Syntheses and Luminescence of Three Zinc Complexes Constructed by Rigid 4⁃Substituted Bis(1,2,4⁃triazole)Ligand
PENG Yan-Fen LIU Tian-Bao WU Qiu-Yan WU Meng-Qing
(College of Materials and Environmental Engineering,Key Laboratory of Micro⁃Nano Powder and Advanced Energy Materials of Anhui Higher Education Institutes,Chizhou University,Chizhou,Anhui 247000,China)
Abstract:Three new coordination polymers,{[Zn(4-Nbdc)(btx)]·2H2O}n(1·2H2O),[Zn(btx)1.5(1,3-bdac)]n(2)and{[Zn(mbtx)(1,3-bdc)(H2O)2]·H2O}n(3)(btx=1,4-bis(4H-1,2,4-triazole)benzene,mbtx=1,3-bis(4H-1,2,4-triazole)benzene,4-Nbdc=4-nitrophthalate,1,3-bdac=1,3-phenylenediacetate,1,3-bdc=1,3-benzenedicarboxylate),were synthesized under room temperature condition and characterized by single-crystal X-ray diffraction,elemental analyses,powder X-ray diffraction.Sing-crystal X-ray structural analysis show that complex 1 is 2D waved(4,4)network.2 and 3 exhibit 1D chain,and the 1D chains are connected into a 3D network by π-π interaction in 2.The adjacent 1D chains are connected into a 2D network by intermolecular hydrogen bond in 3.Two sets of equivalent 2D networks catenate with each other to give a 2D+2D→2D network through intermolecular hydrogen bond.Luminescence and thermogravimetric analysis of the three complexes were discussed.CCDC:2088317,1;2088318,2;2088319,3.
Keywords:1,3-bis(4H-1,2,4-triazole)benzene;1,4-bis(4H-1,2,4-triazole)benzene;π-π interaction;luminescence
0 Introduction
The synthesis and characterization of coordination polymers(CPs)or metal-organic frameworks(MOFs)have been one of the hot topics in the present research because of their interesting topological structures and some physical properties with potential applications in ion-exchange or cation sensing,catalysis,luminescence,and gas absorption[1-11].In crystal engineering,supramolecular interactions such as intermolecular ππ interactions and hydrogen bonding are just as important as coordination bonds[12].In some complexes,a 1D chain or 2D network can be connected into a 3D network by π -π stacking or hydrogen bond.The metal ions play an important role in the synthesis of complexes.Adopting the d10configuration,the Zn(Ⅱ)metalorganic frameworks may be as new advanced luminescent materials because of attractive fluorescence properties[13-14].On the other hand,judicious selection of organic ligands is very crucial for obtaining frameworks with fascinating structures in crystal engineering.More and more O-donor ligands and N-donor ligands were widely used in the synthesis of coordination polymers.In previous work,we synthesized some coordination polymers based on 1-substituted bis(1,2,4-triazole)ligand under the assistance of oxygen ligands[15-17].In contrast to bis(1H-1,2,4-triazole)ligand,the 4-substituted-1,2,4-triazole derivatives have more coordination sites.We have synthesized some coordination polymersbased on rigid 4-substituted-bis(1,2,4-triazole)ligand 1,4-bis(4H-1,2,4-triazole)benzene(btx)[18-19],flexible ligand 1,2-bis(4H-1,2,4-triazole)ethane(btre)[20-22],1,4-bis(1,2,4-triazole-4-ylmethyl)benzene(btrb)[23]For example,[Zn(btx)0.5(fum)(H2O)]n(fum=fumarate)displays a 2D→3D inclined polycatenation motif consisting of two sets of equivalent 2D(6,3)layers.{[Zn4(OH)2(btre)(1,2,3-btc)2(H2O)4]·4H2O}n(1,2,3-btc=1,2,3-benzenetricarboxylate)exhibits(3,8)-connected 3D network.
