Process Optimization of RTS Technology for Ultra-Low Sulfur Diesel
Ge Panzhu; Ding Shi; Xi Yuanbing; Zhang Le; Nie Hong; Li Dadong
(SINOPEC Research Institute of Petroleum Processing, Beijing 100083)
Abstract: The RTS technology can produce ultra‐low sulfur diesel at lower costs using available hydrogenation catalyst and device.However, with the increase of the mixing proportion of secondary processed diesel fuel in the feed, the content of nitrogen compounds and polycyclic aromatic hydrocarbons in the feed increased, leading to the acceleration of the deactivation rate of the primary catalyst and the shortening of the service cycle.In order to fully understand the reason of catalyst deactivation, the effect of mixing secondary processed diesel fuel oil on the operating stability of the catalyst in the first reactor was investigated in a medium‐sized fixed‐bed hydrogenation unit.The results showed that the nitrogen compounds mainly affected the initial activity of the catalyst, but had little effect on the stability of the catalyst.The PAHs had little effect on the initial activity of the catalyst, but could significantly accelerate the deactivation of the catalyst.Combined with the analysis of the reason of catalyst deactivation and the study of RTS technology, the direction of RTS technology process optimization was put forward, and the stability of catalyst was improved obviously after process optimization.
Key words: RTS technology; nitrogen compound; polycyclic aromatic hydrocarbons; catalyst deactivation; process optimization
1 Introduction
With the deterioration of crude oil quality and the increasingly strict environmental laws and regulations,the production of ultra‐low sulfur diesel has become the focus of current research.The RTS technology has been developed by the SINOPEC Research Institute of Petroleum Processing (RIPP) for producing ultra‐low sulfur diesel[1‐3], which has been applied in a number of industrial plants.The RTS technology is characterized by the removal of most sulfides, polycyclic aromatic hydrocarbons, and almost all nitrogen compounds in the first reactor at high temperature and high space velocity.The second reactor can remove residual sulfides and improve the color of prduct oil under the condition of low temperature and high space velocity.The RTS technology was originally designed to process the straight‐run diesel or 20% of secondary processed diesel mixed with a straight‐run diesel.However, in the actual industrial production process, the proportion of secondary processed diesel fuel oil is much higher than the design value, reaching 30%‒70% of the total feed, and the quality of feed is worse.The increase in the content of nitrogenous compounds, polycyclic aromatic hydrocarbons (PAHs), and refractory sulfides in the raw materials makes the processing more difficult, which can lead to a faster deactivation rate of the primary catalyst and a shorter running period.
At present, there are many studies on the effects of nitrogen compounds and polycyclic aromatic hydrocarbons on hydro‐desulfurization activity[4‐8].However, under the condition of long cycle operation,there are few studies working on the effects of nitrogen compounds and polycyclic aromatic hydrocarbons on the ultra‐deep hydro‐desulfurization activity and stability of catalysts, and no systematic understanding has been formed.Therefore, in combination with the existing problems of RTS technology, the effects of nitrogen compounds and PAHs on the operating stability of the catalyst were investigated by adding nitrogen compounds and PAHs into common diesel (straight‐run diesel mixedwith 20% of FCC diesel).Combined with the analysis of the causes of catalyst deactivation and the study of RTS technology, feasible optimization measures are put forward.The operating stability of RTS technology before and after optimization is compared comprehensively.This paper can provide some technical guidance information for the development of diesel ultra deep hydro‐desulfurization catalyst and the process in the future.
2 Experimental
2.1 Feedstocks
The common diesel fuel oil used in the test is obtained from a refinery, and the ratio of straight‐run diesel to FCC diesel is 4:1, which is labeled as the feedstock oil A.The properties of feedstock oil A are shown in Table 1.According to the literature review[9], the nitrogen compounds in FCC light cycle oil are mainly quinoline,indole and carbazole.Due to the low solubility of indole and carbazole in diesel, the feedstock oil A+BN was obtained by adding 350 μg/g of quinoline (measured by nitrogen content) to the feedstock oil A.Due to the high content of PAHs in FCC diesel fraction, which is mainly composed of bicyclic aromatics, naphthalene, and tricyclic aromatics.Therefore, the feedstock oil A+PAH is obtained by adding 3% of naphthalene, 12% of 1‐methylnaphthalene and 1.2% of phenanthrene to the feedstock oil A.

