Effects of Hydrotreating Severity on Hydrocarbon Compositions and Deep Catalytic Cracking Product Yields
Deng Zhonghuo; Dai Lishun; Niu Chuanfeng; Jia Yanzi; Wei Xiaoli; Cai Xinheng
(SINOPEC Research Institute of Petroleum Processing, Beijing 100083)
Abstract: Residue deep hydrotreating (RDHT) process was developed by the Research Institute of Petroleum Processing(RIPP) to provide high quality feedstock for deep catalytic cracking (DCC) process.In this research work, the effects of RDHT process and reaction severity on heteroatom removal, hydrogen content increase, hydrocarbon composition improvement, and DCC product yields were studied.It was showed that the RDHT process can effectively reduce heteroatoms, increase hydrogen content and improve the hydrocarbon compositions, which can contribute to an increase of light olefins yield in DCC unit.
Key words: residue hydrotreating; hydrocarbon composition; propylene; olefins; deep catalytic cracking
1 Introduction
Propylene is an important basic organic synthesis raw material, which can be used to produce a variety of organic chemical products, such as polypropylene,acetone, acrylic acid, etc.Different from the sluggish growth of transport fuels, the average annual growth rate of propylene is expected to reach 4% in the next few years[1].Therefore, the transition from a fuel‐oriented refinery to a chemical‐oriented refinery has become a research hotspot recently.
The sources of light olefins (ethylene, propylene,and butene) are relatively diversified, such as steam cracking[2], fluid catalytic cracking (FCC)[3‐4], coal‐to‐olefins process[5], etc.With the current crude oil price fluctuating between US$30/barrel and US$60/barrel,the oil‐to‐olefins process is a very competitive route[6].However, China’s olefin industry is also facing the continuous pressure of low‐cost bulk petrochemical imports from North America, the Middle East, and other regions, and it is urgent to reduce the production cost of oil‐to‐olefins process.At present, the raw materials of deep catalytic cracking (DCC) are mainly paraffin based vacuum gas oil or atmospheric residue[7], or the product of vacuum gas oil hydrogenation unit mixed with atmospheric residue[8], which would inevitably weaken the margins.However, if the feedstock of DCC unit can be extended to hydrogenation product of intermediate based residue, the raw material cost of DCC unit can be greatly reduced and its economic margin can be strengthened accordingly.
Compared with the FCC process, the DCC process has higher requirements on the quality of raw materials.To meet these requirements, the Research Institute of Petroleum Processing (RIPP) has developed a residue deep hydrotreating (RDHT) process[9].To further understand the influence of RDHT process on the product yields of DCC unit, this work focused on the effects of hydrotreating severity on hydrocarbon compositions and DCC product yields.
2 Experimental
2.1 Feedstock
The feedstock used in the RDHT experiments is an atmospheric residue provided by the Dalian West Pacific Petrochemical Co., Ltd.and is denoted as XTAR, the properties of which are listed in Table 1.Herein, the sulfur content of XTAR is 2.92%, which is a typical feature of the Middle East high‐sulfur atmospheric residual oil.
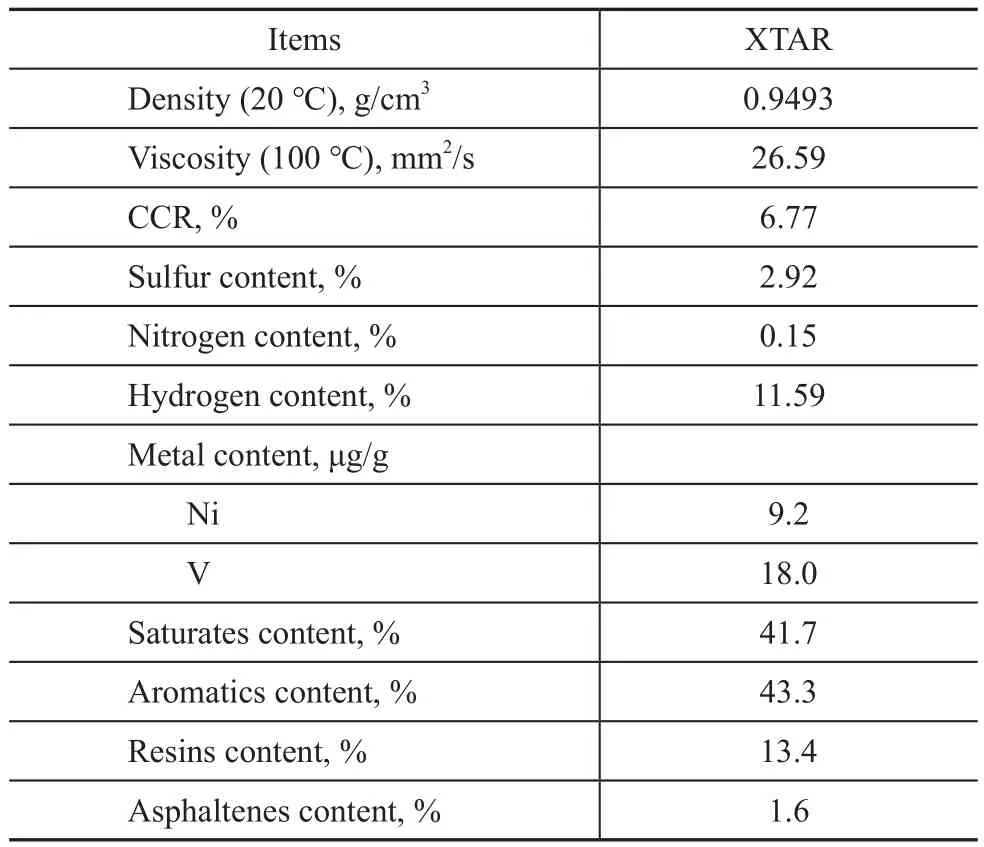
Table 1 Properties of feedstock
2.2 RDHT and DCC Process
The RDHT experiments were carried out in a pilot plant consisting of four 1000‐mL reactors connected in series and a separation system including an atmospheric fractionator, as shown in Fig.1.The hydrogenated samples were collected from the bottom of atmospheric fractionator, with the initial boiling point of hydrogenated oil equating to around 320 ℃.A RDHT catalyst system consisting of a combination of guard catalyst, hydrodemetallization (HDM) catalyst,hydrodesulfurization (HDS) catalyst, HDM‐HDS catalyst,and Conradson carbon residue reduction (HDCCR)catalyst was used in the present study.The detailed information about the RDHT catalyst system has been reported in the previous work[9].The RDHT tests were carried out after catalyst sulfidation and one of the samples was obtained at a temperature of 385 ℃ at the beginning of the experiment, while the other samples were collected after 400 hours of stability test, which operated at a temperature of 370‒395 ℃.The other test conditions for the above samples covered: an LHSV of 0.17 h−1, a hydrogen pressure of 17.0 MPa, and a hydrogen/oil volume ratio of 800.The sample obtained at the beginning of test was studied in order to reveal the correlation between the DCC product yields and the hydrocarbon composition.The hydrogenated samples collected after the stability test at different reaction temperature were denoted as “T+temperature”, such as T370, T375, etc.
