Metal-organic Framework Membranes for Efficient Separation of Small Molecules and Ions†
JIANG Xiao-Tian,YIN Qi,LIU Tian-Fu,CAO Rong
(State Key Laboratory of Structural Chemistry,Fujian Institute of Research on the Structure of Matter,Chinese Academy of Sciences,Fuzhou 350002,China)
Abstract This review summarizes the recent research progress of metal-organic framework(MOF)membrane for the efficient separation of small molecules and ions.Owing to their high crystallinity,designable structures,tunable pore sizes and easy functionality,MOF membranes have attracted great research attention and being considered as excellent candidates for separation technology.Given the tremendous progresses made in recent years,it is timely and worthy to systematically summarize the recent advances and shed light on the future trend in this multidisciplinary field.In this review,four common fabrication methods of MOF membranes are firstly summarized,including layer-by-layer assembly(liquid-phase epitaxy and Langmuir-Blodgett deposition),vacuum-based fabrication(chemical vapor deposition and atomic layer deposition),electrochemical deposition and powder MOF-based deposition.Furthermore,three emerging separation applications of MOF membranes are minutely described,including gas separation,liquid separation,and ion/proton conductivity.Finally,a summary proposed some key scientific challenges and the possible solution,showing future perspectives of the development of this filed.
Keywords Metal-organic framework;Membrane;Fabrication;Separation
Membrane materials are primarily used for separation and have aroused great interest in both fundamental researches and be adopted for several practical applications over the last six decades[1].Compared with other traditional separation methods,membrane separation technologies have the advantages of low cost,low energy consumption and high efficiency,being widely used in various separation industries[2-4].However,traditional membrane materials have suffered the disadvantages such as low selectivity,low chemical stability,and high operating temperature.Therefore,it is still a great challenge and demands to explore novel membrane materials with high permeability,desired selectivity and excellent durability for the wide development and utilization of membrane separation technology.
In recent several decades,metal-organic frameworks(MOFs) coordinated by metal ions/clusters and organic ligands have attracted increasing attention as a class of excellent candidates for membrane separation due to their characteristics of high crystallinity,permanent porosity,high thermal and chemical stability,easy functionality,and adjustable structure/pore[5-8].The preparation methods of MOF membranes have been developed rapidly and a kind of MOF membranes fabricated by different MOFs with excellent permeability and selectivity have been successfully prepared on porous substrates for efficient separation with the thickness ranging from tens of micrometers to sub-100 nm[9-20].The remarkable development of structural designability and pore adjustability have made MOF membranes more applicable in practical industrial conditions than traditional membrane materials specifically for separation technology.
In this review,the developments of MOF thin films will be summarized with respect to three parts.First,the commonly used fabrication methods for MOF membranes,being one of the important issues for membrane separation,will be described.Secondly,the recent advances of MOF membranes in typical separation applications will be presented,including gas separation,liquid separation and ion/proton conduction.Finally,the current challenges and future perspectives of MOF thin films in this field will be evaluated.
1 Fabrication Methods
Four kinds of common fabrication methods with their particular advantages for fabricating MOF membranes will be discussed in this section,including layer-by-layer assembly(liquid-phase epitaxy and Langmuir-Blodgett deposition),vacuum-based fabrication(chemical vapor deposition and atomic layer deposition),electrochemical deposition and powder MOF-based deposition.
1.1 Layer-by-layer Assembly(LBL)
Layer-by-layer assembly(LBL)method,including liquid-phase epitaxy(LPE)process and Langmuir-Blodgett(LB)deposition,has been developed for preparing MOF membranes on various substrates,expanding a wide range of applications especially the surface-supported metal-organic frameworks(SURMOFs)films with steerable and uniform thickness and surface reaction[21].The LBL methods can precisely monitor the thickness of MOF thin films through controlling the number of MOF unit cells on the substrate.
LBL assembly technique is one of the most commonly used synthesis strategies for SURMOFs,and several techniques have been established for the SURMOFs preparation based on epitaxial growth,such as high-throughput spray[22],large-area spray[23],spin coating[24],dipping robot[25],and flow-based automation[26]as shown in Fig.1.The SURMOFs films synthesized by LBL method not only retain the powder characteristics,but also realize the design of MOF thin film structure by multi-heteroepitaxy.Numerous efforts have been contributed to the fabrication of SURMOF thin films on functionalized substrates and many valuable applications have been realized on this basis.Due to the brittleness of the crystalline coordination compounds,it is difficult for MOF crystals to form a satisfactory binding force with the inert porous substrate for the subsequent film growth.Owing to the inherent properties of MOFs,the surface modification of the substrate is crucial to solve this interfacial interaction problem,improving or changing the physical and chemical properties of the substrates and enhancing the interaction between MOFs and the substrates[27].There are several different modification methods for the preparation of SURMOF membranes,including organic molecular modification[28,29],inorganic compound modification[30-32],and so on[33-35].The spray device[Fig.1(A)]is suitable for fabricating 2D SURMOFs,while spin coating,dipping robot and flow automation[Fig.1(B—D)]are applied to preparing 3D SURMOFs.In addition,2D MOFs layered structure can be prepared by controlling the conditions of layer-by-layer growth[36].