Table 1 Properties of feedstock oil A
Quinoline, naphthalene, 1‐methyl naphthalene, and phenanthrene are analytically pure products provided by the Sinopharm Chemical Reagents Co., Ltd.
2.2 Catalysts
The hydrofining catalyst used in the experiment was a Ni‐Mo‐W/γ‐Al2O3catalyst developed by the SINOPEC Research Institute of Petroleum Processing.The physical properties of catalysts are shown in Table 2.

Table 2 Physical properties of Ni-Mo-W/Al2O3 catalyst
3 Results and Discussion
3.1 Deactivation rate of catalyst
Under the conditions covering a hydrogen partial pressure of 6.4 MPa and a LHSV of 2.0 h‐1, the reaction temperature was adjusted with the target of producing ULSD.In order to analyze the deactivation rate of catalyst more accurately, according to literature research[10‐12], a reaction ordernof 1.2, an activation energyEaof 80 kJ/mol[13], and a sulfur mass fraction in the target product of 9 μg/g were used to normalize the actual reaction temperature.
Figure 1(a) and 1 (b) shows the changes in the operating stability of the catalyst before and after adding nitrogen compounds and PAHs to the feedstock.According to the temperature increase of reaction temperature in Figure 1,the phase with a running time of 48‒248 h was defined as the initial stage of operation, and the activity of catalyst in this stage was defined as the initial activity of catalyst.The period with a running time of 248‒448 h was defined as the early stage of operation.The period with a running time of 448‒1048 h was defined as the middle stage of operation.The target data of each stage in Figure 1 is the deactivation rate of the catalyst at this stage.
It can be seen from Figure 1 (a) that compared with processing oil A, the deactivation rule of the catalyst is basically similar after the addition of nitrogen compounds in the raw material.In both cases, the reaction activity loss of the catalyst is the most obvious at the beginning of operation, and then the catalyst gradually shifts from the initial rapid deactivation stage to the middle slowdeactivation stage.In the initial stage of operation,the reaction temperature increased obviously after the addition of nitrogen compounds, indicating that the addition of nitrogen compounds significantly affected the initial activity of the catalyst, but had a slight effect on the stability of the catalyst.This might be due to the strong competitive adsorption of nitrogen-containing compounds, and the addition of nitrogen‐containing compounds to the raw material has a strong inhibitory effect on hydrodesulfurization reaction, thus reducing the initial activity of the catalyst significantly[14‐15].However,after the initial stage of reaction, the active centers with strong adsorption capacity have been occupied, and the adsorption capacity is weakened, so that the competitive adsorption of nitrogen compounds is not obvious in the middle stage of reaction, resulting in a slight influence on the stability of catalyst.

Figure 1 Influence of nitrogen compounds or PAHs on the operating stability of the catalyst
It can be seen from Figure 1 (b) that the addition of PAHs to the raw material has little effect on the initial activity of the catalyst, and the reaction temperature of the catalyst in the initial stage of operation is increased by 1‒4 ℃.However, the addition of PAHs after the initial stage of operation significantly accelerated the deactivation of the catalyst, and especially the deactivation rate of the catalyst after the addition of PAHs in the middle stage of reaction was 5 times as much as that of oil A,indicating that PAHs were the main reason for the rapid deactivation of the catalyst.Therefore, during processing the low‐quality diesel fuel oil, the main reason for the rapid deactivation of the catalyst in the first reactor of RTS technology is the increased content of PAHs in low‐quality diesel fuel oil.
3.2 Study on the influence of process conditions of RTS technology
3.2.1 Influence of reaction temperature in the second reactor on RTS technology
In the actual industrial operation process, due to the increase of the proportion of secondary processed diesel fuel in the raw material, the operation of the first reactor of RTS technology is more rigorous, and the catalyst deactivation rate of the first reactor is accelerated, which leads to the problem of the mismatch between the first reactor and the second reactor.Therefore, it is very important to control the proper desulfurization depth of the first reactor and effectively play the role of ultra‐deep hydrodesulfurization in the first reactor.
Under the conditions covering a hydrogen partial pressure of 6.4 MPa, a LHSV of 2.0 h‐1in the first reactor, and a LHSV of 6.0 h‐1in the second reactor, coupled with a standard hydrogen/oil ratio, the influence of reaction temperature of the second reactor operating with RTS technology on the catalyst desulfurization activity was investigated by using different desulfurization depths in the first reactor.The test results are shown in Table 3.
