Insights into the Reaction Network and Mechanism of Green Aerobic Oxidative Esterification of Methacrolein over Different Heterogeneous Catalysts
Li Chenhao; Xia Changjiu; Liu Yujia; Huang Kaimeng; Peng Xinxin;Liu Jinsheng; Lin Min; Zhu Bin; Luo Yibin; Shu Xingtian
(State Key Laboratory of Catalytic Materials and Reaction Engineering, Research Institute of Petroleum Processing, SINOPEC, Beijing 100083)
Abstract: The oxidative esterification of methacrolein (MAL) is an important way to prepare high‐valued methyl methacrylate (MMA), but this process is ultra‐complex due to the high reactivity of both C=O and C=C bonds in MAL molecule.In order to further improve MMA selectivity, the reaction network and relevant mechanisms have been proposed and profoundly investigated in this paper.Five kinds of fundamental reactions are involved in this process, including (a)the acetal reaction; (b) the aerobic oxidation of hemiacetal; (c) the alkoxylation of C=C double bond; (d) the Diels‐Alder reaction; and (e) the hydrogenation reaction of unsaturated double bond.Among them, the Diels‐Alder reaction of MAL is non‐catalyzed, and the Brönsted acid sites or the Lewis acid sites favor promoting acetal reaction of MAL with methanol,while the alkoxylation of C=C bond with methanol is enhanced under alkaline condition.In particular, by employing the Pd‐based catalysts, hydrogenation products are formed in alkaline methanol solution, hence with lower than those obtained by the Au‐based catalysts.Notably, it is necessary to match the hemiacetal fromation and aerobic oxidation of hemiacetal,which is relevant with the amount and strength of acid and redox sites.Consequently, this work can provide a good guidance for the further design of both catalysts and processes in future.
Key words: methacrolein; oxidative esterification; mechanism; reaction network; methyl methacrylate
1 Introduction
Methyl methacrylate (MMA) is an important organic chemical intermediate, which is the specialty monomer of polymethyl methacrylate (PMMA)[1], and is also applied in many industries, including paints and coatings,electronics, PVC modifier, and bone inserts[2‐3].MMA production mainly lies in the ACH process[4‐5](named by the intermediate acetone cyanohydrin), the C‐2 routes(including the BASF process[6]and the Alpha process[7‐8])and the C‐4 route (two‐step oxidation process), which are based on light hydrocarbon raw material, as shown in Scheme 1.Notably, the former two routes suffer from serious environmental and economic drawbacks, such as the use of highly toxic chemicals (i.e.HCN) and the harsh conditions of transportation and storage of ethylene,which urgently need to be replaced by clean and efficient routes.Compared with them, the two‐step oxidation process, including the oxidation of isobutane to MAL and the aerobic oxidative esterification of MAL with CH3OH to MMA in oxygen atmosphere, markedly overcomes the above mentioned problems.The first step is easily to be carried out by a mature oxidation method already performed in the commercial scale[9], therefore, more attention is paid to the search for catalysts with great catalytic performance on oxidative esterification of MAL.Although the two‐step oxidation process has been already commercialized in an industrial scale, the low selectivity of target product still serves as a major challenge in catalyst development, due to the high reactivity of C=C and C=O bonds derived from the conjugated effect between them[10], with reaction network and mechanismbeing far beyond well understanding.The by‐products of the reaction in different literature reports are listed in Table 1, which can be divided into four classes, viz.:(a) the oxidative products of solvent methanol[11](with methyl formate (MF), etc., being beyond the scope of this article), (b) saturated products (acetals of MAL (ACE),isobutyl aldehyde (IBA), methyl isobutyrate (MIBA),3‐methoxy‐2‐methyl‐propanal (OMA) and isobutyric acid(IBAC)), (c) oligomerization products of MAL (DIM),and (d) decarbonylation to CO2and propene[12].The catalysts in Table 1 mainly cover the Pd‐ and Au‐based compounds, and the Au‐based catalysts generally exhibit higher activity than the Pd‐based catalysts.Furthermore,high molar ratio of methanol to MAL and high metal loading are still demanded for good product yield,resulting in high cost of the process.