With this background information,in this paper,we report three new coordination polymers{[Zn(4-Nbdc)(btx)]·2H2O}n(1·2H2O),[Zn(btx)1.5(1,3-bdac)]n(2),and{[Zn(mbtx)(1,3-bdc)(H2O)2]·H2O}n(3)based on rigid 4-substituted bis(1,2,4-triazole)ligand btx and mbtx(1,3-bis(4H-1,2,4-triazole)benzene)and three aromatic dicarboxylate ligands 4-H2Nbdc,1,3-H2bdac,and 1,3-H2bdc(4-Nbdc=4-nitrophthalate,1,3-bdac=1,3-phenylenediacetate,1,3-bdc=1,3-benzenedicarboxylate)(Scheme 1).The syntheses,structures,thermal stability,and luminescence properties of complexes 1·2H2O,2 and 3 are studied.
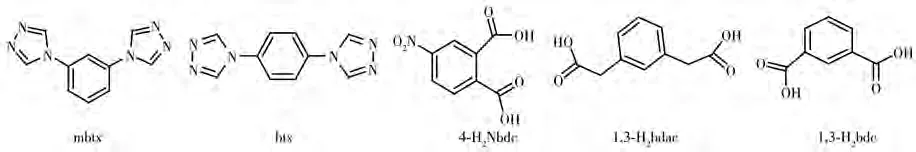
Scheme 1 Structure of ligands mbtx,btx,4-H2Nbdc,1,3-H2 bdac,and 1,3-H2 bdc
1 Experimental
1.1 Material and methods
The ligands btx and mbtx were synthesized according to the literature method[24].All other reagents(such as Zn(NO3)2·6H2O,4-H2Nbdc,1,3-H2bdac,1,3-H2bdc)were of analytical grade and used without further purification.Elemental analyses for C,H,and N were performed on a Perkin-Elmer 240C analyzer.Powder X-ray diffraction(PXRD)was performed on a D/MAX-3C diffractometer with the Cu Kα radiation(λ=0.154 06 nm,U=40 kV,I=40 mA)over a 2θ range of 5°-50°at room temperature.The luminescence measurements were carried out in the solid-state at room temperature and the spectra were collected with a Perkin-Elmer LS50B spectrofluorimeter.Thermogravimetric analysis(TGA)was carried out using a Thermal Analyst 2100 TA Instrument and SDT 2960 Simultaneous TGA-DTA Instrument in flowing nitrogen.
1.2 Synthesis of{[Zn(4⁃Nbdc)(btx)]·2H2O}n(1·2H2O)
A solution of 4-H2Nbdc(0.2 mmol,42 mg)in 10 mL of H2O was adjusted to pH=6 with 1.0 mol·L−1NaOH solution,and Zn(NO3)2·6H2O(0.2 mmol,59 mg)was added with stirring.The btx(0.2 mmol,42 mg)was dissolved in 5 mL of DMF and added to the mixture and continuously stirred for 10 min.The mixture solution was filtered and stood for two weeks to give colorless single crystals of 1·2H2O.Anal.Calcd.for C18H11ZnN7O6·2H2O(%):C,41.36;H,2.89;N,18.75.Found(%):C,41.45;H,2.80;N,18.69.IR data(cm−1):3 429s,3 121w,1 582m,1 487w,1 384m,1 198w,1 170w,1 128w,1 079w,1 023w,772w,647w.
1.3 Synthesis of[Zn(btx)1.5(1,3⁃bdac)]n(2)
The synthesis method of 2 was similar to that of 1,except that 4-H2Nbdc was replaced by 1,3-H2bdac.Anal.Calcd.for C25H20ZnN9O4(%):C,52.14;H,3.50;N,21.89.Found(%):C,52.20;H,3.48;N,21.76.IR data(cm−1):3 436w,3 126w,3 090s,1 583s,1 541s,1 402s,1 311w,1 231m,1 098m,1 049m,855m,746w,642w.
