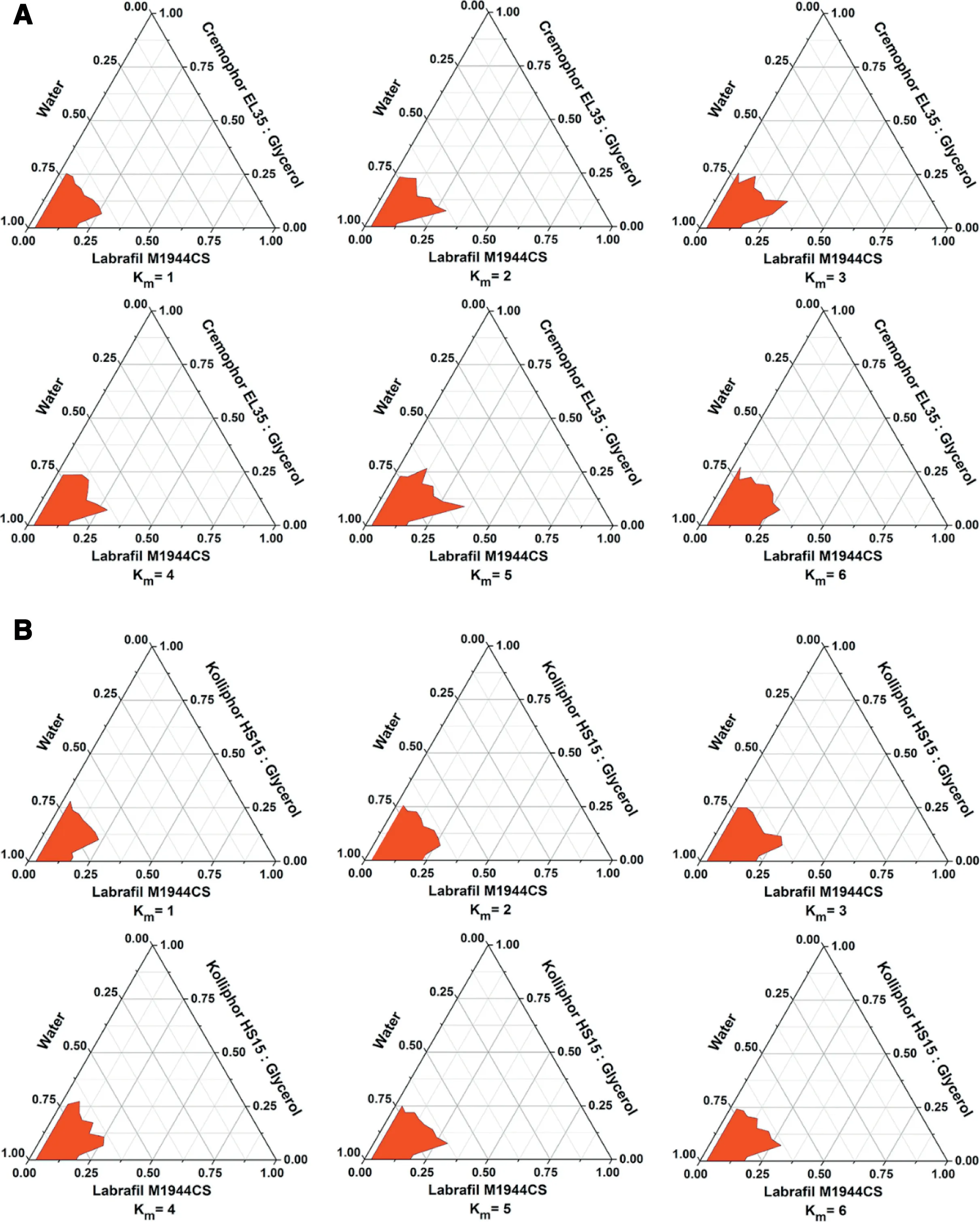
Fig 2-Pseudo-ternary phase diagram of Cremophor EL35(A)and glycerin(B).
