Preparation and Characterization of Copper Complexes of Schiff Base Ligands Synthesized In Situ from Spiropyran Derivative
ZHANG Shao-ZhuangMA Chi-XiaoGUO Hai-YangSHE Jian-Hui ZHANG Jun-Yong*, SHI Yan-Bo LI Guo-Dong REN Xiao-Ming XIE Jing-Li*,,
(1State Key Laboratory of Materials⁃Oriented Chemical Engineering,College of Chemistry and Molecular Engineering,Nanjing Tech University,Nanjing 211816,China)
(2College of Biological,Chemical Sciences and Engineering,Jiaxing University,Jiaxing,Zhejiang 314001,China)
(3Central Laboratory of Jiaxing Traditional Chinese Medicine Hospital,Jiaxing Key Laboratory of Diabetic Angiopathy Research,Jiaxing,Zhejiang 314000,China)
(4State Key Laboratory of Inorganic Synthesis and Preparative Chemistry,College of Chemistry,Jilin University,Changchun 130012,China)
Abstract:By virtue of Schiff base ligands bis(2-(methyliminomethyl)-4-nitro-phenol)dianion(L1)/2-((3-aminopropylimino)-methyl)-4-nitro-phenol monoanion(L2)that derived from photochromic molecule 2-(3′,3′-dimethyl-6-nitrospiro(chromene-2,2′-indolin)-1′-yl)ethanol,two metal-organic complexes[Cu(L1)](1)and[Cu(L2)(1,3-DAP)]NO3(2)have been successfully fabricated through in situ ligand reaction in the presence of Cu2+.These complexes were characterized by IR,single-crystal X-ray diffraction,and powder X-ray diffraction.Additionally,certain photocatalytic properties of the two complexes for the degradation of organic dye molecules have been observed.CCDC:2053028,1;2104827,2.
Keywords:Schiff base ligand;in situ reaction;spiropyran;photocatalytic activity
The pursuit of new complexes containing Schiff base remains at the forefront of molecular science because of their potential applications in various fields such as pharmaceutical,catalysis,analytical chemistry,corrosion,and photochromic.Specifically,(1)Schiff bases have antibacterial,bactericidal,antitumor,antiviral biological activities[1-3];(2)metalcomplexes containing Schiff bases have been used as catalysts[4-7];(3)Schiff bases could be exploited as suitable ligands to identify metal ions and then quantitatively analyze metal ionization[8-11];(4)aromatic Schiff bases have been applied as corrosion inhibitors for steel[12-14];(5)several kinds of Schiff bases have shown their advantages in the photochromic field[15-17].Needless to say,photochromic materials have been applied in many fields such as chemo-and biosensing applications[18-21],photoelectric devices[22-23],information storag[24-25],molecular logic switches[26-29],ion recognition[30],molecular self-assembly[31-32],drug release[33-37],and super-resolution imaging[38-39].
Progress has been made in the past using Schiff base unit to obtain functional materials but usually,those synthetic methods are limited to the multistep reaction,i.e.,the condensation between aldehydes and amines should be executed firstly,and the target molecule could be achieved subsequently.Often overlooked though is the impressive ability of in situ ligand reaction to assemble the final product in a feasible way[40-45].
In this work,through solvothermal process,2-(3′,3′-dimethyl-6-nitrospiro(chromene-2,2′-indolin)-1′-yl)ethanol reacted with ethylenediamine or 1,3-propanediamine in situ to form two Schiff base ligands:bis(2-(methyliminomethyl)-4-nitro-phenol)dianion(L1)/2-((3-amino-propylimino)-methyl)-4-nitro-phenol monoanion(L2)(Scheme 1),and two complexes[CuL1](1)and[Cu(L2)(1,3-DAP)]NO3(2)have been obtained.

Scheme 1 Structures of Schiff base ligands
1 Experimental
1.1 Materials and measurement
All the chemicals were purchased and used withoutfurtherpurification.PowderX-raydiffraction(PXRD)pattern was collected on a DX-2600 X-ray diffractometer with Mo Kα radiation(λ=0.071 073 nm)at 293 K(U=40 kV,I=30 mA,2θ=0°-60°).The IR spectra were obtained on a Varian 640 FT/IR spectrometer with KBr pellet in 4 000-400 cm−1region.The UV-Vis absorption spectra were obtained on a TU-1901 spectrometer.
1.2 Synthesis of[Cu(L1)](1)
Through a one-pot strategy,the product has been prepared in a convenient way(Scheme 2).2-(3′,3′-dimethyl-6-nitrospiro[chromene-2,2′-indolin]-1′-yl)ethanol(0.1 mmol),Cu(NO3)2·3H2O(0.3 mmol),n-propanol(NPA,9 mL),N,N-dimethylformamide(DMF,3 mL)and ethylenediamine(100µL)were mixed and stirred for 2 h.The solution in the screw-capped vial was heated under 80℃for 3 d.After cooling to room temperature,the product was washed with water and dried in a vacuum.Yield:72.0%(based on Cu).Elemental analysis Calcd.for C16H12CuN4O6(%):C,45.77;H,2.88;N,13.34.Found(%):C,44.98;H,2.79;N,13.65.