1.4 Synthesis of{[Zn(mbtx)(1,3⁃bdc)(H2O)2]·H2O}n(3)
The synthesis method of 3 was similar to that of 1,except that btx and 4-H2Nbdc were replaced by mbtx and 1,3-H2bdc,respectively.Anal.Calcd.for C18H18ZnN6O7(%):C,43.61;H,3.66;N,16.95.Found(%):C,43.72;H,3.58;N,16.87.IR data(cm−1):3 370s,3 164w,3 103w,1 615s,1 548m,1 403m,1 372s,1 287w,1 220w,1 093w,1 050w,880w,741m,679w.
1.5 X⁃ray crystallography
Single crystal X-ray diffractions analysis of the three complexes was carried out.The diffraction data of 1 were collected on Bruker Smart ApexⅡ,and 2 and 3 on Rigaku Mercury CCD diffractometer with graphite monochromated MoKα(λ=0.071 073 nm)radiation.Intensities data were collected by theφ-ωscan technique for 1,2,and 3.The structures were solved by direct methods with SHELXS and refined with fullmatrix least-squares onF2with SHELXL.All nonhydrogen atoms were refined anisotropically and hydrogen atoms were determined with theoretical calculations and refined isotropically.The lattice water of 1·2H2O is highly disordered and could not be modeled properly and was removed by the SQUEEZE routine in PLATON[25].The number of lattice water molecules for 1·2H2O was deduced from the TG and elemental analysis.The parameters of crystal data collection and refinement of 1,2,and 3 are given in Table 1.Selected bond lengths and bond angles of 1,2,and 3 are listed in Table 2.
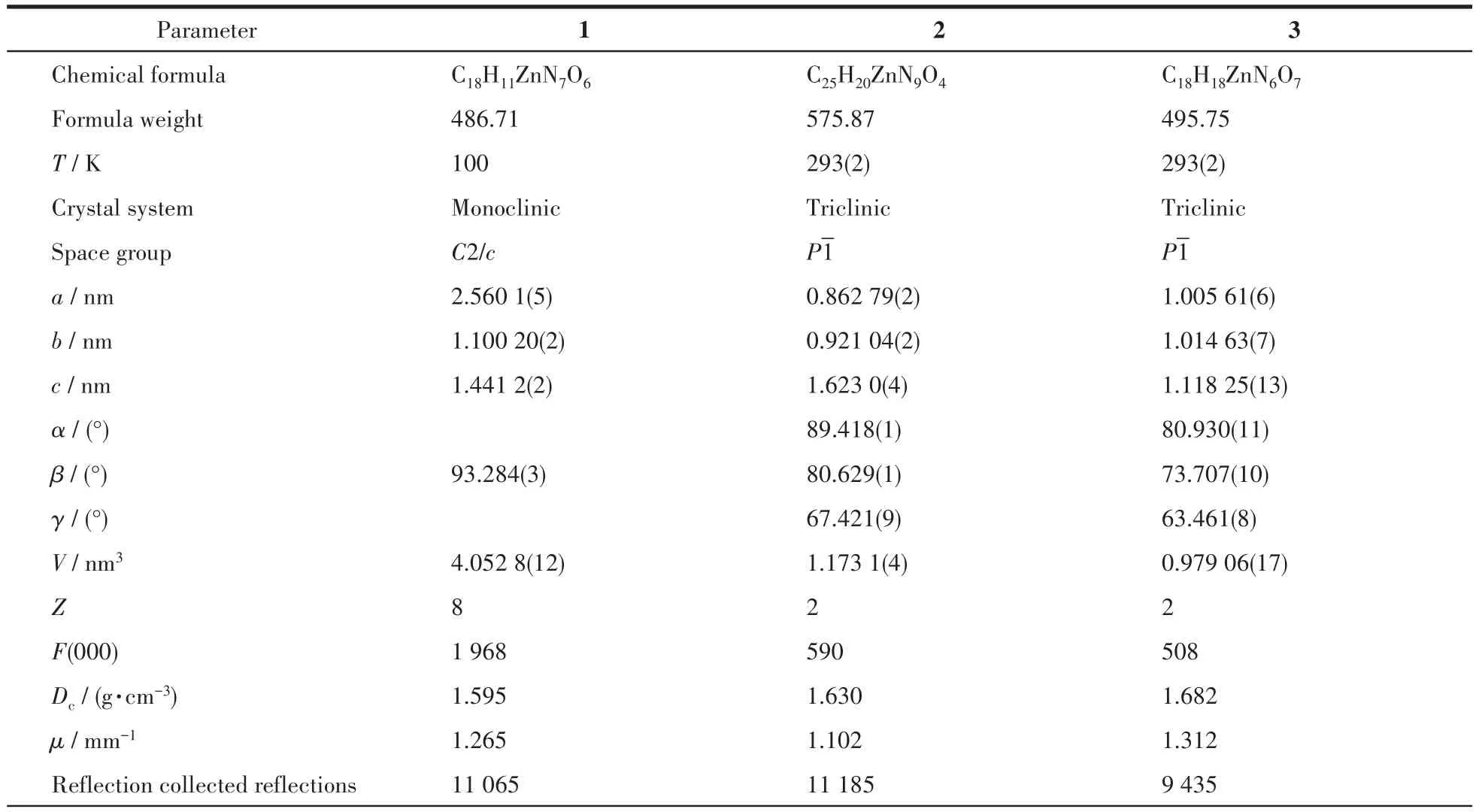
Table 1 Crystallographic data and structure refinements of 1,2,and 3
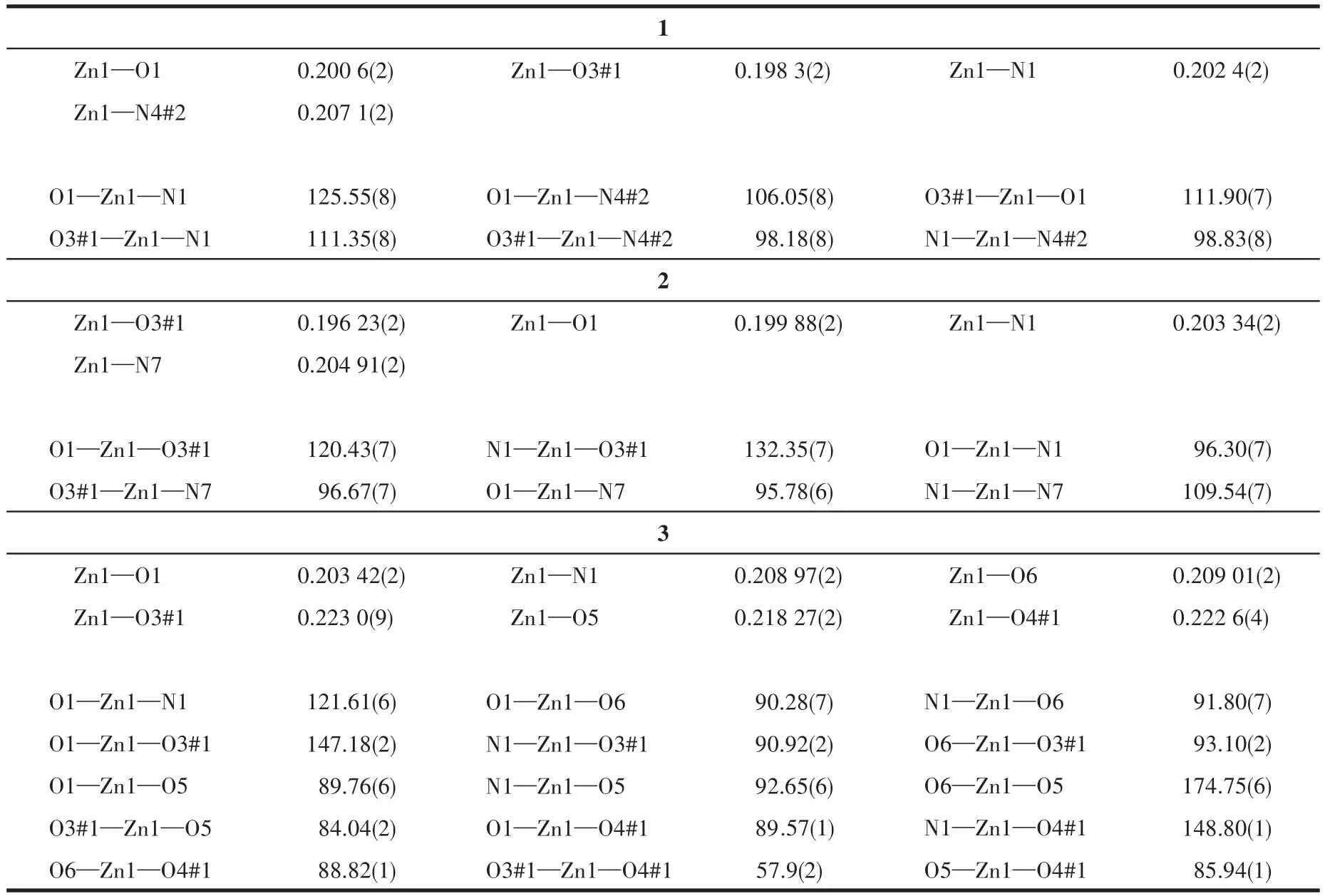
Table 2 Selected bond distances(nm)and bond angles(°)for 1,2 and 3
CCDC:2088317,1;2088318,2;2088319,3.