It can be seen from Table 3 that when the reaction temperature in the first reactor of RTS technology is 340 ℃, the mass fraction of nitrogen at the first reactor outlet has been decreased to 3.2 μg/g, and the mass fraction of sulfur is 149 μg/g.The products of the first reactor can directly enter the second reactor without separation.When the reaction temperature of the second reactor was raised by 50℃ higher than the basic temperature, the sulfur content of the products still didnot meet the requirements for producing ULSD.When the reaction temperature of the first reactor was further raised to 355 ℃, the mass fraction of nitrogen and sulfur in the product at the first reactor outlet was 1.1 μg/g and 29.9 μg/g, respectively, and the reaction temperature of the second reactor was by 30℃ higher than the basic temperature to meet the requirements for producing ULSD.According to the above analysis, the first reactor has to reduce the sulfur mass fraction in raw material to less than 30 μg/g and the nitrogen mass fraction to less than 2.0 μg/g, whereas the reaction temperature of the second reactor is still by 30 ℃ higher than the basic temperature, so that the sulfur mass fraction in the product can be reduced to less than 10 μg/g, and the color of the product formed after the increase of the temperature in the second reactor would become significantly darker.
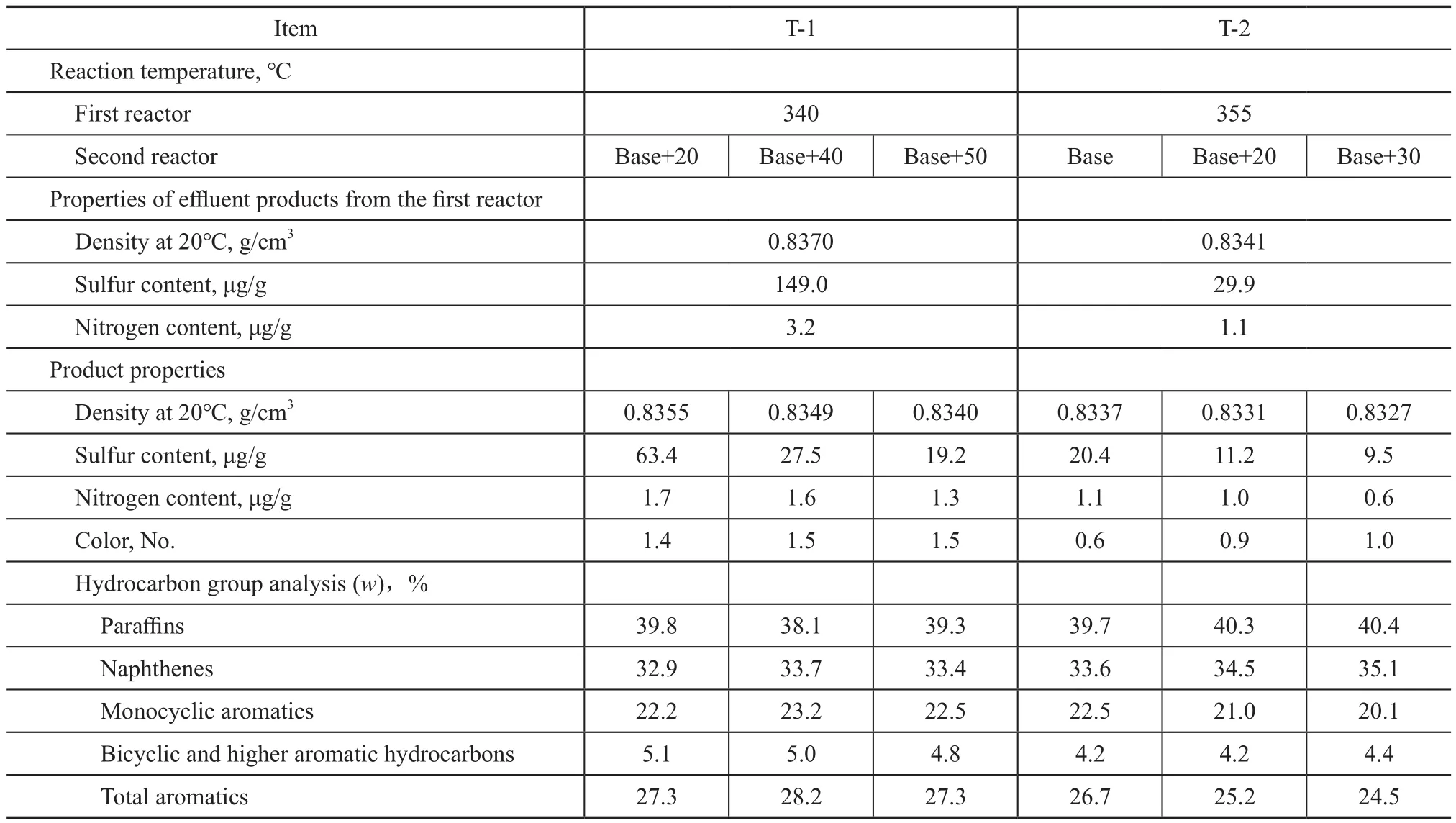
Table 3 Effect of reaction temperature in the second reactor of RTS technology on desulfurization activity of catalyst
Due to the increase of the proportion of secondary processed diesel fuel in the feed, the content of refractory sulfides in the raw material increases, and the difficulty of desulfurization by the RTS technology increases obviously, which makes it very difficult for the second reactor to play the role of ultra‐deep hydrodesulfurization at low temperature and high space velocity.In order to deeply analyze the types of sulfides in the products leaving the first reactor, sulfide types were analyzed for the effluent products delivered from the first reactor, with the results presented in Table 4.

Table 4 Sulfide types of the effluent products delivered from the first reactor operating with RTS technology