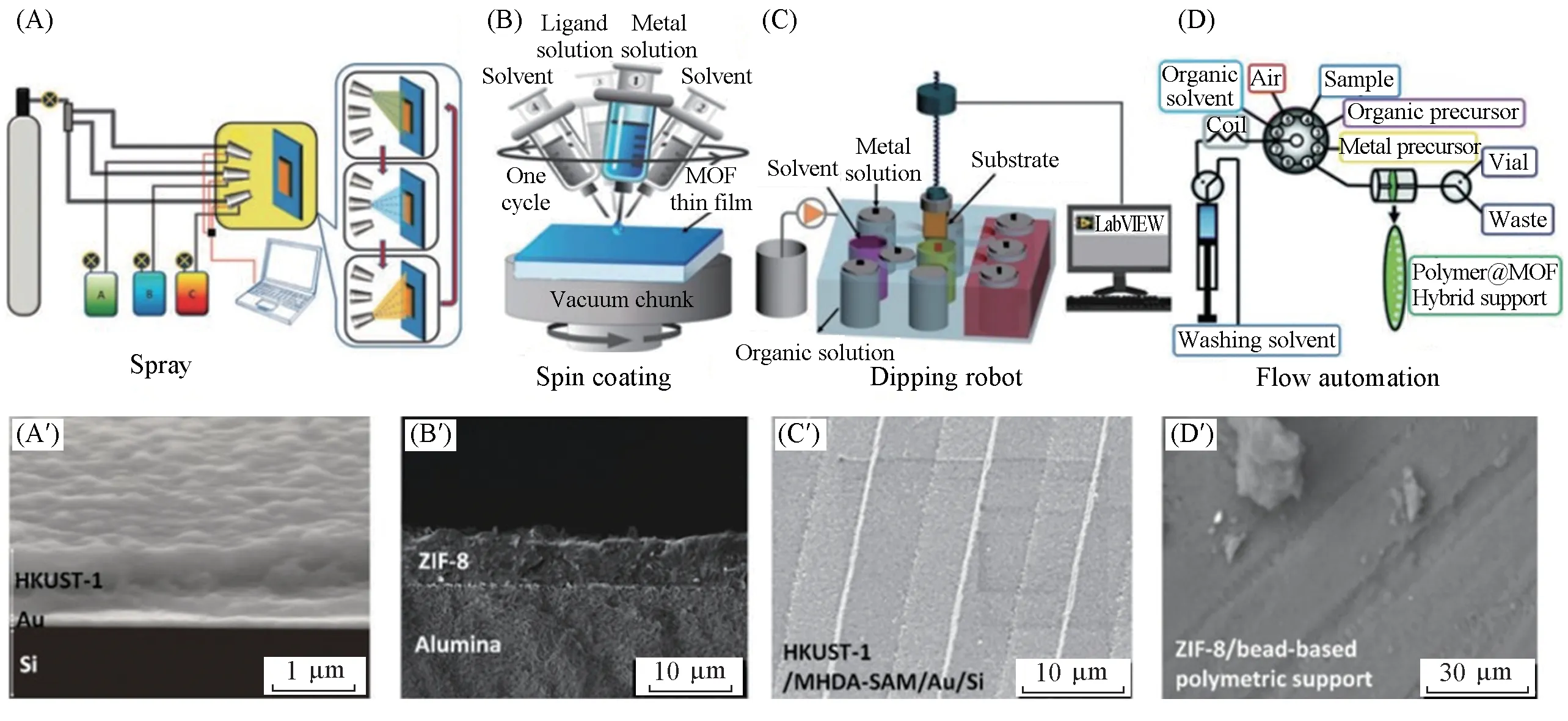
Fig.1 Established setups for the fabrication of SURMOF thin films by utilizing LBL assembly(A―D)and the resulting SURMOF thin films onto various substrates with corresponding setups(A′―D′)
During the LPE process illustrated in Fig.2(A),the substrates are sequentially immersed in the precursor solution of metal ions and organic ligands several times[37].Between each immersion step,the sample is rinsed with the solvent to remove the uncoordinated precursors.Rigid MOFs must be attached to a porous substrate with a certain mechanical strength for subsequent separation applications.In some cases,different functional groups of the modified substrate can regulate the growth orientation of the MOF films[Fig.2(B)]or adjust the density of the functional group to form a perfectly oriented MOF films[28].The LPE process is beneficial to grow oriented,continuous and homogenous MOF thin films with single growth orientation through restricting the assembly onto the substrate surface to enhance the heterogeneous nucleation of MOFs.
Beinet al.[28]grew SURMOF HKUST-1[Cu3(BTC)2]films on modified gold surfaces through a layerby-layer LPE fabrication methods.The OH- and COOH-terminated self-assembled monolayers(SAMs)on modified gold surfaces were discussed and had different growth directions during the growth of MOF films.During the fabrication process,the gold substrates were modified with HS(CH2)10CO2H or HS(CH2)10CH2OH monolayers and placed in a clear Cu3(BTC)2crystallization solution.Considering the geometric differences ofcoordination structure,highly ordered HKUST-1 thin films with different crystal orientations were obtained on the -CO2H- and -OH functionalized SAMs.Fig.2(B)showed a schematic illustration that the HKUST-1 film grew on the OH-modified surface oriented along the[111]direction and on the COOH-SAMs oriented along the[100]direction.
Fischer and coworkers[36]demonstrated the growth of HKUST-1 films on functionalized substrates in 2005.The gold substrate was modified by using SAMs of 16-mercaptohexadecanoic acid and 1H,1H,2H,2H-perfluorododecane thiol to make the Au(111)surface contain abundant carboxyl groups.Then the modified gold substrate was immersed into a MOF-5 supersaturated reactant mixture of Zn(NO3)2·4H2O and terephthalic acid(BDC),which could effectively anchor the precursor unit of MOF-5([Zn4O(BDC)3])on the substrates,and then successfully obtained continuous MOF-5 films[shown on Fig.2(C)].The AFM imaging of the crystallites demonstrated that the MOF-5 thin films could attach to the surface well even after repeated washing with ethanol.