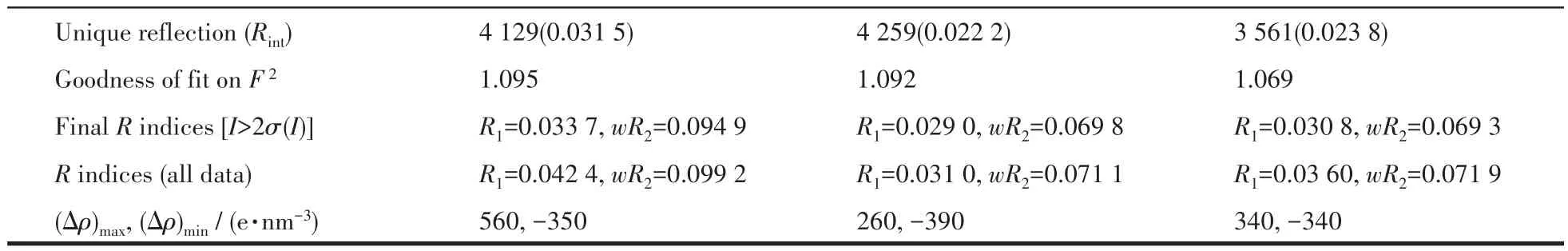
Continued Table 1
2 Results and discussion
2.1 Crystal structure of[Zn(4⁃Nbdc)(btx)]n(1)
Single crystal diffraction shows that 1 crystallizes in the monoclinic system with theC2/cspace group.The asymmetric unit of 1 consists of one Zn(Ⅱ)ion,one 4-Nbdc,onebtxligand.TheZn(Ⅱ)ionisfour-coordinated by two carboxylate oxygen atoms(O1,O3#1)from two 4-Nbdc ligands,two nitrogen atoms(N1,N4#2)from two btx ligands to give a[ZnO2N2]distorted tetrahedron geometry(Fig.1a).The Zn—O/N bond lengths are in a range of 0.198 3(2)-0.207 1(2)nm,which is similar to those observed in the other Zn—O/N complexes[26].Each btx ligand represents bis-monodentate coordinated mode,and bridges two Zn(Ⅱ)ions with the distance of 1.332 64(2)nm,and expands to a 1D zigzag chain[Zn(btx)]n(Fig.1b).The dihedral angle of two triazole rings of btx is 75.949°.Ligand 4-Nbdc adopts bismonodentate coordinated mode and bridges two Zn(Ⅱ)ions with a distance of 0.612 84(9)nm.Ligands 4-Nbdc connect adjacent 1D zigzag chains to form a twodimensional waved network(Fig.1c).Each Zn(Ⅱ)connects two btx and two 4-Nbdc ligands and acts as a 4-connected node.Ligands btx and 4-Nbdc act as a twoconnected linker.So,the two-dimensional waved network can be simplified to a 2D(4,4)network(Fig.1d).