It can be seen from Table 4 that when the mass fraction of sulfur in the products delivered from the first reactoris reduced to 20‒150 μg/g, the remaining sulfides are mainly refractory sulfides such as 4,6‐DMDBT, and C2-DBTs ‒ C5‐DBTs, which are difficult to remove.Studies have shown[16]that in order to keep the sulfur content of products below 10‒15 µg/g, it is necessary to almost completely remove such refractory sulfides.Due to steric hindrance effect, it is very difficult to remove all refractory sulfides.
Judging from the above analysis, it can be seen that the catalyst of the second reactor operating with RTS technology can be effectively utilized when the reaction temperature of the second reactor is properly raised to bring into full play the role of ultra‐deep hydrodesulfurization.
3.2.2 The effect of H2/Oil ratio on RTS technology
Under the conditions covering a hydrogen partial pressure of 6.4 MPa, a reaction temperature of 350 ℃ in the first reactor, a reaction temperature of by 20 ℃ higher than the basic temperature in the second reactor, a LHSV of 2.0 h‐1in the first reactor, and a LHSV of 6.0 h‐1in the second reactor, the influence of H2/oil ratio on the properties of the products was investigated.The test results are shown in Table 5.

Table 5 Influence of H2/oil ratio change on product properties
It can be seen from Table 5 that with the increase of H2/oil ratio, the sulfur content, nitrogen content, and density of the products were significantly reduced, the total aromatic hydrocarbon content and the content of bicyclic and higher aromatic hydrocarbons decreased, indicating that the increase of H2/oil ratio could effectively promote the removal of sulfur compounds, nitrogen compounds, and aromatic hydrocarbons.
3.2.3 Influence of LHSV of the second reactor on RTS technology
To bring into full play the role of the second reactor, and reduce the color grade number of the product, under the condition covering a hydrogen partial pressure 6.4 MPa, a temperature of 350 ℃ in the first reactor, and a LHSV of 2.0 h‐1in the first reactor, the LHSV of the second reactor is appropriately reduced to make the second reactor function at a relatively low reaction temperature.It not only can play the role of ultra‐deep hydrodesulfurization in the second reactor, but also can ensure the properties of the product.Table 6 shows the influence on desulfurization performance of the second reactor, when the LHSV of the second reactor is 6.0 h‐1and 4.0 h‐1,respectively.