The DCC experiments were carried out in a small fixed‐bed fluidized reactor operating with a commercial DCC catalyst developed by RIPP.The reaction temperature was 580℃ and the catalyst to oil mass ratio was 10.
2.3 Analytical methods
The feedstock and hydrogenated samples were analyzed by a Fourier transform ion cyclotron resonance mass spectrometer (FT‐ICR MS) to get the molecular level compositions.The hydrocarbon compositions were analyzed according to the SH/T 0659 method.Other common physical and chemical properties of samples were analyzed by corresponding standard methods.The samples analyzed by FT‐ICR MS were usually characterized by the molecule form of CcHhNnOoSs, where the subscripts represent the number of atoms of each element.
3 Results and Discussion
3.1 Effect of hydrotreating severity on hydrocarbon composition
The heteroatom content, hydrocarbon composition and hydrogen content of feedstock are the key factors affecting the propylene yield of DCC unit[10].Therefore, it is necessary to effectively remove heteroatoms, increase hydrogen content and optimize hydrocarbon compositions in the RDHT process to produce high quality DCC feedstock.From a deeper perspective, the essence of removing heteroatoms and increasing hydrogen content is also the process of hydrocarbon conversion.
Fig.2 shows the influence of reaction temperature on sulfur, Conradson carbon residue (CCR) and nitrogen contents of XTAR after RDHT process.It could be seen that the sulfur content decreased from 2.92% to less than 0.25%, the CCR content decreased from 6.77% to less than 2.00%, while the nitrogen content decreased from 0.15% to less than 0.06%.Concomitantly, the afore-mentioned hetero-atoms contents all decreased rapidly with an increasing reaction temperature.Fig.3 indicates the impact of reaction temperature on (Ni+V)and hydrogen contents of XTAR after RDHT process.It illustrates that the (Ni+V) content decreased from27.2 μg/g to less than 2.5 μg/g, the hydrogen content increased from 11.59% to more than 12.69%, and the hydrogen content increased linearly with an increasing reaction temperature.As shown in Fig.2 and Fig.3, it was verified that the RDHT process achieved efficient removal of heteroatoms and increase of hydrogen content,and the above effects can be improved by increasing the reaction severity.
Fig.4 illustrates the distribution of hydrocarbons (HC)and heteroatom compounds of XTAR before and after RDHT process.Before RDHT process, the relative abundance of HC class was less than 31%, while the sum of relative abundance of S1, S2, S3, N1 and N1S1 compound classes was more than 67%, indicating that XTAR was rich in heteroatoms.After RDHT process, the relative abundance of HC class increased from less than 31% to more than 87%, and it increased linearly with a rising reaction temperature.Therefore, with the efficient removal of heteroatoms and increase of hydrogen content,the hydrocarbon compounds were dominant in the hydrogenated residue.
According to the research on the potential content of propylene[11], the yield of light olefins of DCC unit is closely related to the hydrocarbon compositions of the feedstock.Further research[12]showed that the dominant hydrocarbon structures of the feedstock to produce propylene are alkanes, one‐ring to four‐ring cycloalkanes and alkylbenzenes.The hydrocarbons could be divided into four classes due to different potential content of propylene[9], as shown in Table 2.Fig.5 illustrates the influence of reaction temperature on hydrocarbon class I and hydrocarbon class II contents.It depicts that with arising reaction temperature, the alkanes content increased slowly, and the one‐ring to four‐ring cycloalkanes content increased rapidly, while the alkylbenzenes, the naphthenic benzenes and the dicycloalkylbenzenes contents all decreased slowly.As a result, with an increase in temperature, the hydrocarbon class I content increased,while the hydrocarbon class II content decreased.In more detail, the hydrocarbon class I content increased from ca.40% to 61%‒72% after RDHT process, indicating that the hydrotreating severity has a positive impact on the hydrocarbon compositions of DCC feedstock.