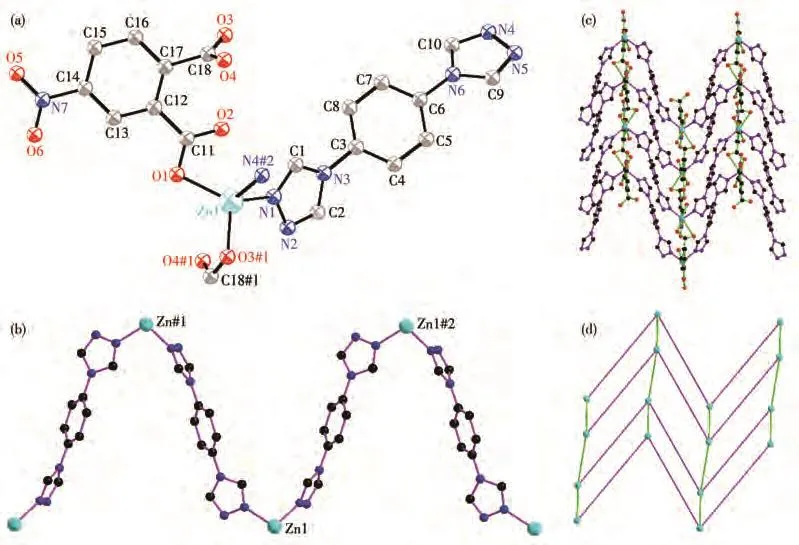
Fig.1 (a)Coordination environment of Zn(Ⅱ) ion in 1;(b)1D zigzag chain construced by Zn(Ⅱ) and btx;(c)2D network of 1;(d)Schematic depiction of 2D(4,4)network in 1 with pink and bright green lines representing btx and 4-Nbdc,respectively
2.2 Crystal structure of[Zn(btx)1.5(1,3⁃bdac)]n(2)
Single crystal diffraction shows that 2 crystallizes in the triclinic system with theP1 space group.The asymmetric unit of 2 consists of one Zn(Ⅱ)ion,one 1,3-bdac,one and a half of btx ligands.The Zn(Ⅱ)ion is fourcoordinated by two carboxylate oxygen atoms(O1,O3#1)from two 1,3-bdac ligands,two nitrogen atoms(N1,N7)from two btx ligands to give a[ZnO2N2]distorted tetrahedron geometry(Fig.2a).The Zn—O/N bond lengths are in a range of 0.196 23(2)-0.204 91(2)nm,which are in the normal range.The bond angles around Zn (Ⅱ) are in a range of 95.79(6)°-120.44(7)°.Each 1,3-bdac ligand shows bis-monodentate coordinated mode,bridges two Zn(Ⅱ)with the distance of 0.991 21(2)nm,and expands to a 1D chain[Zn(1,3-bdac)]n.There are two kinds of btx in 2.One kind of btx exhibits bis-monodentate coordinated mode and connects the adjacent 1D chain[Zn(1,3-bdac)]nto form a 1D ladder chain.The only nitrogen atom of the other kind of btx is coordinated to Zn(Ⅱ).The other btx ligands are distributed on both sides of the ladder chains like wings(Fig.2b).The dihedral angles of two triazole rings of two kinds of btx ligands are 0.00°and 81.611(13)°.
There are strong π-π interactions between two triazole rings Cg(6)and Cg(5)(Symmetry code:1−x,1−y,−z)of btx ligand,benzene ring Cg(7)and triazole ring Cg(5)(Symmetry code:−x,2−y,−z)of btx,triazole ring Cg(6)(Symmetry code:x,1+y,z)and benzene ring Cg(9)of1,3-bdacligand[27](Fig.2c).Thecenteriod-to-centeriod distances and dihedral angles of Cg(6)and Cg(5),Cg(7)and Cg(5),Cg(6)and Cg(9)are 0.345 6 nm and 15.328°,0.393 1 nm and 32.018°,0.399 7 nm and 35.976°,respectively.The Cg(5),Cg(6),Cg(7),and Cg(9)represent the rings N4—N5—C10—N6—C9,N7—N8—C15—N9—C14,C1-C6,C16-C21,respectively.The 1D chains are further connected to the 3D network by the strong π-π interactions(Fig.2d).