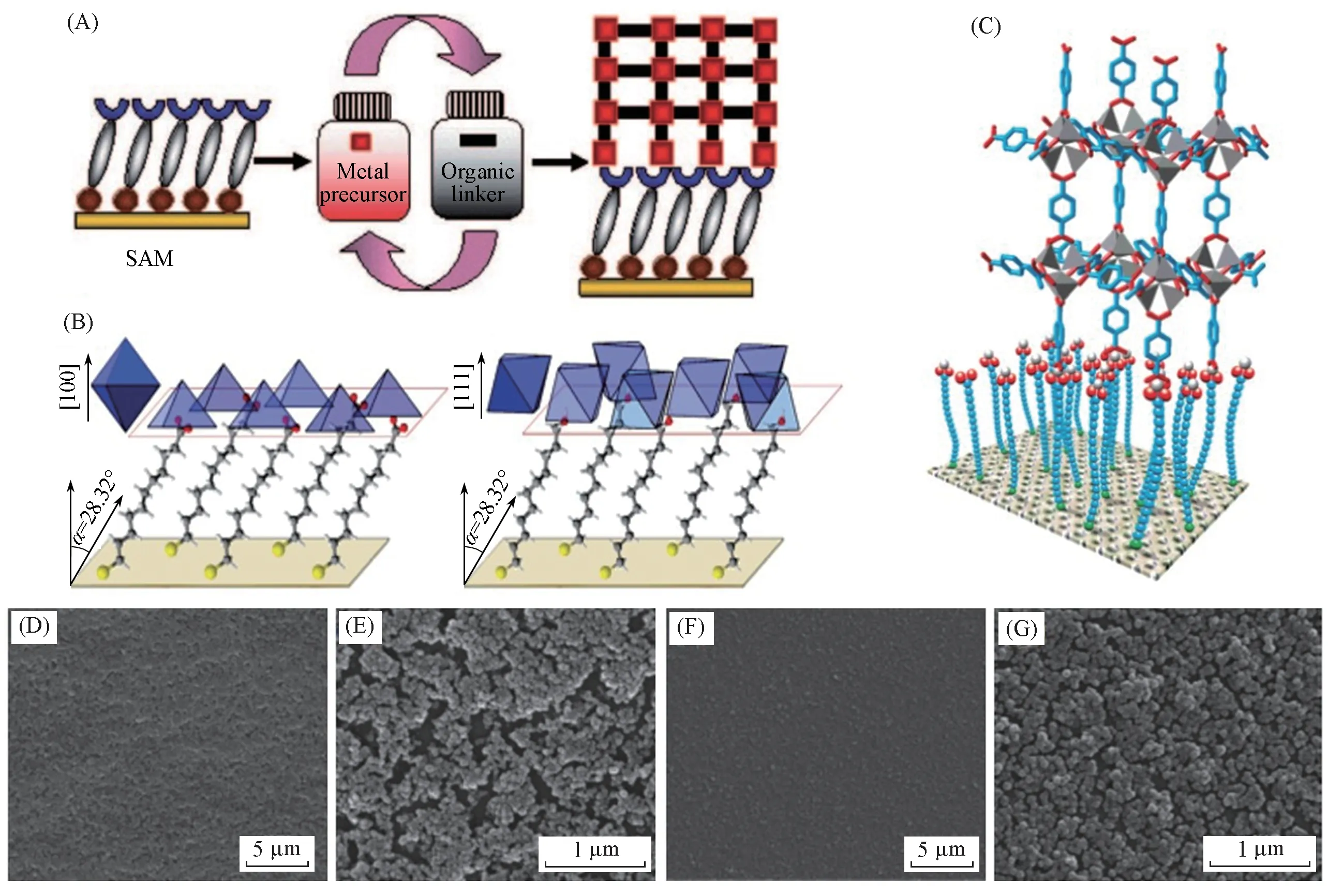
Fig.2 LPE approach and LB method for fabricating MOF thin films
Langmuir-Blodgett(LB)method is another interesting approach of LBL assembly technique for MOF membranes fabrication,which was firstly applied to constructing highly oriented MOF membrane based on metalloporphyrin building units and metal ions by Kitagawa and co-workers[38]in 2010.This fabrication process allows the preparation of MOF monolayers at the phase interface and the subsequent deposition of films through weak intermolecular forces,such asπstacking between reactant groups,assembling thin films onsubstrate[12].Based on Langmuir-Blodgett devices,the thickness of MOF thin films could be precisely controlled by varying the number of LB deposition periods.Gasconet al.[39]constructed and characterized the LB film of MIL-101(Cr)on quartz crystal microbalance(QCM)substrates.During this process,MIL-101(Cr)nanoparticles synthesized from CrCl3·6H2O and H2BDC in microwave route were spread into different mixture solvents of chloroform and methanol[solvents with different ratios and sometimes with benzoic acid(BA)as modulators],and the MIL-101(Cr)monolayers were obtained at the air-water interface.Then the LB monolayers were transferred onto solid substrates(glass,quartz,silicon)for depositing and stacking of MOF films.The resultant LB films MIL-101(Cr)could be generated with compact and dense monolayers by optimizing the solvent proportion and the appropriate volume of BA[Fig.2(D—G)].
1.2 Vacuum-based Fabrication:CVD,ALD
At present,most of the explored methods for preparing MOF thin films involve solvothermal chemical reactions route in organic systems.However,solution-based MOF thin film manufacturing has some limitations including the potential contamination of the synthetic solution during the manufacturing process.To overcome this drawback,vacuum-based fabrication technology,including chemical vapor deposition(CVD)and atomic layer deposition(ALD),has been explored.In this case,two kinds of vacuum-based fabrication technology are attentively discussed.
CVD is a cornerstone fabrication technology that relies on the reaction of vaporized substance and substrate to form uniform thin films[40].This method of deposition expands optional substrate range and improves the accurate maneuverability for morphology and thickness of fabricated thin films,and can be used to prepare thin film with definite requirements on curved surfaces or inside pipes.Ameloot and coworkers[40]firstly used the CVD method to fabricate ZIF-8 membrane with controllable and uniform thickness coating on silicon pillar arrays in 2016.In 2019,they[41]reported highly oriented CuBDC and CuCDC membranesviaCVD method based on Cu(II)and organic linkers H2BDC and H2CDC(trans-1,4-cyclohexanedicarboxylic acid),respectively(Fig.3).This MOF membrane synthesis method consisted of two CVD steps:first,Cu or CuO films were deposited onto silicon or glass substrates as a metal sourceviaphysical vapor deposition(PVD),and then the vapor-solid reaction step placed the Cu- or CuO- coated substrates in vaporized organic linker for 16 h at 200 ℃ for growing MOF films[Fig.3(A)].The resulting CuBDC and CuCDC films are the first crystalline and oriented MOF films deposited completely from the vapor phase,which were demonstrated by synchrotron grazing-incidence X-ray diffraction(GIXRD).The attenuated total reflection Fourier-transform infrared spectroscopy(ATR-FTIR spectroscopy)was used to confirm the(100)orientation of CuBDC and CuCDC films.Both of them had pores perpendicular to the surface and were therefore ideal for accessing guest molecules[Fig.3(B)].Then SEM images exhibited that homogeneous CuBDC and CuCDC thin films were obtained[Fig.3(C)—(D)].
