由4,4′-二甲基-2,2′-联苯二甲酸配体构筑的锌 (Ⅱ)和镉 (Ⅱ)双核配合物的合成、晶体结构、荧光及光催化性质
陈金伟 赵振宇 黎 彧*, 邹训重 冯安生
(1广东轻工职业技术学院,轻化工技术学院/广东省特种建筑材料及其绿色制备工程技术研究中心,广州 510300)
(2深圳信息职业技术学院,智能制造与装备学院,深圳 518172)
0 Intro duction
During the past few decades,extensive endeavors have been focused on the rational design and controllable synthesis of coordination compounds,since they displaying intriguing network structures and potential applications in gas adsorption and separation,catalysis,sensing,luminescence,and magnetism[1-7].From the viewpoint of the synthetic strategy of crystal engineering,the suitable selection of organic ligands and metal ions plays a dominating role on the construction of coordination compounds[8-9].Moreover,other factors will also be taken into account,such as solvent,template,temperature,and pH value[10-13].
In this context,biphenyl polycarboxylate ligands have been extensively utilized to synthesize various functional coordination compounds owing to their strong coordination ability in diverse modes and the fact that they are able to satisfy the geometric requirement of the metal centers,which leads to higher dimensional frameworks[11,13-15].
In this work,we selected 4,4′-dimethyl-2,2′-biphenyldicarboxylic acid(H2dbda)as an organic ligand owing to the following features:(1)it can twist and rotate freely to generate different angles between the two phenyl planes via the C-C single bond to furnish a subtle conformational adaptation;(2)it has four potential coordination sites(four carboxylate O donors),which can lead to diverse coordination patterns and high dimensionalities;(3)this acid ligand remains poorly used for the generation of coordination compounds.Given these features,the main objective of the present study consisted in the exploration of H2dbda as a biphenyl dicarboxylate ligand for the assembly of diverse coordination compounds.
Hence,in this work,we report the syntheses,crystal structures,luminescent and photocatalytic properties of three Zn (Ⅱ) and Cd (Ⅱ) coordination compounds constructed from the biphenyl dicarboxylate ligand.
1 Experimental
1.1 Reagents and physical measurements
All chemicals and solvents were of AR grade and used without further purification.Carbon,hydrogen and nitrogen contents were determined using an Elementar Vario EL elemental analyzer.IR spectra were recorded using KBr pellets and a Bruker EQUINOX 55 spectrometer.Thermogravimetric analysis(TGA)data were collected on a LINSEIS STA PT1600 thermal analyzer with a heating rate of 10 ℃·min−1.Excitation and emission spectra were recorded on an Edinburgh FLS920 fluorescence spectrometer using the solid samples at room temperature.
1.2 Synthesis of[Zn2(μ-dbda)2(phen)2(H2O)2](1)
A mixture of ZnCl2(0.027 g,0.20 mmol),H2dbda(0.054 g,0.20 mmol),phen(0.040 g,0.20 mmol),NaOH(0.016 g,0.40 mmol),and H2O(10 mL)was stirred at room temperature for 15 min,and then sealed in a 25 mL Teflon-lined stainless steel vessel,and heated at 160℃for 3 days,followed by cooling to room temperature at a rate of 10 ℃ ·h−1.Yellow blockshaped crystals of 1 were isolated manually,and washed with distilled water.Yield:44%(based on H2dbda).Anal.Calcd.for C56H44Zn2N4O10(%):C 63.23,H 4.17,N 5.27;Found(%):C 63.47,H 4.16,N 5.30.IR(KBr,cm−1):3 435w,3 020w,1 590s,1 558m,1 475 w,1 413m,1 356m,1 253w,1 216w,1 148w,1 102w,1 055w,1 018w,868w,838w,807w,770m,728w,676w,552w.
1.3 Synthesis of[Zn2(μ-dbda)2(2,2′-bipy)2](2)
The preparation of 2 was similar to that of 1 except using 2,2′-bipy(0.031 g,0.20 mmol)instead of phen.After being cooled to room temperature,yellow block-shaped crystals of 2 were isolated manually,and washed with distilled water.Yield:41%(based on H2dbda).Anal.Calcd.for C52H40Zn2N4O8(%):C 63.75,H 4.12,N 5.72;Found(%):C 63.49,H 4.10,N 5.76.IR(KBr,cm−1):1 594s,1 557w,1 475w,1 413m,1 356 w,1 253w,1 211w,1 154w,1 097w,1 050w,1 019w,874w,807w,764m,734w,681w,656w,547w.
1.4 Synthesisof[Cd2(μ-dbda)2(2,2′-bipy)2(H2O)2]·2H2O(3)
A mixture of CdCl2·H2O(0.040 g,0.20 mmol),H2dbda(0.054 g,0.20 mmol),2,2′-bipy(0.031 g,0.20 mmol),NaOH(0.016 g,0.40 mmol),and H2O(10 mL)was stirred at room temperature for 15 min,and then sealed in a 25 mL Teflon-lined stainless steel vessel,and heated at 160℃for 3 days,followed by cooling to room temperature at a rate of 10 ℃·h−1.Yellow blockshaped crystals of 3 were isolated manually,and washed with distilled water.Yield:38%(based on H2dbda).Anal.Calcd.for C52H48Cd2N4O12(%):C 54.51,H 4.22,N 4.89;Found(%):C 54.77,H 4.20,N 4.93.IR(KBr,cm−1):3 430m,2 916w,1 548s,1 475w,1 418 m,1 376m,1 252w,1 216w,1 154w,1 101w,1 060w,1 008w,910w,807m,770m,728w,676w,552w.
The compounds are insoluble in water and common organic solvents,such as methanol,ethanol,acetone,and DMF.
1.5 Structure determination
Three single crystals with dimensions of 0.26 mm×0.24 mm×0.23 mm(1 and 3)and 0.25 mm×0.24 mm×0.23 mm(2)were collected at 293(2)K on a Bruker SMART APEXⅡ CCD diffractometer with MoKαradiation(λ=0.071 073 nm).The structures were solved by direct methods and refined by full matrix leastsquare onF2using the SHELXTL-2014 program[16].All non-hydrogen atoms were refined anisotropically.All the hydrogen atoms were positioned geometrically and refined using a riding model.A summary of the crystallography data and structure refinements for 1~3 is given in Table 1.The selected bond lengths and angles forcompounds 1~3 are listed in Table 2.Hydrogen bond parameters of compounds 1 and 3 are given in Tables 3 and 4.