Table 2 Hydrocarbon classes distribution due to different potential content of propylene
For cycloalkanes and aromatics, the potential content of propylene increases with an increasing side chain carbon number[12].Fig.6 illustrates the side chain carbon number distribution of the hydrogenated residue at a reaction temperature of 385 ℃.It can be seen that the side chain carbon number decreased with an increase in DBE,demonstrating that the more the aromatic ring numbers,the less the side chain carbon number and the less the potential content of propylene.
Figure 7 illustrates the carbon number distribution of XTAR and hydrogenated residues with different double bond equivalents (DBE) and reaction temperature.It can be seen that the carbon number distribution of low DBE hydrocarbons could hardly change with the increase of reaction temperature, implying that the cracking and condensation reactions of low DBE hydrocarbons can be neglected even at high reaction severity.Meanwhile, the condensation reaction was significant for hydrocarbons of DBE 15.And both the cracking and condensationreactions were significant for hydrocarbons with a DBE of more than 25.Moreover, the cracking reaction intensity increased significantly with an increasing reaction temperature.Judging from Fig.6 and Fig.7, it could be concluded that the low DBE hydrocarbons which had longer side chain was not easy to crack, while the high DBE hydrocarbons, which had shorter side chain, tendedto crack at high hydrotreating severity.In summary, to a large extent, the long side chain beneficial to propylene production was retained after RDHT process.