ALD consists of repeated deposition processes on the substrate for several times of a volatile metal precursor layer and a subsequent volatile organic linker layer to grow the MOF thin films.The growth of heterogeneous nucleation on the substrate for fabricating MOF membranes can be promoted by forming the metal oxide layer on the substrate in the first step as the nucleation layer.Comparing with CVD,ALD can achieve more steerable growth of the film with atomic/molecular layer thickness precision based on a continuous atomic/molecular layer-by-layer manner.Karppinen and coworkers[42]prepared a Ca-BDC MOF thin film with the assistance of ALD in 2016.During the fabrication process of Ca-BDC thin film,the metal precursors Ca(thd)2(thd:2,2,6,6-tetramethyl-3,5-heptanedione)and organic ligands BDC were kept in the reactor as volatile precursors respectively to generate a MOF monolayer on the substrate.The resultant Ca-BDC film thickness could be precisely controlled by adjusting the cycle times of ALD[Fig.4(A)].Finally,MOF thin film with “dry”Ca-BDC structure(space groupC2/c)was prepared.Under notably humid conditions,the crystal structure of the thin film was transformed into a trihydrated Ca-BDC(space groupP21/c)due to the water absorption.Both crystal structures were 3D coordination networks formed by the coordination of calcium atoms with organic moleculesviaoxygen atoms[Fig.4(B)].
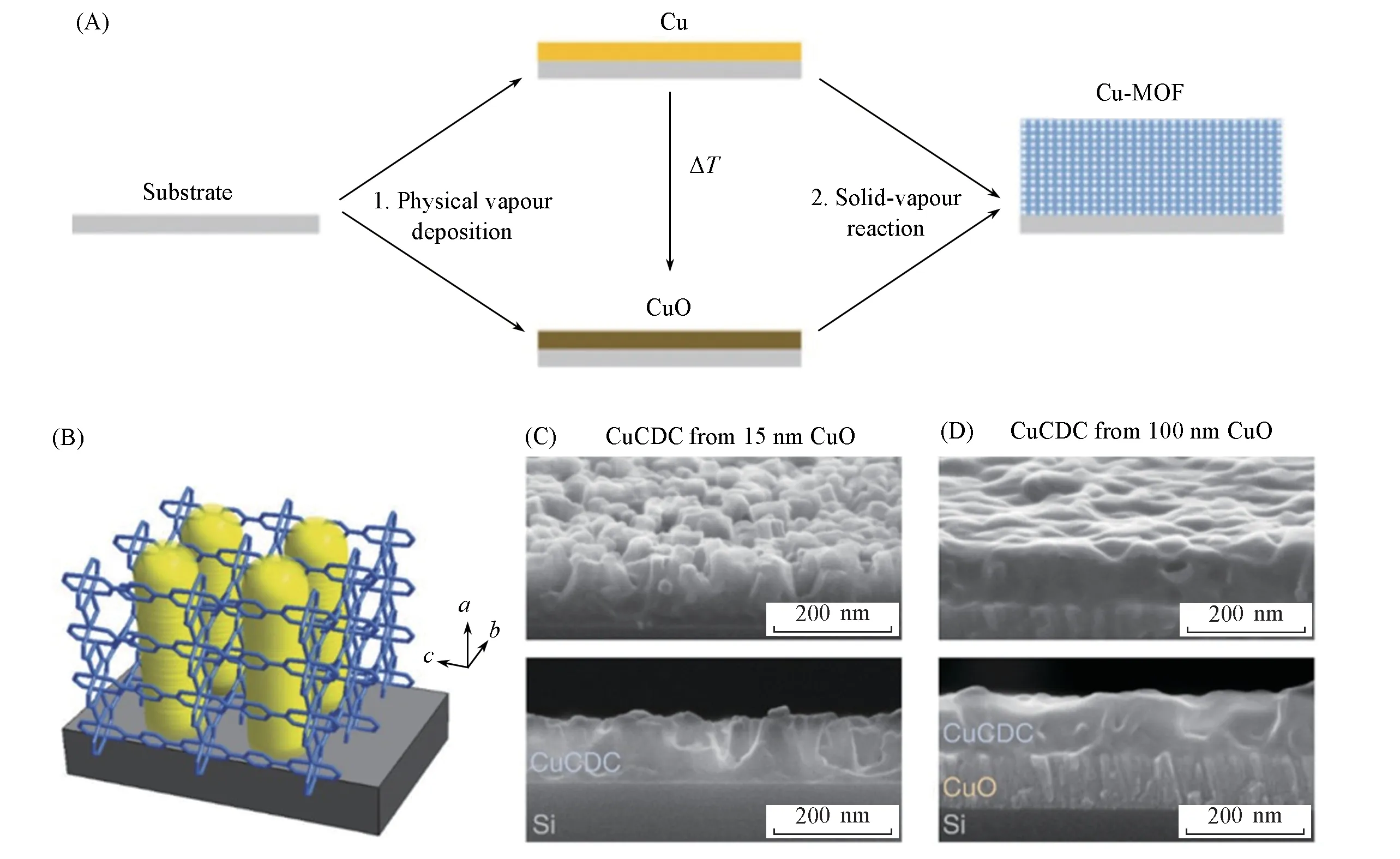
Fig.3 Chemical vapor deposition of CuBDC and CuCDC films[41]
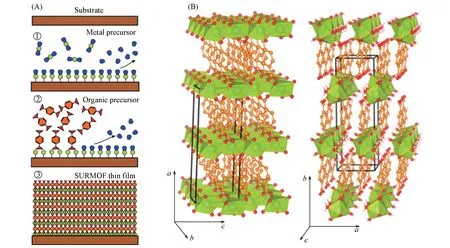
Fig.4 ALD process of fabricating Ca-BDC MOF thin films[42]
1.3 Electrochemical Deposition
The electrochemical methods are a kind of fabrication technologies based on electric power as a driving force to produce MOFs thin films,avoiding the use of salts and reducing the synthesis time,which can be widely utilized in shaped coatings on electrodes of essentially any geometric and areal surface andin siturenovating of defects such as cracks and pinholes during film deposition process only at the electrode-solution interface without other instrumental setups.They are divided into anode deposition,cathode deposition,and electrophoretic deposition according to different routes of MOF film growth[Fig.5(A)—(C)].