Scheme 2 Synthetic route of complex 1
In the spiropyran molecule,the indoline ring and the benzopyran ring are connected by a central spiro carbon atom,and the structure is orthogonal but not conjugated[46].Under a mild basic condition in presence of ethylenediamine,in situligand reaction is achieved,and complex[Cu(L)]could be formed.
1.3 Synthesis of[Cu(L2)(1,3⁃DAP)]NO3(2)
As show in Scheme 3,2-(3′,3′-dimethyl-6-nitrospiro(chromene-2,2′-indolin)-1′-yl)ethanol(0.1 mmol),Cu(NO3)2·3H2O(0.3 mmol),NPA(3 mL),DMF(1 mL),and 1,3-diaminopropane(1,3-DAP,200µL)were mixed and stirred for 30 min.The solution in the screwcapped vial was heated under 80℃for 3 d.After cooling to room temperature,green cubic crystals were washed with distilled water and dried in a vacuum.Yield:68.0%(based on Cu).Elemental analysis Calcd.for C13H22CuN6O6(%):C,37.01;H,5.26;N,19.92.Found(%):C,36.76;H,5.15;N,19.68.

Scheme 3 Synthetic route of complex 2
1.4 X⁃ray crystallography
Suitable crystals of complexes 1 and 2 were selected and the crystal data were collected on an Oxford Diffraction Gemini R Ultra diffractometer with graphitemonochromated CuKα(1:λ=0.154 184 nm)and MoKα(2:λ=0.071 073 nm)at 293(2)K.The structures were solved by direct methods and refined onF2by fullmatrix least-squares methods using the SHELXTL package.A summary of the crystal data of the two complexes is provided in Table 1.
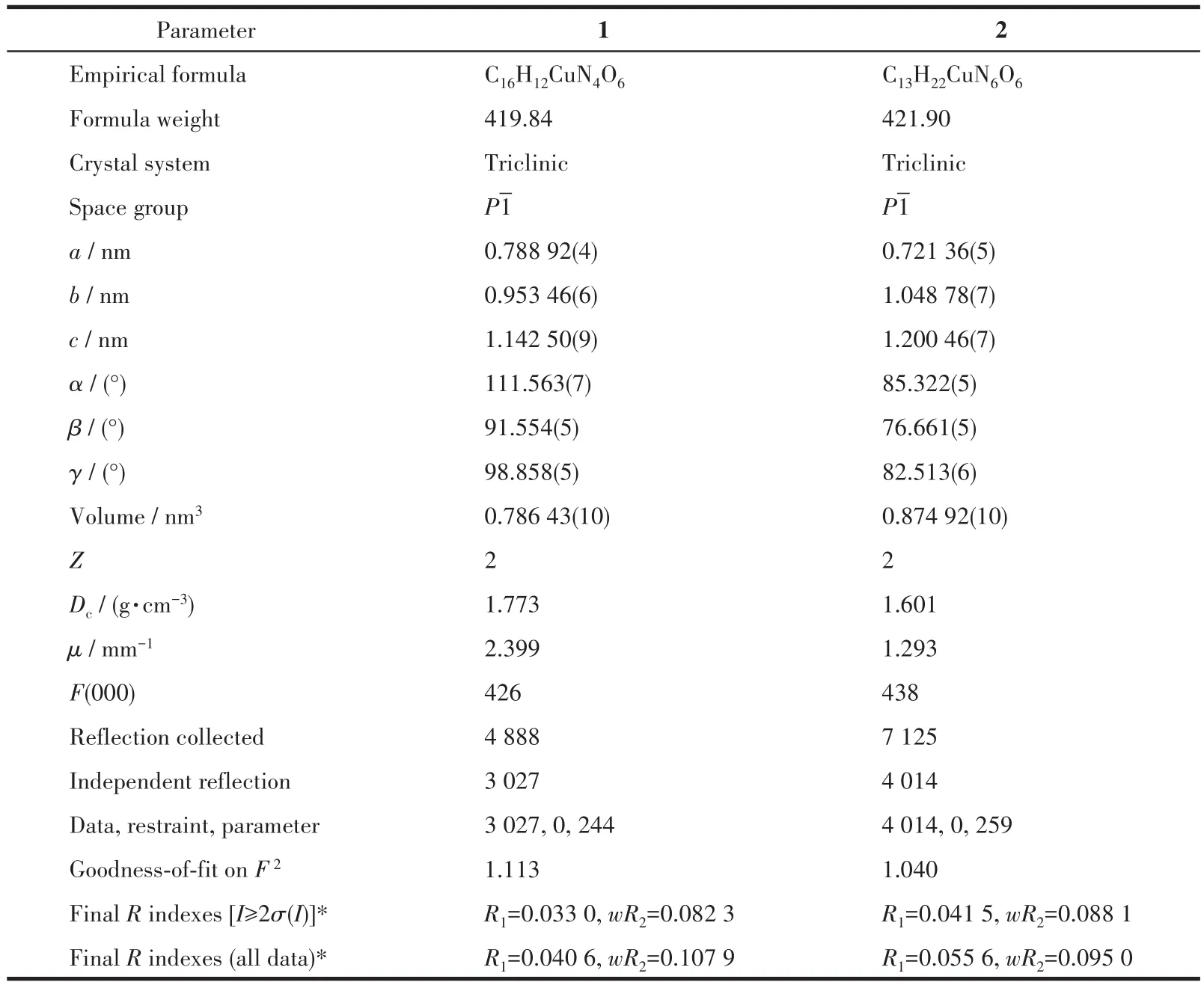
Table 1 Crystal data and structure refinements for complexes 1 and 2
CCDC:2053028,1;2104827,2.
1.5 Experiment of photocatalytic degradation
Complex 1(8 mg)was added to an aqueous solution(40 mL)of pararosaniline hydrochloride(PH,c=10 mmol·L−1)or methylene blue(MB,c=10 mmol·L−1)and stirred for 30 min in the dark to ensure the adsorptiondesorption equilibrium of the resulting solution.Then the solution was exposed to UV irradiation from a 100 W Hg lamp(λ=365 nm)and it was kept for stirring during the irradiation.At every 30 min interval,4 mL solution was taken out for the UV-Vis measurement.