3. Results and discussions
3.1. Preparation and characterization of PLDC
PTX-loaded PLDC was prepared by a solvent evaporation method.The optimal molar ratio of soybean phospholipid and PTX was 1:1. Several formulation factors including the reaction solvents, reaction temperature, reaction time, and total reaction concentration were optimized. As shown in Fig S1,anhydrous alcohol was selected as the reaction solvents,and the optimal reaction concentration,reaction temperature and reaction time were 10 mg/ml,60°C and 6 h,respectively.
The prepared PLDC of PTX was well characterized by IR,DSC, XRD and SEM. As shown in Fig. 1A, the IR spectra of the physical mixture of PTX and phospholipid did not show significant difference when compared with that of PTX and phospholipid,suggesting that no distinct interaction occurred between PTX and phospholipid in their physical mixture. In contrast,the characteristic peaks of phospholipid(1089.5 and 1239.7) and PTX (1072.5, 1243.7 and 1647.2) have changed when compared with PLDC. Moreover, as shown in Fig. 1B,the DSC results showed that one new decalescence peak(-147°C) appeared in the DSC curves when compared with PTX,phospholipid and their physical mixture.Similar results were found in the XRD results (Fig.1C),there still are typical peaks of PTX in the XRD diffractogram of the physical mixture,but the characteristic peaks of PTX disappeared in PLDC.All these results demonstrated that PTX loaded PLDC was successfully prepared,and the physicochemical properties of PTX have been significantly changed after being formulated into PLDC. The morphology of PTX loaded PLDC was observed by SEM(Fig.1D).
3.2. Preparation and characterization of PLDC-SNEDDS
After PTX-loaded PLDC was done, we then prepared PLDCSNEDDS.Several formulation factors including oil phase,surfactants, cosurfactants and drug loading were systemically optimized.As shown in Fig.S2,PTX-PLDC had higher solubility in Labrafil M 1944CS than other oils.Moreover,as shown in Table S1 and S2 and Fig.S2,when Labrafil M 1944CS and glycerin were used as oil phase and cosurfactant, PLDC-SNEDDS prepared by using Cremophor EL35 as surfactant showed smaller particle size and PDI than other surfactants. Then, the optimal ratio of Cremophor EL35 and glycerin were investigated by pseudo-ternary phase diagram (Fig. 2), it turned out that the optimal ratio of Cremophor EL35 and glycerin was 5:1 (w/w).Finally, the final formulation was composed of Labrafil M 1944CS (600 mg),Cremophor EL35 (500 mg),glycerin (100 mg),and PTX(20 mg).
PLDC-SNEDDS will spontaneously form O/W nanoemulsions when exposed to aqueous medium. After deionized water (10 ml) was added in PTX-PLDC-SNEDDS (100 μl),PTX-PLDC-SNEDDS colloidal dispersion with light blue opalescence was obtained. The average size of PTX-PLDCSNEDDS dispersed particles was ~30 nm and zeta potential were around -14 mV.The morphology of PTX-PLDC-SNEDDS dispersed particles showed spherical structure (Fig. 3). In addition,we found that different aqueous medium(deionized water, HCl solution or PBS), temperature (25, 37 and 45°C),and stirring speed (100, 200 and 300 rpm) showed negligible effects on the fabrication and particle size of nanoemulsions(Table S3 and S4 and S5).These results suggested that PLDCSNEDDS could readily form uniform O/W nanoemulsions when exposed to aqueous medium.
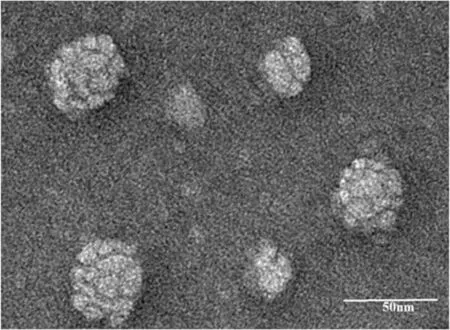
Fig 3-The morphology of PTX-PLDC-SNEDDS dispersed particles observed by TEM.