Table 1 The reaction activity of oxidative esterification of MAL over different catalysts

Scheme 1 Industrial route for synthesis of MMA
It can be seen that both active components and supports have an obvious influence on product distribution.The point of our discussion here is the effect of additives and supports on catalytic performance of catalysts.Generally, a second doping metal and different support types can promote the oxidation ability of metal activesites through electronic effect and steric effect[13‐17].As for the impact of doping metal, electronic interaction between the active metal and the doping metal, which are often formed via the inter‐metallic compounds or core‐shell structure[18‐20], can strengthen oxidation capability of the active metal or inhibit side reactions.For example,Yamamatsu, et al.[9]discovered that after doping Pb the inter‐metallic compound Pd3Pb is formed and the decarbonylation reaction is markedly suppressed.Metal doping also improves the dispersion of the active metal and changes its particle size[21].Han, et al.[22]suggested that the preloaded Pb species not only can improve the dispersion of Pd precursors, but also can increase the number of active sites.Upon considering the influence of supports, their acid‐base property has an important impact on the reaction, which may promote the adsorption of reactants and the formation of intermediates, thus constituting a synergistic effect with the oxidation ability of the metal active sites[23].For instance, the group of Wan[24]disclosed that the enhancement of the intermediate formation by the high density of basic sites is probably the key reason for the superior activity of the Au/MgO catalyst.In addition, there is often a strong‐interaction between the metal and the support leading to the anchoring effect, which promotes the dispersion of the metal nanoparticles and reduces its size to an extent favoring the oxidation reaction[25‐27].The support can also influence the electronic atmosphere of the metal sites and change its valence state so as to promote its oxidation ability.Tsutsumi, et al.[28]found that there was a higher population of Au (I) on Au/CeO2‐nano and Au/CeO2-rod than on Au/CeO2‐cube due to a higher concentration of lattice defects in the former two supports.
Herein, we prepared two typical and easily available catalysts of high reactivity (Pd5Pb5/Al2O3and Au4CuO/Al2O3) which can readily exhibit the nature and characteristics of the Pd‐ and Au‐based catalysts.Moreover, we designed experiments on them to have a good grasp of the effect of different reaction conditions on product distribution and to have a comprehensive viewpoint on the reaction network and the key impact factors on both major reaction and side reactions, which can provide a good guidance on the development of catalysts and is of promising meanings on the further development of this green MMA manufacture route, in order to meet the vigorous demands in many areas.
2 Experimental
2.1 Chemical reagents
Palladium chloride (PdCl2), sodium chloride (NaCl),lead nitrate (Pb(NO3)2), sodium hydroxide (NaOH),hydrogen tetrachloroaurate (HAuCl4·3H2O), copper nitrate(Cu(NO3)2·3H2O), methanol, formaldehyde, and urea were purchased from the Damao Chemical Reagent Factory (Tianjin, China).MAL and MMA were obtained from the Aladdin Industrial Corporation (Shanghai,China).
2.2 Catalyst preparation
The Pd5Pb5/Al2O3catalyst was prepared using a conventional reduction deposition‒precipitation method with some adjustments.Firstly, PdCl2powder was dissolved in NaCl solution under stirring to form a tan Na2PdCl4solution.Al2O3support was added into the solution and was stirred for 1 hour at 70 ℃.Then Pb(NO3)2was added into the aforementioned mixture and was stirred for another hour.After that, NaOH was added to the suspension liquid and the color of the mixture quickly became black.After 15 minutes, HCHO solution was added into the mixture and reduction was carried out for 3 hours under rigorous stirring at 70 ℃.The resultant suspension was filtered, washed with deionized water until no Cl-ion was detected (by using AgNO3test), and was dried at 70 ℃ for 10 hours.Both the weight ratio of Pd and Pb to Al2O3in Pd5Pb5/Al2O3was 5%.
The Au4CuO/Al2O3catalyst was also prepared by a deposition‒precipitation method similar to that referred to in the literature[30]with necessary modifications.Firstly,Cu(NO3)2was impregnated into Al2O3by conventional impregnation method.The obtained blue powder after calcination was labeled as CuOx/Al2O3.Then CuOx/Al2O3was added to HAuCl4solution and urea was used as the precipitator at an Au/urea molar ratio of 1:100.The obtained mixture was transferred to an oil bath and stored in the dark under rigorous stirring and refluxing for 4 h at 90 °C.The resultant suspension was filtered, washed with deionized water until no Cl-ion was detected, and wasthen dried at 80 ℃ for 10 hours.Then the gold powder was put in the muffle furnace and was subjected to calcination at 300 ℃ for 4 hours.The weight ratio of Au to Al2O3in Au4CuO/Al2O3was 4% and the weight ratio of Cu to Al2O3was 1%.