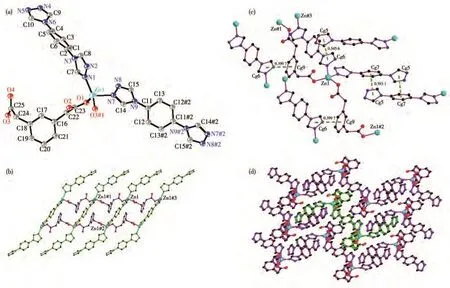
Fig.2 (a)Coordination environment of Zn(Ⅱ) ion in 2;(b)1D ladder-like infinite chain of 2;(c)π-π interactions represented by bright green dot lines in 2;(d)3D network constructed by π-π interactions in 2
2.3 Crystal structure description of{[Zn(mbtx)(1,3⁃bdc)(H2O)2]·H2O}n(3)
Complex 3 crystallizes in the triclinic system with the P1 space group.The asymmetric unit of 3 consists of one Zn(Ⅱ)ion,one 1,3-bdc,one mbtx ligand,two coordination water molecules,and one lattice water molecule.The Zn(Ⅱ)ion is six-coordinated by three carboxylate oxygen atoms(O1,O3#1,and O4#1)from two 1,3-bdc ligands,two coordination water(O5 and O6),and one nitrogen atom N1 to give a[ZnO5N]distorted octahedron geometry(Fig.3a).The two carboxyl groups(C17O1O2 and C18O3O4)of 1,3-bdc ligand show monodentate and bidentate chelation coordinated mode,respectively.The Zn(Ⅱ)ions were linked by 1,3-bdc to form a 1D chain[Zn(1,3-bdc)]n.The distance of two Zn(Ⅱ)ions bridged by 1,3-bdc is 1.005 61(6)nm.Only nitrogen atom N1 of mbtx is coordinated to Zn(Ⅱ)(Fig.3b).The dihedral angle between two triazole rings of mbtx ligands is 44.185(7)°.
There are some intermolecular hydrogen bonds in 3.The adjacent 1D chains were connected to form a 2D network through the intermolecular hydrogen bonds between N5#3…O7,O6…O7#1(Fig.3c).Two sets of 2D networks were linked by hydrogen bonds between O6…N2#2 and polycatenated with each other to give a 2D+2D→2D network(Fig.3d).The hydrogen bond parameters are listed in Table 3.
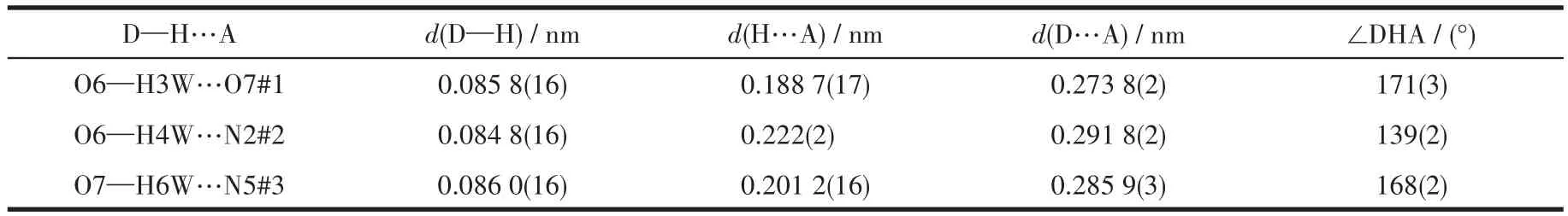
Table 3 Hydrogen bond parameters of 3

Fig.3 (a)Coordination environment of Zn(Ⅱ)ion in 3;(b)1D chain of 3;(c)2D network constructed by hydrogen bonds in 3;(d)2D+2D→2D network constructed by hydrogen bonds in 3 with dot lines representing hydrogen bonds
2.4 PXRD patterns and TGA
To characterize the purity of complexes 1·2H2O,2,and 3,the as-synthesized samples were measured by PXRD at room temperature.As shown in Fig.4,the peak position of the measured patterns is in good agreement with the simulated patterns,indicating the purity of the samples.TGA of 1·2H2O,2,and 3 was performed by heating the crystalline samples from room temperature to 800℃with a heating rate of 10℃·min−1under nitrogen atmosphere.As shown in the TG curve of 1·2H2O(Fig.5),1·2H2O was stable from 20 to 50℃,and the lattice water was lost from 50 to 171℃(Calcd.6.89%,Obsd.6.81%).The framework of 1 was thermally stable above 316℃.Then the decomposition happened quickly until 528℃.From 528 to 800℃,the residue was