The anode electrochemical method is based on the delivery of the metal ions electrochemically by anodic dissolution.During the anodic fabrication process[43][Fig.5(A)],metal ions are generated by anodic dissolution of metal substrate and could react with organic ligands in an electrolyte solution,resulting in the growth of MOF film on the anode.Various MOF films have been prepared on metal substrates by anodic electrochemical deposition,such as Zn(TPTC)on Zn substrate[44][Fig.5(D)],MIL-100(Fe)on Fe anodes[45],and HKUST-1 on Cu electrodes[46].

Fig.5 Electrochemical deposition methods of MOF thin films
As for the cathodic deposition method[43][Fig.5(B)],during the fabrication process,metal ions in electrolyte solution firstly form metal oxide films on the cathode surface and then deprotonated organic ligands near the cathode react with metal ions attracted by the negative potential,forming MOF films on the substrateviaan oxide-hydroxide intermediate.Taking MOF-5[Zn4O(BDC)3]thin film as an example[47],the key of MOF-5 thin film fabrication was to form a partial alkaline zone near the cathode.Then the fabrication of MOF-5 thin films were introduced by the reaction of metal ions and deprotonated organic ligands[Fig.5(E)].
The electrophoretic deposition method occurs in a system with direct-current(DC)electric field as force and nonpolar suspension containing the surface-charged MOF particles as an electrolyte solution[48],triggering particle transport and deposition onto a conductive substrate to form MOF thin films[Fig.5(C)].Farha and coworkers[47]fabricated HKUST-1,Al-MIL-53,UiO-66,and NU-1000 membranes by depositing MOF particles on conductive fluorine-doped tin oxide(FTO)substrates[49][Fig.5(F)].
1.4 Powder MOF-based Deposition
The preparation method of thin films based on powder MOF is also a kind of common strategy,including spin coating,dip coating andin situdeposition.Powder MOF modified substrates by uniformly MOF nanoparticles can be used to provide nucleation sites,and then immerse the modified substrates into the mother solutions for the secondary growth of MOF thin films to solve the problem of low nucleation rate on the substrate surface in thein-situdeposition method.At present,the methods of MOF seed modified on substrate mainly include physical crystallization method[50],reaction crystallization method[51],and microwave-assisted crystallization method[33].
Spin coating or dip coating strategies can be used to obtain MOF membranes through directly depositing the pre-synthetic powder or suspension of MOF particles onto the substrate.The spin-coating or dip-coating route achieves controllability of the continuous MOF film thickness by tuning the number of coating cycles and adjusting the concentration of MOF nanoparticle suspension.The obtained films possess a hierarchical structure constructed by the micropore from the MOF itself and the mesopore from the agglomeration of MOF nanoparticles,leading to convenient diffusion and high permeation of guest molecules through MOF thin films.And mixed matrix membranes can be fabricated using the MOF particle suspension containing other particles suspension during the coating route.Qiu and coworkers[52]synthesized a MOF-based mixed-matrix membrane using electrospinning deposition.The schematic illustration of the experimental electrospinning process was shown in Fig.6(A).The nanoparticles of ZIF-8 crystals were synthesized through the solvothermal route of MeOH solution with Zn(NO3)26H2O and mIm at 150 ℃ for 5 h.Then the electrospinning solution consisted of mixed suspension of ZIF-8 and PVP(polyvinylpyrrolidone)was added into MeOH and then the solution was loaded into a syringe for electrospinning.The ZIF-8/PVP composite fiber coating was formed when the solutions were sprayed on the substrate by applying a voltage[Fig.6(B)and(C)].The thickness of the ZIF-8 layer could be precisely mastered by adjusting conventional solvothermal treatment.
In situdeposition is the most straightforward method for fabricating MOF thin films through immersing the substrate into the mother solutions following the published recipe and then heating reaction system as usual solvent-thermal synthesis for a while for the nucleation,crystal growth and intergrowth.Caoet al.[53]fabricated continuous Co-TBTC MOF thin films based on the flexible ligand H3TBTC(1,3,5-tris[4-(carboxyphenyl)oxamethyl]-2,4,6-trimethylbenzene)and Co(NO3)2·6H2O onα-Al2O3substrates byin situgrowth under solvent thermal conditions[Fig.6(D)and(E)].The flexible H3TBTC ligand could interface withα-Al2O3substrate with different conformations in different preparation conditions before coordinated with metal ions.By changing the pre-activation temperature of the substrate,the morphology of the Co-TBTC microcrystals of membrane could be obtained with taper-shaped microcrystal(80°C),sheet-shaped microcrystal(120°C)and thin plateshaped microcrystal(160°C)[Fig.6(F)—(I)].