2.3 Catalytic performance evaluation
Direct oxidative esterification of MAL was conducted in a 25‐mL glass reaction tube with a gas vent on the tube wall.In a typical reaction, the reaction tube was filled with 2 mmol MAL (0.14 g), 10 mg of catalyst,and 2 mL of methanol.Then the tube was sealed with a rubber stopper and was placed in a stirring heater for conducting the reaction.The gas vent was connected with a O2balloon (0.1 MPa).The reaction was carried out for 2 hours under stirring at 60℃.After the reaction,the reaction mixture was then centrifuged, with the supernatant liquor collected.Analysis of the obtained liquor was performed using a gas chromatograph (GC,Agilent 7890 A) equipped with a flame ionization detector and a column.The correction factors of commercial available compounds were determined by an internal standard method.The reaction activity is expressed as conversion rate and selectivity based on the amount of substance.
3 Results and Discussion
3.1 Product distribution and reaction network
As shown in Table 2, apart from major MMA product,several kinds of byproducts, including hemiacetal (HMA),DIM, oxidation products of DIM (DIO), OMA, ACE,isobutylene alcohol (IBEO), isobutanol (IBO), MIBA,IBA, and methyl 3‐methoxy‐2‐methyl‐propionate (MOM),are detected by GC‐MS spectroscopy.And it is observed that different catalysts and pH values have significant impact on the product selectivity and distribution.In the absence of heterogeneous catalysts and additives(entry 1), the selectivity of DIM and ACE is 86.8% and 13.2%, respectively, which is ascribed to the non‐catalytic dimerization of MAL via the Diels‐Alder reaction and acetal reaction of MAL with CH3OH at a relatively high temperature.When adding HCl (entry 2), the MAL conversion was significantly enhanced to 100% with a high ACE selectivity of 86.9%, suggesting the promotion of acetal reaction by the Brönsted acid.In contrast, under alkaline conditions (entry 3), the product is mainly OMA produced by the addition reaction of C=C bond of MAL with CH3OH (alkoxylation reaction) and DIM.Upon adding 10 mg of catalyst to the resulting mixture after the reaction of entry 3 (entry 9), it can be found that oxidative esterification of OMA to MOM is catalyzed by the catalyst showing the transformation of MAL into MOM via the alkoxylation of C=C bond firstly followed by the oxidative esterification of carbonyl group.In the presence of the Pd‐Pb based catalyst (entry 4), the target product MMA appears, indicating the aerobic oxidation capability of metal active sites.Noteworthily, when 0.3 mL of CH3OH solution saturated with NaOH was added (entry 5), the MAL conversion and the MMA selectivity was obviously improved but other side‐products, such as MIBA, MOM, and DIO were obtained via the aerobic oxidative dehydrogenation.In addition, after adding a certain amount of K2CO3, the hydrogenation and alkoxylation of MAL are more favored, resulting in the formation of IBO, MIBA, IBA, OMA and MOM, as shown in entry 6.For entries 5 and 8 depicted in Table 2, it can be seen that after the substitution of solvent methanol by acetonitrile (aprotic solvent), the hydrogenation products(IBA and MIBA) are obviously inhibited, indicating that the hydrogenation is attributed to the interaction between CH3OH and the catalyst, although MAL conversion rate declines to an extent due to low concentration of CH3OH.As regards 1,4‐dioxane (entry 7), no goal product has been found and almost all the MAL has been transformed into DIM, demonstrating that the solvent plays an important role in the oxidative esterification reaction.When no CH3OH participates in reaction (entry 10),no product except DIM exists in the resulting mixture,revealing that MAL is not able to be directly oxidized to methylacrylic acid and the oxidation of MAL can only occur by the help of CH3OH probably via intermediate HMA.Furthermore, to investigate the effects of base on the product distribution, different amounts of K2CO3were added in the Pd5Pb5/Al2O3catalyzed system, as shown in Figure 1.When the molar ratio of K2CO3to MAL is 1.1%, a trace amount of IBEO was generated via the hydrogenation of the carbonyl group (not mentioned inFigure 1).It is observed that the selectivity of MMA is decreased from 43.5% to below 20%, while the selectivity of MIBA and IBO increases along with the increase of the molar ratio of K2CO3to MAL, demonstrating the conversion path from MMA to MIBA.In addition, the conversion of IBA into MIBA accouts for the decrease of IBA yield.In conclusion, the alkaline additive can accelerate the hydrogenation and alkoxylation reactions,which agrees well with these results presented in Table 1.When this reaction is catalyzed by Au4CuO/Al2O3under neutral condition (entry 11), the yield of MMA is much higher (69.5%) than those catalyzed by the Pd‐Pb‐based catalysts without base addition (entry 4), while DIO is the only side product, revealing the strong aerobic oxidation capability of the Au‐based catalysts.Furthermore, being different from the nature of Pd‐Pb based catalysts, the addition of base (entry 11) plays no role in facilitating the oxidative ability of Au4CuO/Al2O3but can promote the alkoxylation reaction.It can be seen in entries 13 and 14 that the catalyst to MAL weight ratio has a remarkable influence on conversion of MAL and selectivity of MMA.Interestingly, the batch feeding of MAL (entry 15) favors promoting the selectivity of MMA possibly because the adverse effect of low MAL concentration on the side reaction (D‐A reaction) is stronger than that on the main reaction (oxidation).