3.3. Stability of PLDC-SNEDDS colloidal dispersions
The colloidal stability of the formed nanoemulsions is of crucial importance for oral drug delivery.In this section,the colloidal stability of nanoemulsions was evaluated by determining the mean diameter and PDI after incubated in HCl solution (0.1 mol/l, pH 1.0) and PBS (pH 6.8). As shown in Fig.4, PTX-PLDC-SNEDDS nanoemulsions showed good colloidal stability in both HCl solution (0.1 mol/l, pH 1.0) and PBS (pH 6.8) with no significant variation of their particle size for 6 h, which is a long time enough for oral absorption in GI tract. These results indicated that the formed colloidal dispersion of PTX-PLDC-SNEDDS showed good physical stability in medium with various pH values and long-term stability at 37°C, which would be beneficial for oral absorption of PTX-PLDC-SNEDDS.
3.4. Pharmacokinetics study in rats
The ultimate goal of oral drug delivery is to improve the bioavailability of therapeutic agents. In this section, the oral absorption efficiency of PTX-PLDC-SNEDDS was investigated in rats when compared with PTX-PLDC and PTX solution.A validated analysis method based on UPLC-MS-MS was developed to determine the plasma concentration of PTX.The concentration-time curves of all the formulations were shown in Fig. 5, and the main pharmacokinetic parameters were summarized in Table S6.The AUC of PTX-PLDC-SNEDDS showed 3.42-fold and 2.13-fold higher than PTX solution and PTX-PLDC, respectively. As a result, the oral absolute bioavailability of PTX solution was only 1.23%,while the oral absolute bioavailability of PTX-PLDC and PTX-PLDC-SNEDDS were 2.62% and 4.21%, respectively. Moreover, the Cmaxvalues of PTX-PLDC (246.95±73.02) and PTX-PLDC-SNEDDS(209.34±39.60) were much higher than that of PTX solution(44.03±24.36).
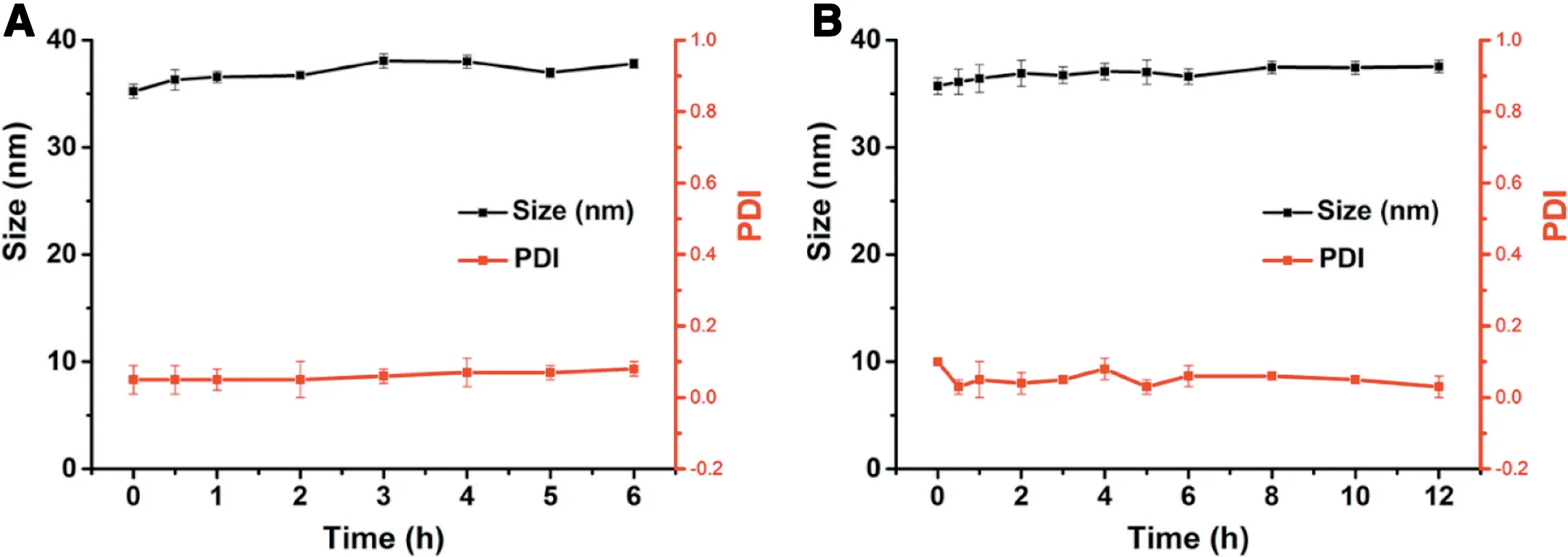
Fig 4-The colloidal stability of PLDC-SNEDDS in(A)HCl solution(0.1 mol/l,pH 1.0)and(B)PBS(pH 6.8).
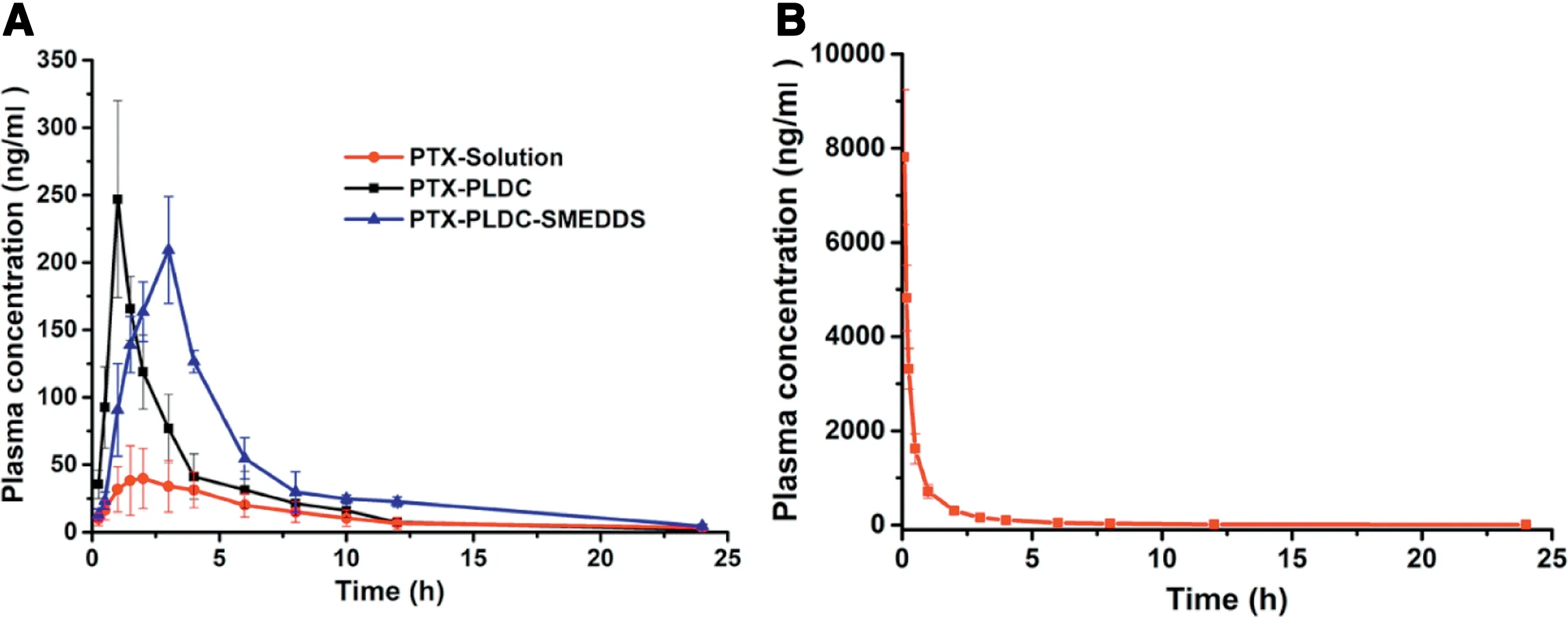
Fig 5-The concentration-time curves of(A)PTX-Solution,PTX-PLDC and PLDC-SNEDDS(oral administration)and(B)PTX-Solution(intravenous administration).