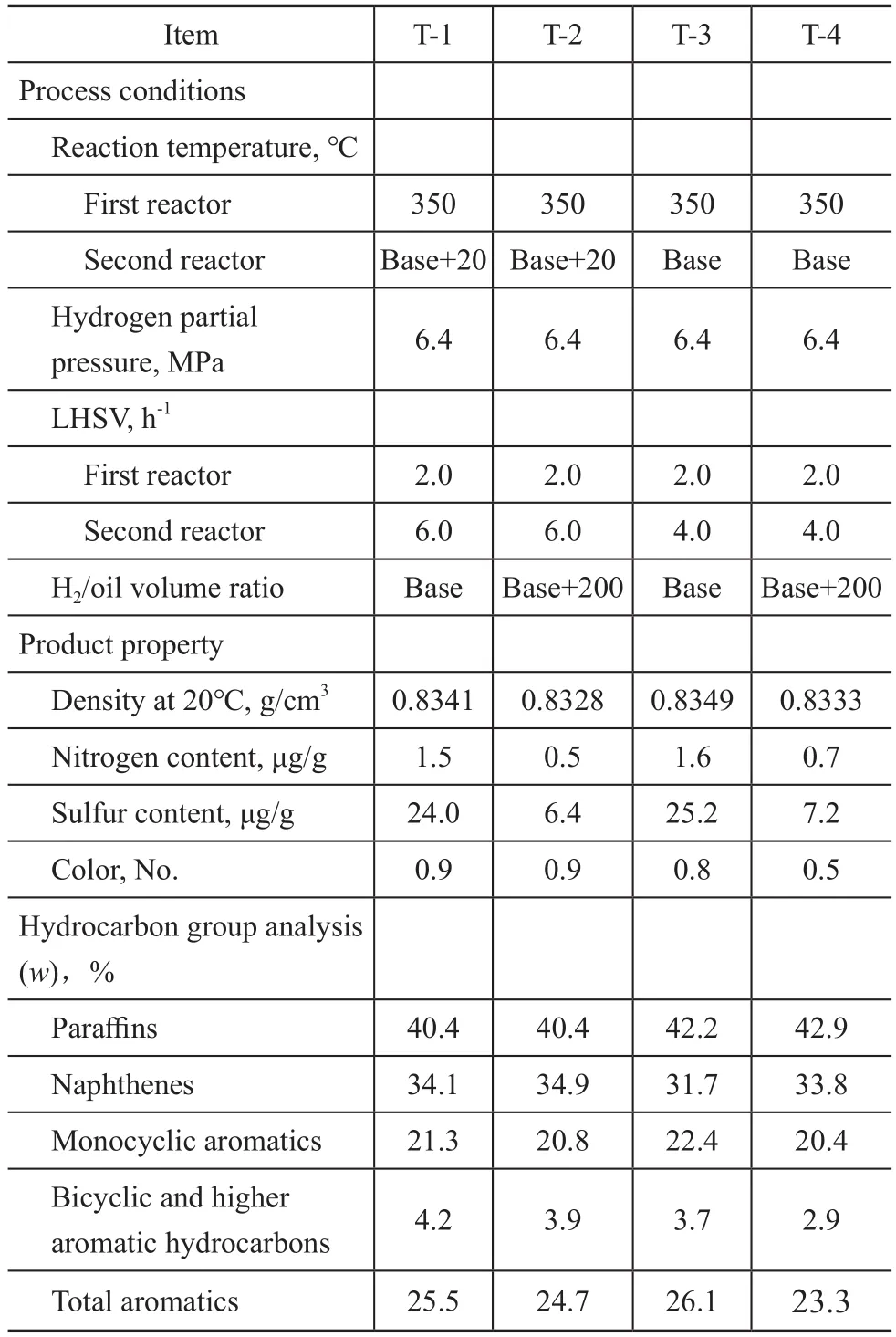
Table 6 Influence of LHSV on desulfurization performance in the second reactor
According to the comparative analysis of the test data in Table 6, under the same process conditions used in the first reactor, the LHSV in the second reactor decreased from 6.0 h‐1to 4.0 h‐1.When the desulfurization depth is basically the same, the reaction temperature of the second reactor can be reduced by about 20 ℃.The color grade of product is significantly reduced, and the content of total aromatic hydrocarbons and polycyclic aromatic hydrocarbons can be significantly reduced.It is indicated that the catalyst of the second reactor can fully play the role of ultra‐deep hydrodesulfurization at a relatively low temperature and the properties of the products can be improved obviously by appropriately reducing the LHSV of the second reactor.