Figure 1 Selectivity variation of different products onincrease of base (K2CO3) addition
Consequently, based on the above results, the nature of metal active sites, the acidity or basicity of reaction media,the solvent type (protonic or aprotic solvent), the catalyst to MAL weight ratio and the reaction concentration of MAL all have influence on the product distribution of the oxidative esterification of MAL.Moreover, the reactions over different catalysts mainly involve five fundamental reactions, as shown in Scheme 3, including:(a) acetal reaction; (b) aerobic oxidation of hemiacetal;(c) alkoxylation of C=C double bond; (d) the Diels‐Alder reaction; and (e) hydrogenation reaction of C=C double bond/carbonyl group.It is widely accepted that the oxidative esterification of MAL proceeds via the hemiacetal reaction of MAL with methanol to HMA and oxidative dehydrogenation of HMA, which are also supported by our experiment.By the help of acid, HMA also tends to react with another methanol molecule to produce ACE,leading to a competitive relationship with oxidative dehydrogenation of HMA.In the presence of methanol and the Pd‐Pb based catalysts, hydrogenation reaction of unsaturated double bond is favored to generate IBA and IBEO.And IBO is obtained by further hydrogenation of either one of them.Alkoxylation of C=C bond of MAL with methanol gives OMA products under alkaline condition.DIM can be produced by the Diels‐Alder reaction of two MAL molecules under most conditions except in the strong protonic acid medium.Unfortunately, the side‐reaction of oxidation inevitably happens and the oxidative esterification of carbonyls of IBA, OMA and DIM occurs via the same way as MAL to generate MIBA, MOM and DIO respectively.Among them, the HMA formation and the oxidative dehydrogenation of HMA to MMA need to be promoted to gain high MMA yield, while other reactions should be suppressed to limit byproducts yield.In the next several chapters, mechanisms of all fundamental reactions are proposed and discussed for deeper understanding of the reaction network.

Scheme 2 Products of oxidative esterification of MAL under different conditions
3.2 Acetal reaction
HMA formation is the key process in oxidative esterification route, involving the addition of carbonyl groups with methanol[31‐32].Yet, HMA is likely toparticipate in subsequent acetal reaction or oxidative dehydrogenation giving birth to ACE or MMA respectively.As shown in entry 2 of Table 2, the introduction of HCl greatly promotes the generation of ACE with an 100% MAL conversion and a 78.2% ACE selectivity, indicating that the Brönsted acid plays an essential role in reactions.In general, the Lewis acid can also accelerate acetal reaction.Consequently, it is essential to control the amount and strength of acid sites in the catalyst to match the oxidative esterification capability for promoting hemiacetal formation and aerobic oxidation and inhibiting subsequent acetal reaction to the largest extent.

Table 2 Product distribution of oxidative esterification of MAL under different conditions
The mechanism diagrams of the acetal reactions of MAL over the Brönsted acid or the Lewis acid are shown in Scheme 4.As for the Brönsted acid catalytic route, the proton would first combine with the electronegative carbonyl O atom to activate the carbonyl C atom.Then,the hydroxyl O atom of methanol with lone pair electrons attacks the carbonyl carbon and the positive charge transfers to the H atom of hydroxyl group of methanol.In the next step, hemiacetal is produced along with the departure of proton.However, the hydroxyl O atom is easily to be attacked by another hydrogen proton, causing the formation of H2O and carbocation species.After the nucleophilic attack of methanol and the departure of proton,the acetal product is produced.It can be also catalyzed bythe Lewis acid following the similar reaction pathways as shown in Figure 3b, while its energy barrier is higher.