1.5 Conclusions
The selection of fabrication methods for MOF thin films depends on the targeting applications(Table 1).The surface modification of substrates is vital to solving the interfacial interaction problem during the LBL process.Meanwhile,the different functional groups located on substrate cause MOF crystals membranes with diversiform orientation.Vacuum-based fabrication technology including CVD and ALD can effectively avoidsolvent contamination and liquid waste,achieving solvent-free,seed-free,precisely thickness-controllable and nano-level homogeneous thin film fabrication.Electrochemical deposition methods are the optimal method to address the interface problem between MOFs and electrode surfaces.Meanwhile,highly intergrown MOF layers can be obtained on the unmodified substrates using an external electric field to guide the nucleation deposition of MOF especially conductive MOFs.The powdery MOF-based deposition methods provide the possibility of producing large scale films with unlimited rigidity on the substrate using advanced technique.Therefore,porous MOF thin films can be obtained through direct constructing MOF structure during the deposition process,or pre-synthesizing MOFs powder followed with material processing route for film fabrication.
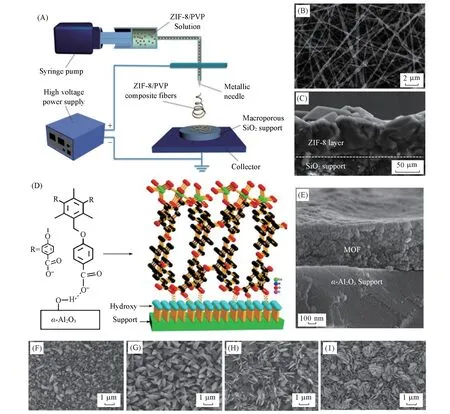
Fig.6 Powder MOF-based deposition for MOF membranes
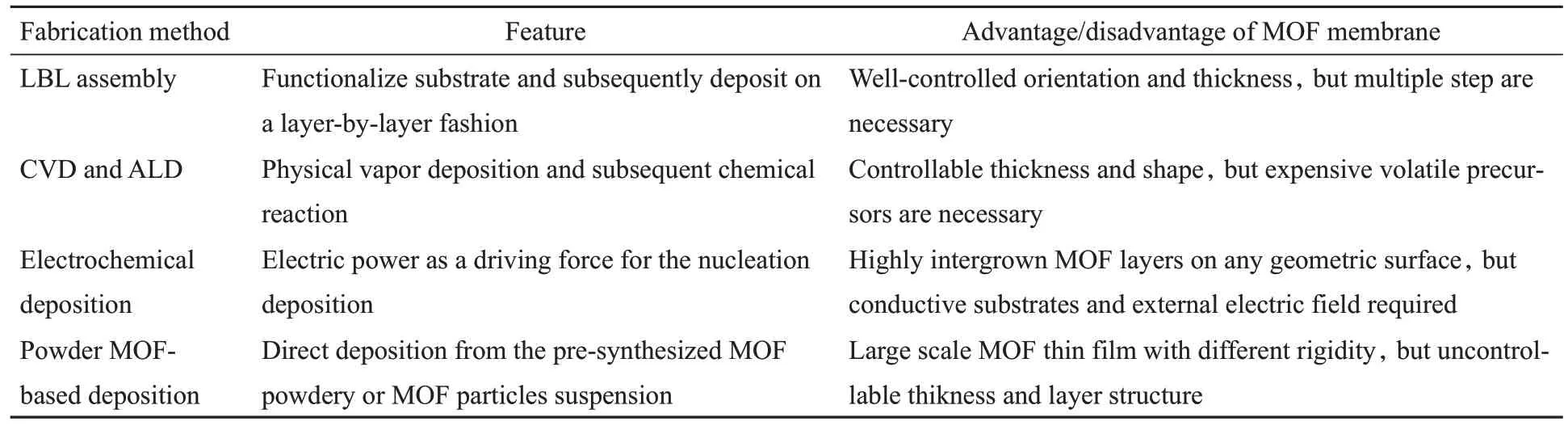
Table 1 Various fabrication methods for MOF membranes
2 Applications
Owing to various characteristics,such as homogeneity,smoothness,coarseness,thickness,as well as physical and chemical properties,MOF thin films fabricated by the above methods can be applied in various fields.In this section,we focus on the small molecule separation based on MOF film materials.With the rapid and continuous development of MOF thin films field,these materials have been widely used in various environmental separation and energy-related transmission processes due to their porosity,high surface area,tunable structure/pore size,various functionalities and low energy power consumption.According to the different separation targets,the applications of MOF membranes are mainly divided into gas separation,liquid separation,and ion/proton-conduction(Table 2).MOFs membranes achieve gas-phase separation usually based on different gas diffusion coefficients and the sieving effect[54-57].The combination of structural diversity,selective permeability and adsorption affinity of MOF membranes account for the liquid separation[58-60].Surface characteristics such as morphology and functional groups on MOF structure and the properties of molecules introduced into MOF membranes play a significant role in determining MOF membranes performance in ion/proton-conduction,including transport properties,sieving effect and selective penetration[61-64].This part will present some recent advances of MOF membranes in these applications.

Table 2 Principle of MOF membrane applications for separation
2.1 Gas Separation
With the development of gas separation technology,porous MOF membranes have been more and more applied in gas purification and separation field due to the advantages of low cost and high efficiency compared with traditional separation technologies.The desired separation membrane materials should possess good gas permeation parameters related properties such as free volume,diffusivity,thermodynamic sorption parameters,especially selectivity and permeability as key factors to limit the application for gas separation.The gas separation performance of the MOF membrane is mainly determined by the molecular sieving effect based on the host-guest interactions between the frameworks and the gas molecules influenced by the inherent property of MOF block materials and membrane state(pore size,homogeneity,smoothness,coarseness,thickness)related with fabrication technologies.Therefore,the separation efficiency of MOF membrane is mainly related to the intrinsic structural properties and varies with the preparation conditions[65-67].