Table 1 Crystal data for compounds 1~3
CCDC:1962704,1;1962705,2;1962706,3.

Table 2 Selected bond distances(nm)and bond angles(°)for compounds 1~3
Table 3 Hydrogen bond parameters of compound 1

Table 4 Hydrogen bond parameters of compound 3
1.6 Photocatalytic activity study
Photocatalytic degradation ofmethylene blue(MB)in the presence of catalysts 1~3 was investigated using a Cary 5000 UV-Vis-NIR spectrophotometer.The catalyst(50 mg)was dispersed in 100 mL aqueous solution of MB(10 mg·L−1)under stirring for 30 min in the dark,aiming to ensure an adsorption-desorption equilibrium.The obtained mixture was then exposed to a continuous UV irradiation using an Hg lamp(125 W)for 150 min with continuous stirring.Reaction samples(5 mL)were taken out every 15 min,centrifuged,and then analyzed by UV-Vis spectrophotometry,monitor-ing an intensity decrease of the MB absorption band at 668 nm.A control experiment was also performed under the same reaction conditions,showing that no MB degradation takes place in the absence of catalyst.
2 Results and discussion
2.1 Description of the structure
2.1.1 Structure of[Zn2(μ-dbda)2(phen)2(H2O)2](1)
This compound reveals a discrete dimeric structure with the asymmetric unit containing a Zn (Ⅱ)ion,aμ-dbda2−ligand,a phen moiety,and one coordinated water molecule.The Zn (Ⅱ)center is penta-coordinated and reveals a trigonal bipyramidal{ZnN2O3}environment,which is completed by two O atoms from twoμdbda2−blocks,one O atom from the H2O ligand,and a pair of N atoms from the phen moiety(Fig.1).The lengths of the Zn-O and Zn-N bonds are 0.196 1(3)~0.214 8(3)and 0.209 8(3)~0.212 9(4)nm,respectively;these are within the normal values for related Zn (Ⅱ)derivatives[11,17-18].Theμ-dbda2−block acts as aμ-linker via monodentate COO−groups(Fig.2).In theμ-dbda2−block,the dihedral angle of two benzene rings is 68.36°.Theμ-dbda2−blocks connect two Zn1 ions to give a Zn2molecular unit having a Zn…Zn distance of 0.574 3(3)nm(Fig.2).These discrete Zn2units are interconnected by O−H…O hydrogen bonds to form a 3D supramolecular framework(Fig.3).