Scheme 3 Reaction network of aerobic oxidative esterification of MAL
3.3 Aerobic oxidation of hemiacetal
According to the above results depicted in Table 2, there is no MMA product obtained in the absence of catalysts,indicating the important role of aerobic oxidation capability of metal active sites for MMA formation.But the selectivity of MMA is relatively low, due to high reactivity of C=C and C=O bonds and the conjugation effect between them.In addtion, a small amount of Lewis acid accounts for the formation of HMA and we propose that it can also take part in the aerobic oxidation of hemiacetal.Based on the above results and other researchers’ work[28,33‐37], a comprehensive hemiacetal oxidation mechanism is proposed (taking HMA as example), as shown in Scheme 5.Firstly, the hydroxyl group of HMA is adsorbed by the Lewis acid site, thus the O‐H bond becomes weakened enough to react with latticeoxygen species easily.Then, the proton transfers from HMA to lattice oxygen species yielding a more reactive alkoxide, and another hydrogen connected with adjacent carbon interacts with the lattice oxygen species.As a result,the water molecule is formed leaving an oxygen vacancy and a MMA molecule is also released from the LA site.The oxygen vacancy is recovered by the chemisorbed oxygen and thus a catalytic process is finished.

Scheme 4 Brönsted acid (H+) and Lewis acid promoted mechanism of acetal reaction between MAL and methanol
3.4 Alkoxylation of C=C bond with methanol
The alkoxylation of MAL to OMA involving the 1,4‐addition of C=C bond with methanol[28,38], especially happens in the presence of alkaline additives, as shown in Table 2.The reaction mechanism of MAL alkoxylation is as follows.At the beginning, the hydroxyl oxygen of methanol with lone electron pair attacks positive carbon of MAL, which undergoes a conjugate transformation at first.And negative oxygen captures the proton yielding the hydroxyl species.Thus, OMA is obtained by keto‐enol tautomerism, as shown in Scheme 6.
3.5 The Diels-Alder reaction
Two notable side‐products including DIM and DIO are generated via the Diels‐Alder (D‐A) cycloaddition reaction in which MAL serves as both diene and dienophile.Electrophilic dienophiles with electron‐withdrawing groups is favored in the D‐A reaction, which means that MAL is a good dienophile[39].Hence, the D‐A reaction of MAL is performed easily to produce a six‐membered‐ring dimer[40],as shown in Scheme 7.Obviously, three kinds of electron shifts simultaneously happen in this reaction process: π electrons of C1‒C2 attack adjacent σ bond (C2‒C3) to generate a π bond (B bond); π electrons of C3‒O4 move to O4‒C5 to generate a σ bond (C bond); π electrons of C5‒C6 move to C1‒C6 to generate a σ bond (A bond).The D‐A reaction is a one‐step synergistic reaction without any intermediates and participation of ions, which suggests that the Brönsted/Lewis acid and base are unnecessary, in line with the results of Table 2.In addition, batch feeding of MAL plays a role in decreasing MAL concentration in the reaction mixtures, which is likely to reduce the probability of mutual collision of MAL molecules, thus significantly reducing the D‐A reaction rate and the selectivity of dimerization products.
3.6 Hydrogenation reaction of C=C bond/ carbonyl group
There are several hydrogenation products generated underthe influence of the Pd‐Pb based catalyst, rather than the Au‐based catalysts.The reason is that Pd species has high capability of hydrogen abstraction and hydrogen spillover,then favoring the transfer of active hydrogen species.As a result, the active hydrogen species would easily react with unsaturated C=C and C=O bonds of both MAL and MMA,giving birth to IBO, IBEO, IBA and MIBA.Judging from the above scenario (Chapter 3.1), it is concluded that the hydrogenation reaction is attributed to the interaction between CH3OH and Pd‐Pb catalyst with the aid of the alkali.The hydrogenation process is proposed, as shown in Scheme 8[41].