ZIF-90([Zn(ICA)2],ICA=imidazolate-2-carboxaldehyde)has a pore diameter of 0.35 nm,which isbetween the kinetic diameters of H2(0.29 nm),N2(0.36 nm)and CH4(0.38 nm),having potential applications in H2purification[68].Caro’s group[50]prepared the continuous ZIF-90 membrane on a modified porousα-Al2O3substrate with excellent H2permselectivity.Because the substrate modified with an organic molecule has been demonstrated to increase heterogeneous nucleation and growth,the ZIF-90 membrane was prepared by using 3-aminopropyltriethoxysilane(APTES)as the covalent linkers between ZIF-90 crystal layers and Al2O3substratesviathe imines condensation reaction between free aldehyde groups and amine groups[68].During this preparation process,the ethoxy groups of APTES reacted with the hydroxyl group on the Al2O3surface,followed by the imines condensation reaction of the amino groups with the aldehyde groups of ICA[Fig.7(A)].Then thenucleation and crystal growth began and the modified Al2O3surface was completely covered with a well-grown ZIF-90 layer after the solvothermal reaction at 100 ℃ for 18 h[Fig.7(B)].The single gas permeance through this activated ZIF-90 membrane was shown in Fig.7(D).It was found that the separation factors of H2/CO2,H2/N2,H2/CH4and H2/C2H4were 7.3,11.7,15.3 and 62.8,respectively,and the H2permeability was about 2.4×10-7mol/(m2·s·Pa)at 200 ℃,1 kPa,indicating that the ZIF-90 membrane had excellent H2separation performance.
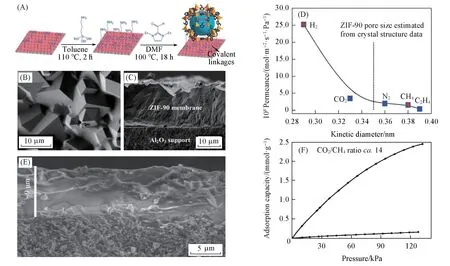
Fig.7 Gas separation of MOF membranes
Carreon’s group[69]fabricated continuous and reproducible ZIF-8 membrane located on modified tubularα-Al2O3using secondary growth method and exhibiting promising separation capacity of CO2and CH4.After the hydrothermal seed provided nucleation sites for the membrane growth,ZIF-8 membrane was synthesized byin-situgrowth on theα-Al2O3tube.The membranes coated with eight-layer ZIF-8 was about 9 μm thick[Fig.7(E)].The CO2adsorption was about 14 times more than CH4at 100 kPa[Fig.7(F)].It was found thatZIF-8 film could effectively separate CO2/CH4at 22 ℃,139.5 kPa and the permeability of CO2could reach 2.4×10-5mol/(m2·s·Pa),indicating that the prepared ZIF-8 membrane not only had good selectivity for CO2and CH4,but also had a high permeability of CO2.
2.2 Liquid Separation
In order to solve the environmental challenges of water availability,membrane separation technologies such as ultrafiltration,nanofiltration and reverse osmosis have been developed and widely used in seawater desalination and sewage purification.Thanks to the great diversity in the structure and pore size tunability,adsorption affinity,and selective penetration,MOF membranes are great candidates for liquid separation.Great progress has been made in exploiting liquid separation membranes meeting application requirements in MOF field.Efficient MOF membranes have not only excellent selectivity and high permeability,but also chemical,water and thermal stability for practical application.
Liuet al.[70]found UiO-66 membrane with excellent stability on Al2O3hollow fiber support applied for water softening requisition,showing promising multivalent ion reactance(e.g.,98.0% for Mg2+,86.3%for Ca2+,and 99.3% for Al3+)when the osmotic pressure was 0.14 L·m-2·h-1·bar-1[Fig.8(C),1 bar=0.1 MPa].Thein-situsolvothermal synthesis method was used to prepare UiO-66 membrane on the outer surface of Al2O3hollow fibers placed vertically into a transparent precursor solution.The UiO-66 polycrystalline film was deposited by optimizing the recipe of mother solution,synthesis duration and temperature,and microstructure of Al2O3hollow fiber[Fig.8(A)and(B)].Recently,Honget al.[71]prepared a nanoscaled 2D Zn-TCP(Fe)membrane(pore size range:1.2—1.24 nm)made of iron porphyrin complex[TCP(Fe)]interacted with Zn2+for methyl red(MR)removal in aqueous environment,exhibiting great permeation rate(2120 L·m-2·h-1·bar-1)and ideal rejection ratio(98%).The fabrication of this polycation-regulated 2D Zn-TCP(Fe)membrane was presented in Fig.8(D).The 2D Zn-TCP(Fe)nanosheets were synthesized through soft template assistance and then the Zn-TCP(Fe)nanosheets suspension was added into the polycationic polymer aqueous solutions to prepare Zn-TCP membrane on nylon support for molecular separation.By contrast with other advanced membranes,the water permeation of Zn-TCP membrane was about 3 times higher than the GO membranes,and the energy consumption was lower than the commercial nanofiltration membrane about 3 orders of magnitude[Fig.8(G)].
2.3 Ion/Proton-conduction
During water purification process,MOF membranes should exclude ions but permeate water molecules.However,it is also essential to realize the accurate separation of ions in many applications,such as lithium ion recovery,proton conductivity and pollutant removal like heavy metal ions from wastewater.Because of porosity,high surface area,adjustable aperture and functionality,MOFs are great candidates for these purpose and MOF blocks have been demonstrated their potential in ion conductive application as a prototype in recent research.Considering the requirements of practical application,it is significantly challenging but important to fabricate MOF membranes for the effective application of proton conductivity and ion selectivity.
In the methanol fuel cells industry,in order to get desired proton exchange membrane(PEMs)for methanol fuel cells,it’s urgent to explore new membrane materials with suitable pore size and structural stability which allow for the rapid transportation of protons but inhibit the permeation of methanol during proton exchange process.Penget al.[61]proposed a new ultra-thin hybrid ZIF-8 membrane as a PEM for methanol fuel cells since ZIF-8 has a smaller aperture(0.34 nm)than methanol molecules(i.e.0.39 nm)which can dramatically reduce methanol permeability.The DNA//ZHNs(zinc hydroxide nanosheets) membrane prepared by filtering the mixture of ZHNs and single-strand DNA(ssDNA)onto AAO(anodic alumina oxide)substrate was immersed into mIm solution at room temperature to form DNA@ZIF-8 membrane[Fig.9(A)].Because the hydrophilic DNA was introduced,the proton conductivity was significantly increased in hybrid ZIF-8 membrane and had a remarkable value of 0.17 S/cm at 97%RH and 75 ℃.