Fig.1 Drawing of the asymmetric unit of compound 1 with 30%probability thermal ellipsoids

Fig.2 Di-zinc (Ⅱ)unit in 1

Fig.3 Perspective of 3D supramolecular framework along b and c axes in 1
2.1.2 [Zn2(μ-dbda)2(2,2′-bipy)2](2)
This compound also has a discrete dimeric structure.Its asymmetric unit containing one Zn (Ⅱ)ion,aμdbda2−ligand,and a 2,2′-bipy moiety.The Zn1 center is tetra-coordinated and reveals a distorted tetrahedral{ZnN2O2}geometry formed by two carboxylate O donors from two individualμ-dbda2−moieties and two N donors from the 2,2′-bipy moiety(Fig.4).The Zn-O(0.189 8(3)~0.191 1(2)nm)and Zn-N(0.205 2(3)~0.205 5(3)nm)bonds are within standard values[17-19].The dbda2−block acts as aμ-linker with its carboxylate groups adopting a monodentate mode(Fig.5).Within theμ-dbda2−block,the dihedral angle between two aromatic rings is 64.00°.Theμ-dbda2−blocks link two Zn1 ions to give a Zn2unit having a Zn…Zn distance of 0.393 0(3)nm(Fig.5).These discrete Zn2units are interconnected to form a 3D supramolecular framework(Fig.6).

Fig.4 Drawing of the asymmetric unit of compound 2 with 30%probability thermal ellipsoids
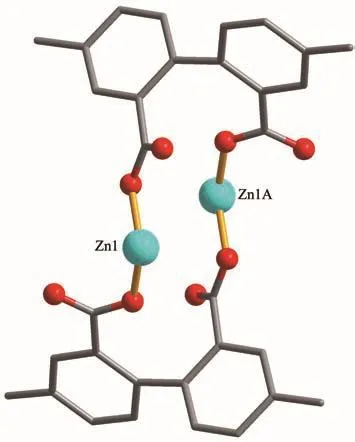
Fig.5 Di-zinc (Ⅱ)unit in 2

Fig.6 Perspective of 3D supramolecular framework along a and b axes in 2
2.1.2 [Cd2(μ-dbda)2(2,2′-bipy)2(H2O)2]·2H2O(3)
This compound has a discrete Cd (Ⅱ)dimer structure.There are one Cd1 ion,oneμ-dbda2−block,one 2,2′-bipy moiety,one H2O ligand,and one lattice water molecule in the asymmetric unit of 3(Fig.7).The Cd1 ion is penta-coordinated with a distorted{CdN2O3}trigonal bipyramidal environment.It is filled by two carboxylate O atoms from twoμ-dbda2−blocks,one O atom from the H2O ligand,and a pair of N atoms from the 2,2′-bipy moiety.The Cd-O(0.218 1(4)~0.229 1(4)nm)and Cd-N(0.231 9(5)~0.233 4(3)nm)bond lengths are in good agreement with those distances observed in some other Cd (Ⅱ) compounds[9,11,13].Theμ-dbda2−block is bidentate and behaves as aμ-linker,interconnecting the adjacent Cd1 ions into a dicadmium (Ⅱ)molecular with a Cd…Cd separation of 0.463 3(5)nm.Discrete Cd2units are further assembled into a 3D supramolecular framework(Fig.9).

Fig.7 Drawing of the asymmetric unit of compound 3 with 30%probability thermal ellipsoids