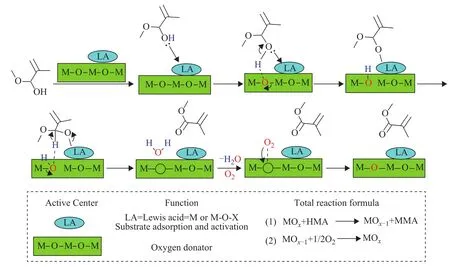
Scheme 5 The aerobic oxidation reaction mechanism of HMA to MMA
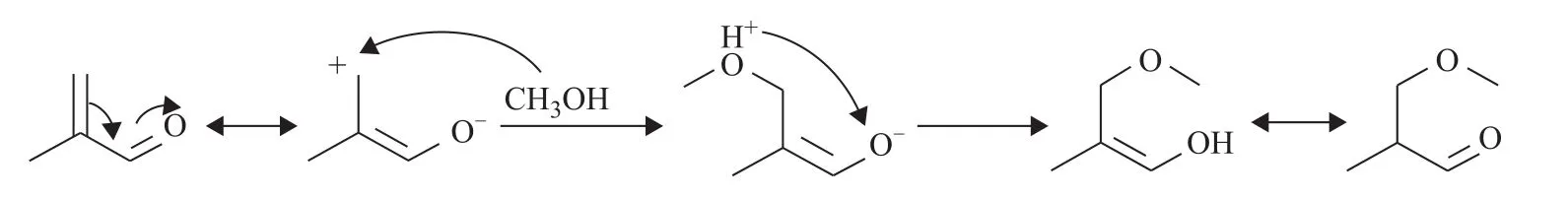
Scheme 6 Schematic of mechanism of MAL alkoxylation

Scheme 7 Schematic of the D-A reaction of MAL

Scheme 8 Schematic of hydrogenation of C=C and C=O bonds over Pd-based catalysts
4 Conclusions
Owing to the structural and electronic influence, the highly reactive C=C bond and C=O bond makes the target product selectivity relatively low in the oxidative esterification of MAL.In this paper, we focus on studying the reaction network and mechanism by designing experiments of different reaction conditions and evaluating the product distribution.It is confirmed that the nature of metal active sites, the acidity or basicity of reaction media, the solvent type (protonic or aprotic solvent), the catalyst to MAL weight ratio and the reaction concentration of MAL all have big influence on product distribution of the oxidative esterification of MAL.One of the five fundamental reactions, dimerization happens even without adding any catalysts, however, it can be obviously inhibited under acidic or alkaline conditions.Acetal reaction of MAL is promoted by acid, while alkoxylation and hydrogenation of MAL is facilitated in the presence of alkali.The catalytic oxidation ability of the Pd‐Pb based and the Au‐based catalyst allows for the oxidative dehydrogenation of hemiacetal of MAL, converting the hydroxyl group of secondary alcohol to carbonyl.As a conclusion, the synergistic effect between the Brönsted and the Lewis acid sites and lattice oxygen of catalysts results in oxidative esterification of MAL.Hence,it is of great importance to coordinate acid strength and the intensity of oxidation capacity to promote the synergistic effect between them, which is one of the key factors in obtaining high activity and MMA selectivity in the oxidative esterification of MAL.Inaddition, the selectivity of MMA catalyzed by the Pd‐based catalysts is lower than those catalyzed by the Au‐based catalyst because of considerable hydrogenation effect of Pd metal active sites on unsaturated double bonds in alkaline methanol solution.The present study can provide insights into the study of other highly active metal catalysts for the oxidative esterification processes to promote industrialization of new catalysts.
Acknowledgements:The authors acknowledge supports from the National Key Basic Research Development Plan “973” Project(2006CB202508), the SINOPEC Project (411058, 413025), and the National Natural Science Foundation (21808244).
- 中国炼油与石油化工的其它文章
- Study on Distribution of Electrocatalytic Reaction Efficiency in a Three-Dimensional Electrocatalytic Reactor
- Synthesis of Bimodal Mesoporous TiO2 -PTA/BMMS and Its Enhanced Performance in the Photocatalytic Oxidative Desulfurization
- Chemoselective Catalytic Hydrogenation of Nitroarenes Using MOF-Derived Graphitic Carbon Layers Encapsulated Ni Catalysts
- Controlling the Pore Structure and Photocatalytic Performance of the Flexible FeⅢ Metal-Organic Framework MIL-53(Fe) by Using Surfactants
- Preparation of Modified Enteromorpha-Immobilized Microbial Agent and Research on Diesel Removal Performance
- Acidity Evaluation of Industrially Dealuminated Y Zeolite via Methylcyclohexane Transformation