The pharmacokinetics results demonstrated that the oral bioavailability of PTX tended to be highly variable as well as low, hindering the intravenous-to-oral switch in cancer chemotherapy. Formulating PTX into PLDC was an efficient strategy to facilitate the oral absorption of PTX by “masking”the inferior properties of PTX, with a 2-fold improvement of bioavailability. More interestingly, PTX-PLDC-SNEDDS, integrating PLDC technology and SNEDDS into one system,could further improve the oral absorption of PTX.As mentioned earlier, large polarity molecules with insufficient lipid solubility(e.g. PTX), would suffer from inferior formulation of SNEDDS due to the poor compatibility between drugs and SNEDDS.After formulated into PLDC,PTX-PLDC showed totally different properties from PTX,the lipophilic phospholipid layer of PLDC could significantly increase the liposolubility of PTX,thereby improve the its compatibility in SNEDDS formulation. As a result, PTX-PLDC-SNEDDS showed much higher oral bioavailability than that of PTX-PLDC and PTX solution.
4. Conclusion
To sum up, a novel oral DDS of PTX was developed by combining PLDC and SNEDDS into one system to improve the oral absorption of PTX. PTX, with large polarity and poor liposolubility,suffers from inferior formulation of SNEDDS due to the poor compatibility between drugs and SNEDDS.In the present study, PTX was formulated into PLDC in response to the inferior physicochemical properties of PTX.Then,the prepared PLDC was further formulated in to SNEDDS by integrating these two drug delivery technologies into one system(PLDC-SNEDDS). PTX-PLDC-SNEDDS showed distinct advantages in terms of oral absorption efficiency, with a 3.42-fold and 2.13-fold higher bioavailability than both PTX-PLDC and PTX solution,respectively.Therefore,integration of PLDC into SNEDDS showed a great potential in improving the oral delivery of large-molecule drugs with high polarity.
Declaration of interest
The authors declare no conflicts of financial interest in this research.
Acknowledgements
This work was financially supported by the National Nature Science Foundation of China (No. 81703451) and the China Postdoctoral Science Foundation(No.2017M611269 and 2018T110233).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.ajps.2018.10.003.
 Asian Journal of Pharmacentical Sciences2019年5期
Asian Journal of Pharmacentical Sciences2019年5期
- Asian Journal of Pharmacentical Sciences的其它文章
- Complex formulations,simple techniques:Can 3D printing technology be the Midas touch in pharmaceutical industry?
- Nanocarriers as a powerful vehicle to overcome blood-brain barrier in treating neurodegenerative diseases:Focus on recent advances
- Design,mechanism,delivery and therapeutics of canonical and Dicer-substrate siRNA
- Improving the protective effects of aFGF for peripheral nerve injury repair using sulfated chitooligosaccharides
- Exploring the relationship of hyaluronic acid molecular weight and active targeting efficiency for designing hyaluronic acid-modified nanoparticles
- Redox-sensitive micelles for targeted intracellular delivery and combination chemotherapy of paclitaxel and all-trans-retinoid acid