Fig.8 Di-cadmium (Ⅱ)unit in 3
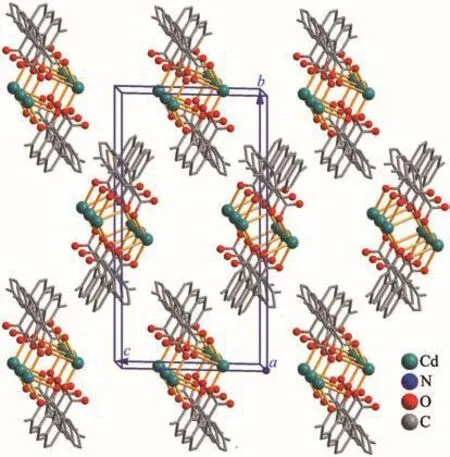
Fig.9 Perspective of 3D supramolecular framework along b and c axes in 3
2.2 TGA analysis
To determine the thermal stability of compounds 1~3,their thermal behaviors were investigated under nitrogen atmosphere by thermogravimetric analysis(TGA).As shown in Fig.10,TGA curve of compound 1 showed that there was a loss of two H2O ligands between 78 and 126℃(Obsd.3.6%,Calcd.3.4%);further heating above 231℃led to a decomposition of the dehydrated sample.Compound 2 did not contain solvent of crystallization or H2O ligands and remained stable up to 210℃,followed by a decomposition on further heating.Compound 3 lost its two lattice water molecules and two H2O ligands in a range of 46~129 ℃(Obsd.6.6%,Calcd.6.3%),followed by the decomposition at 245℃.

Fig.10 TGA curves of compounds 1~3
2.3 Luminescent properties
Solid-state emission spectra of H2dbda and compounds 1~3 were measured at room temperature(Fig.11).The spectrum of H2dbda revealed a weak emission with a maximum at 453 nm(λex=310 nm).In comparison with H2dbda,the coordination compounds 1~3 exhibited more extensive emissions,namely at 390 nm for 1,411 nm for 2,and 387 nm for 3(λex=310 nm).These emissions correspond to intraligandπ-π*ornπ*transition of H2dbda[9,11,18].Enhancement of the luminescence in 1~3 vs H2dbda can be explained by the coordination of ligands to Zn (Ⅱ) or Cd (Ⅱ).The coordination can augment a rigidity of ligands and reduce an energy loss due to radiationless decay[13,17,19].
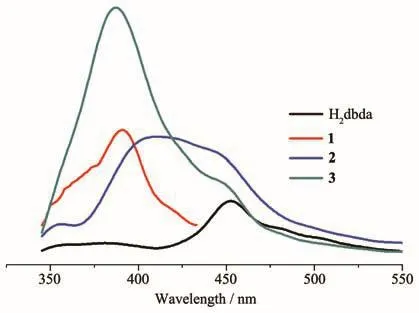
Fig.11 Solid-state emission spectra of H2dbda and compounds 1~3 at room temperature
2.4 Photocatalytic activity for dye degradation

Fig.12 Photocatalytic degradation of MB solution under UV light using catalysts 1~3 and the blank experiment
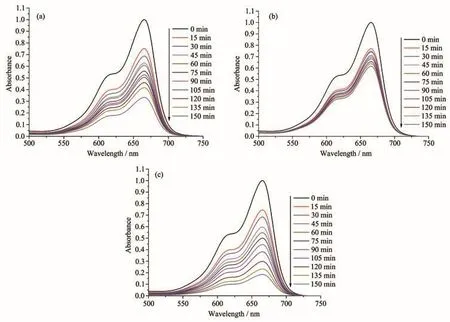
Fig.13 Time-dependent UV-Vis spectra of the reaction mixtures in the course of MB photodegradation catalyzed by 1(a),2(b),and 3(c)
Prior research showed that various transition metal coordination polymers are promising photocatalysts for the degradation of different organic dye pollutants[20-24].To study the photocatalytic activity of 1~3,we selected methylene blue(MB)as a model dye contaminant in wastewater.The obtained results(Fig.12 and 13)indicated that the MB degradation rate attained 81.4%after 150 min in the presence of 3 that is the most active catalyst.For 1 and 2,the MB degradation rates were inferior,being 66.7%and 38.5%,respectively.Under similar conditions,blank test showed that the MB degradation efficiency was only 11.8%after 150 min.The research demonstrates that the photocatalytic activity depends on various factors,such as the number of water ligands,the coordination environment of metal centers,and the optical band gap of catalyst[21,24-25].
3 Conclusions
In summary,we have successfully synthesized and characterized three new zinc and cadmium coordination compounds by using one unexplored biphenyl dicarboxylic acid as ligand under hydrothermal condition.All compounds feature a 0D dimeric structure.Besides,the luminescence and photocatalytic properties were also investigated and discussed.The results show thatcompound3candegrademethyleneblueefficiently.