3.3 Optimization of RTS process conditions
Through the analysis of the causes of catalyst deactivation and the study of RTS technology, this section will optimize the process conditions of RTS technology mainly from two aspects.On the one hand, increasing the H2/oil ratio at the inlet of the first reactor can reduce the concentration of impurities in the reaction mixture, promote the removal of nitrogen compounds, polycyclic aromatic hydrocarbons and reactive sulfides, and improve the activity and stability of the catalyst.In view of the increase of refractory sulfides content in the feedstock, the second reactor can not fully play the role of ultra‐deep hydrodesulfurization performance at low temperature and high space velocity.On the other hand, in order to effectively coordinate the deactivation rate of the catalyst in the first reactor and the second reactor, the temperature of the second reactor can be moderately increased to reduce the LHSV of the second reactor, so as to give full play to the role of the catalyst during the ultra‐deep hydrodesulfurization in the second reactor.Table 7 shows the comparison of process conditions and product properties before and after RTS process optimization.Figure 2 shows the variation of reaction temperature in the first reactor with the operating time before and after process optimization with RTS technology.RTS‐I represents the original RTS technology, and RTS‐II represents the optimized RTS technology.

Figure 2 Operating stability of catalyst before and after RTS process optimization
As shown in Table 7, under the conditions coveringa reaction temperature of 350 ℃ in the first reactor, a hydrogen partial pressure of 6.4 MPa, and a total LHSV of about 1.3 h‐1, the optimized RTS technology can produce ULSD, and the color of product is close to that of clear water.After the optimization of RTS technology,the reaction temperature in the first reactor was reduced by more than 10 ℃, and the reaction temperature in the second reactor was increased by 30 ℃.The effect of catalyst in the second reactor was brought into full play, while the reaction harshness of the first reactor was reduced, and the synergistic effect of the catalysts in thefirst reactor and the second reactor was realized.

Table 7 Comparison of process conditions and product properties of RTS technology before and after optimization
It can be seen from Figure 2 that after the process optimization of RTS technology, the reaction temperature of the first reactor basically does not show a process of rapid temperature increase within the operating time of 48‒1048 h, and the reaction start and stop temperatures are lower than the original RTS technology.By comparing the deactivation rates of the catalysts in different running stages, it can be seen that the deactivation rate of the catalyst decreases significantly after the process optimization of RTS technology.It shows that the stability of catalyst is improved obviously after the RTS process optimization.
4 Conclusions
1) The initial activity of the catalyst was mainly affected by the addition of nitrogen compounds to the feedstock oil, and the reaction temperature was increased obviously at the beginning of operation, but the stability of the catalyst was not significantly affected.The addition of PAHs has little effect on the initial activity of the catalyst, but can accelerate the deactivation of the catalyst significantly, and can also reduce the stability of the catalyst significantly.Therefore, the increase of polycyclic aromatic hydrocarbons (PAHs) content is the main reason for the decrease of catalyst stability in the processing of inferior diesel.
2) Based on the analysis of the reason for catalyst deactivation and the study of RTS technology, the optimization direction of RTS technology was put forward.On the one hand, the H2/oil ratio at the inlet of the first reactor was increased.On the other hand,increasing the reaction temperature and decreasing the LHSV in the second reactor can effectively bring into full play the role of the catalyst in the second reactor.The stability of the catalyst is improved obviously after the process optimization of RTS technology.
Acknowledgement:This work was financially supported by the Technology Development Project of SINOPEC(121025).All of the staff in our laboratory had provided a lot of support in the analysis of oil samples and catalyst characterization.
- 中国炼油与石油化工的其它文章
- Controlling the Pore Structure and Photocatalytic Performance of the Flexible FeⅢ Metal-Organic Framework MIL-53(Fe) by Using Surfactants
- Acidity Evaluation of Industrially Dealuminated Y Zeolite via Methylcyclohexane Transformation
- Study on Distribution of Electrocatalytic Reaction Efficiency in a Three-Dimensional Electrocatalytic Reactor
- Preparation of Ultra-High Molecular Weight Polypropylene Using Ziegler-Natta Catalyst via Combining Internal Electron Donor and Cocatalyst Loading
- Preparation of Modified Enteromorpha-Immobilized Microbial Agent and Research on Diesel Removal Performance
- Chemoselective Catalytic Hydrogenation of Nitroarenes Using MOF-Derived Graphitic Carbon Layers Encapsulated Ni Catalysts