Complex 2(20 mg)was added to an aqueous solution(40 mL)of methylene blue(MB,c=12 mmol·L−1)and stirred for 30 min in the dark to ensure the adsorption-desorption equilibrium.Then the solution was exposed to a 100 W Hg lamp(λ=365 nm)and it was kept for stirring during the irradiation.At every 30 min interval,4 mL solution was taken out for the UV-Vis measurement.
2 Results and discussion
2.1 Structural analysis of 1
Complex 1 crystallizes in the triclinic crystal system with space group P1.The complex is composed of a Cu(Ⅱ)ion and a Schiff base ligand L1.As shown in Fig.1,N1 and N2 atoms,O1 and O2 atoms are coordinated with the central ion,to form a four-coordinated structure,in which the Schiff base unit is achieved through the in situ ligand reaction(Fig.1a).Aided with those C—H…O hydrogen bondings,a 1D supramolecular chain could be observed(Fig.1b).Moreover,considering interchain hydrogen bondings,each[Cu(L1)]molecule is connected with five[Cu(L1)]molecules(Fig.1c)to form a 3D network and it could be simplified to 5-connected bnn topology(Fig.1d).

Fig.1 Crystal structure of 1:(a)coordination environment of Cu(Ⅱ)ion;(b)1D supramolecular chain formed by hydrogen bonding;(c)each[Cu(L1)]connecting with five[Cu(L1)]molecules;(d)diagram of bnn topology
2.2 Structural analysis of 2
Complex 2 crystallizes in the triclinic crystal system with space group P1.This complex is composed of a Cu(Ⅱ)ion,one 1,3-DAP,and a Schiff base ligand L2.As shown in Fig.2a,four N atoms and one O atom are coordinated with the Cu2+to form a five-coordinated tetragonal pyramid configuration[CuNO],and the NO−43acts as the counter anion.Noticeably,the bond length of Cu1—N1,Cu1—N2,Cu1—N4,and Cu1—O1 are 0.200 1(2),0.204 7(2),0.202 1(3),and 0.197 77(18)nm,respectively,whereas the bond length of Cu1—N3 is relatively long(0.219 7(3)nm),indicating the John-Teller effect of Cu2+ion.Through hydrogen bonding interactions(Fig.2b),each[Cu(L2)(1,3-DAP)]NO3molecule is interacted with four neighboring molecules(Fig.2c)to achieve 3D supramolecular network,and it couldbesimplifiedto4-connectedbnntopology(Fig.2d).