Fig.9 Ultrathin MOF membranes for proton conductivity
To further improve the power density in methanol fuel cells,Penget al.[62]introduced zwitterion sulfobetaine methacrylate(SBMA)into a 500-nm thick ZIF-8 membrane.SBMA was firstly enveloped into ZHNs forming the ZHNs@SBMA membrane to prevent SBMA molecules from leaking.Then,well-growth SBMA@ZIF-8 film was obtained byin-situthermal polymerization through immersing ZHNs@SBMA membrane into mIm solution under solvent thermal conditions[Fig.9(E)].By introducing SBMA with cation and anion groups,the prepared ZIF-8 film achieved both high proton conductivity,hydroxide conductivity and high power density(113.77 and 12.65 mW/cm2,respectively)in methanol fuel cells for proton exchange membrane(PEMs)and anion-exchange membranes(AEMs)[Fig.9(F)].
Lithium ions are the pivotal component of lithium batteries,however,the high consumption of lithium batteries leads to lithium source shortage which is a urgent and challenging problem.To realize the renewability of lithium,researchers try to extract lithium ions from seawater as one of the effective lithium ions collection technologies,but some problem still exists in terms of the same valence and similar submicron ion radii of lithium ions and medium alkali ions in seawater.Recently,many advances have been made in the use of functionalized MOF thin films for lithium ions separation.Zhanget al.[63]fabricated ZIF-8 membrane on AAO substrate and realized ultrafast selective transport of alkali metal ions.Through the sieving effect between the uniform subnanopores and alkali metal ions,the Li+could be quickly selected and transit through the ZIF-8 membrane[Fig.10(A)],and the selectivities of ZIF-8 membrane for Li+/Rb+,Li+/K+and Li+/Na+(4.6,2.2 and 1.4,respectively)were far higher than traditional porous membranes(0.6—0.8)[Fig.10(D)].
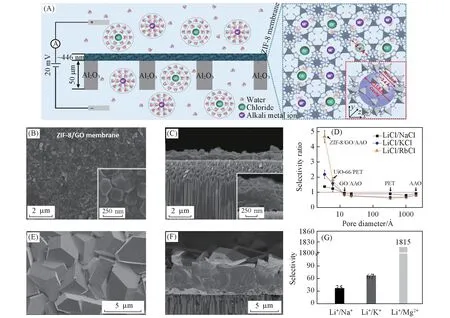
Fig.10 MOF membrane for selective transport of metal ions
In the process of fabricating MOF thin films,some strategies for improving separation capacity by introducing sulfonate groups have been used in lithium ion separation.For example,Guoet al.[64]reported a hybrid polystyrene sulfonate(PSS)/HKUST-1 membrane located on the AAO substrate through anin-situconversion process.PSS was firmly assembled on the surface of copper hydroxide nanofibers(CHNs)based on electrostatic interaction to prepare CHNs/PSS composite thin film through filtering the mixed solution of CHNs and PSS onto the AAO membrane.Then the CHNs/PSS composite thin film was immersed in H3BTC solution for the synthesis of PSS@HKUST-1 hybrid membrane.Changing PSS content in PSS@HKUST-1 thin films greatly influenced the Li+separation performance due to different affinities between PSS and these various alkaline earth metal ions.Therefore,optimal hybrid membrane was obtained which showed high Li+permeation rate(6.75 mol·m-2·h-1)and a desired selectivity for Li+/Na+,Li+/K+and Li+/Mg2+(78,99 and 10296,respectively)[Fig.10(F—H)].
3 Conclusions and Perspective
Desired MOF membranes should have high stability,excellent selectivity,and high permeability to achieve durable performance(long-term acid and alkaline resistance and antifouling ability)for separation and ion transportation.With the development of MOF membrane,great advance has been achieved on preparing efficient and sustainable MOF-based separation membranes for energy- and environment-related applications,including gas separation,liquid separation,and ion/proton conduction.The success in these areas now calls for a more intense research into the manufacture of MOF membranes.However,there still exist several challenges before being directly applied in industry and commerce.First of all,considering the complex relationship between defects and the material properties,the current fabrication methods still lack of morphological integrity,structural defects and satisfactory yield when preparing nanoscale-thick 2D MOF thin films,and even result in the failure to obtain the growth of crystalline structures.Therefore,it is highly desired to exploit more effective fabrication techniques for building membranes with controllable defects and large lateral size.
Moreover,exploration of more simple,universal and low-cost methods for preparing desired MOF membranes is still a challenging task.In view of the diverse MOF structures and components,alternative preparation technologies are desired for preparing ultrathin films based on multiple strategies to achieve synergistic separation effect.
In addition,the prospects offered by MOF-based separation membranes are fascinating since this multidisciplinary field is moving very fast and MOF membranes have achieved significant progress in previous studies.On the one hand,the SURMOFs prepared by the LBL method are oriented and flexible,having great potential to generate the superlattices composed of various types of MOF-layers lattices stacked in an optional order or other molecule lattices embedded in MOFs through LPE process with promising applications in the field of chemical detections[72].On the other hand,the emerging applications[73]of MOF membranes have gone beyond small molecule separation,particularly in particulate matters(PM)capture[74],toxic chemicals purification[75],organic solvent nanofiltration[76]and other high additional value separation processes.It is urgent to explore porous crystalline framework membranes including MOF,covalent organic frameworks(COF)and hydrogen-bond organic frameworks(HOF),and exploit other valuable applications in energy conservation and environment protection.Methodologies based on synthetic coordination chemistry,supramolecular chemistry and molecular topology about MOFs may be quite valuable for the modern separation membrane industry.In this respect,modifying the existing MOF membrane materials,searching for optimal conditions of film syntheses,and developing novel MOF membranes with conventional processing methods are long-lasting challenges for researchers.