Figure 1 Schematic diagram of RDHT process

Figure 2 Influence of reaction temperature on sulfur, CCR and nitrogen contents

Figure 3 Influence of reaction temperature on hydrogen and (Ni+V) contents

Figure 4 Influence of reaction temperature on hydrocarbon and heteroatom compounds distribution

Figure 5 Influence of reaction temperature on hydrocarbon class I content (a), hydrocarbon class II content (b)

Figure 6 Side chain carbon number distribution of hydrocarbons with different DBE

Figure 7 Carbon number distribution of hydrocarbons with different DBE and reaction temperature

Figure 8 DCC product yields of different hydrotreating severity products

Figure 9 Influence of hydrocarbon compositions on propylene yield

Figure 10 Influence of hydrocarbon compositions on light olefins yield

Figure 11 Influence of hydrocarbon compositions on chemicals yield
3.2 Effect of hydrotreating severity on DCC product yields
Using the hydrogenated residues of RDHT process as feedstock, the product yields of DCC unit are shown in Fig.8.It can be seen that with an increase in hydrotreating temperature, the gasoline and liquefied petroleum gas(LPG) yields increased, while the light cycle oil (LCO),heavy cycle oil (HCO), and coke yields decreased.It has been reported that the gasoline yield increases with an increasing hydrogen content and the coke yield decreases with a decreasing CCR value[13].This could occur because with an increasing hydrotreating severity, the contents of heteroatom compounds, condensed aromatics, resins and asphaltenes, which were difficult to crack, were gradually reduced, while the contents of saturates, alkylbenzenes and other hydrocarbons, which were easy to crack, were effectively increased.
To further illustrate the above point of view, Fig.9‐Fig.11 show the influence of hydrocarbon compositions on the yields of propylene, light olefins, and chemicals(light olefins, benzene (B), toluene (T), and xylene (X)),respectively.It can be seen that the yields of propylene,light olefins, and chemicals were more than 19.4%,38.4%, and 43.4%, respectively.Moreover, the yields of propylene, light olefins, and chemicals all increased linearly with an increasing hydrocarbon class I content,with a linear fit goodness equating to about 0.91, 0.89,and 0.95, respectively.Therefore, the yields of propylene,light olefins and chemicals of DCC unit can be estimated from the hydrocarbon class I content of feedstock.
Upon summarizing the relationship between the hydrotreating severity and the hydrocarbon composition of hydrogenated residue, as well as the relationship between the hydrocarbon composition and the product yields of DCC unit, it can be deduced that the RDHT process can effectively improve the hydrocarbon compositions, resulting in an increase of propylene, light olefins, and chemicals yields in the DCC unit.
4 Conclusions
The RDHT process developed by RIPP can effectively remove the heteroatoms and increase the hydrogen content, which can result in efficient increase of the hydrocarbons that are appropriate for DCC unit to produce more chemicals.Moreover, the above effect can be improved by increasing the reaction severity while the long side chain beneficial to propylene production can be retained even at high reaction severity.Using the hydrogenated residues as feedstock, the yields of propylene, light olefins and chemicals of DCC unit were equal to more than 19.4%, 38.4%, and 43.3%,respectively, and they all showed a linear relationship with hydrocarbon class I content, indicating that the RDHT process can provide high quality feedstock for DCC unit.
Acknowledgements:This work was supported by the Science and Technology Project of Sinopec (118015‐1).
- 中国炼油与石油化工的其它文章
- Controlling the Pore Structure and Photocatalytic Performance of the Flexible FeⅢ Metal-Organic Framework MIL-53(Fe) by Using Surfactants
- Acidity Evaluation of Industrially Dealuminated Y Zeolite via Methylcyclohexane Transformation
- Study on Distribution of Electrocatalytic Reaction Efficiency in a Three-Dimensional Electrocatalytic Reactor
- Preparation of Ultra-High Molecular Weight Polypropylene Using Ziegler-Natta Catalyst via Combining Internal Electron Donor and Cocatalyst Loading
- Preparation of Modified Enteromorpha-Immobilized Microbial Agent and Research on Diesel Removal Performance
- Chemoselective Catalytic Hydrogenation of Nitroarenes Using MOF-Derived Graphitic Carbon Layers Encapsulated Ni Catalysts