Fig.2 Crystal structure of 2:(a)asymmetric unit;(b)hydrogen bonding interactions;(c)each[CuL2(1,3-DAP)]NO3 interacting with four neighbouring molecules;(d)diagram of simplified bnn topology
2.3 IR spectra of complexes 1 and 2
As for 1(Fig.3a),the weak absorption peak at 2 923 cm−1is assigned to the C—H stretching vibration.A strong band at 1 643 cm−1can be attributed to the stretching vibration of the—CH=N of Schiff base unit.Those peaks at 1 596 and 1 303 cm−1can be assigned to the—NO2group.In addition,the peak at 902 cm−1is the characteristic absorption peak of=CH— of the phenyl ring,while a peak at 694 cm−1arises from the para-substituted characteristic peak of the phenyl ring.

Fig.3 IR spectra of complexes 1(a)and 2(b)
As for 2(Fig.3b),the weak absorption peak at 2 935 cm−1is assigned to the C—H stretching vibration.A strong band at 1 634 cm−1can be attributed to the stretching vibration of the NH2group.Those peaks at 1 547 and 1 310 cm−1can be assigned to the—NO2group.In addition,the peak at 902 cm−1is the characteristic absorption peak of=CH—of the phenyl ring,while a peak at 699 cm−1arises from the para-substituted characteristic peak of the phenyl ring.
2.4 PXRD of complexes 1 and 2
As shown in Fig.4,the simulated PXRD patterns and the experimental results of 1 and 2 are consistent in the main locations,demonstrating the single-phase purity of those bulk samples.
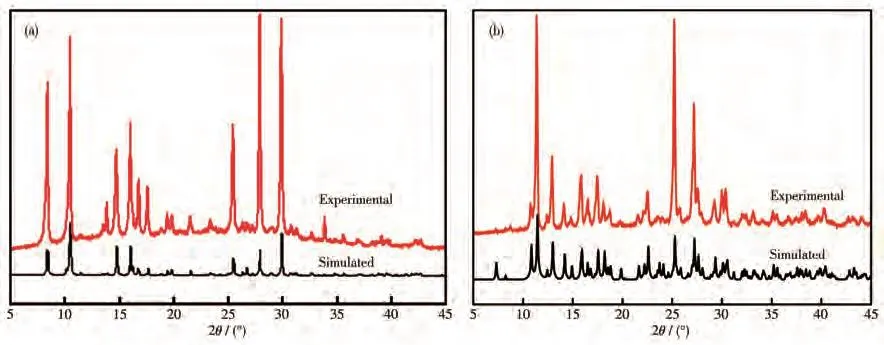
Fig.4 PXRD patterns of complexes 1(a)and 2(b)
2.5 Photocatalytic degradation analysis
Fig.5a and 5b show the changes in UV-Vis spectra of PH and MB solution photocatalytically degraded by complex 1,respectively.The degradation rates of PH and MB were calculated to be 35.9% and 34.6%,respectively.There was no detectable photocatalytic degradation activity of 2 to PH.Nevertheless,complex 2 could degrade MB in a certain way(Fig.5c).The degradation rate of MB was 24.3%.

Fig.5 Changes in UV-Vis spectra of PH(a)and MB(b)solution photocatalytically degraded by complex 1;Changes in UV-Vis spectra of MB(c)solution photocatalytically degraded by complex 2
3 Conclusions
In summary,two complexes containing Schiffbase ligand have been reported.Photocatalytic experiments with pararosaniline hydrochloride and methylene blue have shown those two complexes had certain activity for dye degradation.Notably,the key step in the assembly process is the in situ ligand reaction.Through it,new complexes have been observed and it is anticipated that various Schiff base ligands could be achieved.With elevated temperatures and pressures under hydrothermal/solvothermal experimental conditions,in situ ligand reaction could act as the bridge between coordination chemistry and organic synthetic chemistry because it could harvest new molecules that are inaccessible under mild experimental conditions.To fully utilize the power of in situ ligand reaction and expand the scope of multifunctional complexes,the system of spiropyran and different metal ions in presence of various diamine molecules(including chiral molecules)are actively pursued in the laboratory.