stable.The main residue should be ZnO(Calcd.15.56%,Obsd.18.00%).For 2,the weight was lost slowly from 27 to 320℃.Then decomposition of the framework happened quickly from 320 to 800℃.The main residue should be ZnO(Calcd.14.13%,Obsd.17.82%).Complex 3 was stable before 59℃.The coordinated water and lattice water were lost from 59 to 125℃(Calcd.10.90%,Obsd.10.47%).From 125 to 280℃,the framework of 3 was stable.Then the decomposition happened until 800℃.The main residue should be ZnO(Calcd.16.41%,Obsd.18.95%).

Fig.4 PXRD patterns of complexes 1·2H2O(a),2(b),and 3(c)

Fig.5 TG curves of 1·2H2O,2,and 3
2.5 Photoluminescence properties
Due to the excellent fluorescent properties ofd10metal complexes,the solid-state photoluminescence properties of 1·2H2O,2,and 3 were investigated at room temperature(Fig.6).The free btx[19]and mbtx[12]ligand in the solid-state showed the maxima emission band approximately at 432 and 390 nm upon excitation at 370 and 335 nm,respectively.The free 1,3-H2bdac[28]and 1.3-H2bdc[18]exhibited emissions at 307 and 370 nm,respectively.No emission of 1·2H2O was observed at room temperature.2 exhibited the emission band maximum at 301 nm and a week peak at 397 nm upon excitation at 267 nm.3 had an emission at 381 nm upon excitation at 308 nm.Because the Zn(Ⅱ)ion is difficult to oxidize or to reduce due to thed10configuration,the emissions are neither metal-to-ligand charge transfer (MLCT) nor ligand-to-metal charge transfer(LMCT).The week emission at 397 nm of 2 may be tentatively attributed to the intra-ligand charge transition for 1,3-bdac and btx.The strong emission at 301 nm may be tentatively attributed to the emission of 1,3-bdac.The strong emission at 381 nm of 3 may be tentatively attributed to the mixed intra-ligand mbtx and rigid biscarboxylate 1,3-bdc charge transition[29-30].
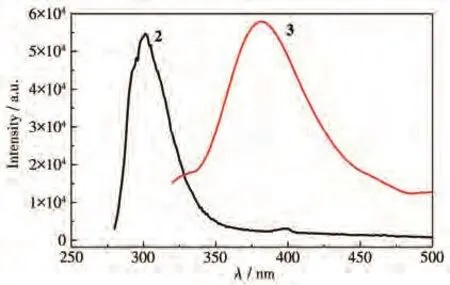
Fig.6 Emission spectra of 2 and 3 in the solid-state at room temperature
3 Conclusions
In summary,three metal-organic frameworks based on the rigid 4-substituted btx,mbtx,and biscarboxylate ligands were synthesized at room temperature.Ligand btx shows bis-monodentate coordination mode in 1,bis-monodentate and monodentate coordination mode in 2.Ligand mbtx exhibits monodentate coordination mode in 3.The adjacent 1D chains are connected into a 3D network byπ-πinteraction in 2.Two sets of equivalent 2D networks catenate with each other to yield a 2D→2D network via intermolecular hydrogen bond in 3.The coordination polymers 2 and 3 can be good candidates for potential fluorescent materials.

