Isolation and Biochemical Characteristics Analyses of Phenoloxidases (POs) in Three Cultured Mollusk Species
SONG Xiangdi, JIANG Jingwei, XING Jing, 2), *, and ZHAN Wenbin, 2)
Isolation and Biochemical Characteristics Analyses of Phenoloxidases (POs) in Three Cultured Mollusk Species
SONG Xiangdi1), JIANG Jingwei1), XING Jing1), 2), *, and ZHAN Wenbin1), 2)
1),,,266003,2),,266235,
Phenoloxidases (POs) are a group of copper proteins including tyrosinase, catecholase and laccase, which play crucial roles in the innate immune response of mollusks. In this research, POs were studied in cultured mollusk species,including scallop, abaloneand clamThe POs were isolated from hemocytes using linear- gradient native-PAGE combined with catechol staining. The PO activities and their characters were investigated. The molecular mass of PO inwas 576kDa, and it was 228kDain. In, four POs were detected and their mole- cular masses were391kDa, 206kDa, 174kDa and <67kDa,which were named as 391-PO, 206-PO, 174-PO and s-PO, respectively.Ki- netic analyses indicated that all of the POs, except for 391-PO had higher affinity to L-DOPA and catechol than to hydroquinone and dopamine. However, all of the POs failed to oxidize tyrosine. The effects of divalent metal ions on POs’activities were assayed, in- cluding Fe2+, Mg2+, Zn2+, Mn2+, Cu2+and Ca2+from FeCl2, MgSO4, ZnSO4, MnCl2, CuSO4and CaCl2. The POs were inhibited by Fe2+at all determined concentrations. Additionally, the inhibition assay showed that all of the POs were inhibited by cysteine, ascorbic acid, sodium sulfite, citric acid, ethylenediaminetetraacetic acid disodium (EDTA) and sodium diethyldithiocarbamate (DETC). The inhibition effects of critric acid and EDTA are dose-dependent.PO and 391-PO were slightly inhibited by sodium azide, andPO, 391-PO and 174-PO were slightly inhibited by thiourea. In conclusion, the POs in the three cultured mollusks are copper-containing laccase-type phenoloxidaseswith similarbiochemical characteristics even though their molecular massesare different.
;;; phenoloxidase; divalent metal ions; inhibitors
1 Introduction
Mollusks comprise approximately 23% of all known ma-rine organisms, which are highly diverse in terms of size,anatomical structure, behavior, and habitat (Rosenberg, 2014; Yao., 2019). Similar to other invertebrates, mollusks lack adaptive immunity and have developed a highly effec- tive and non-specific innate immune response system toadapt to the challenge of pathogens or alien materials (Wang., 2013; Yang., 2021). Invertebrate innate immu- nity consists of cellular and humoral responses, of which the latter involves a battery of hemolymph-based bioactive compounds (Tassanakajon., 2018).
In invertebrates, phenoloxidases (POs) are generated from a reaction cascade called the prophenoloxidase (pro-PO) ac- tivating system, which can be activated by a pathogena- gent (Ma., 2014; Monwan., 2017; Gu., 2019).They can catalyze phenols into unstable quinones, which in turn can polymerise to form melanin (Muñoz., 2006; Anjugam., 2017; Stączek., 2020). The melanin and intermediate metabolites generated in the reactions are involved in melanization, encapsulation, wound heal- ing, phagocytosis, and pathogen extermination (Antonio Luna-González., 2003; Palmer., 2011; Panig- rahi., 2020).Based on the activities, there are three distinct types in the group of POs (Liu., 2006; Luna-Acosta., 2017), including tyrosinase (EC 1.14.18.1), catecholase (EC 1.10.3.1) and laccase (EC 1.10.3.2). All three types can catalyze the oxidation of o-diphenols, while only tyrosinase can catalyze the orhtohydroxylation of mo- nophenols, and only laccase can catalyze the oxidation of m- and p-diphenols (Luna-Acosta., 2017).
In bivalves, POs distribute across different tissue, such as haemocytes, plasma, gills, foot and mantle, and are like- ly to play various roles (Bharathi and Ramalingam, 1983; Thomas-Guyon., 2009; Yang., 2017). POs may be involved in antiviral and bactericidal defenses and play a crucial immune role (Xing., 2012;Bris., 2015; 2008). When contaminant enters the organism, POs can be considerate as a certain type of stressor, enhancing the re- lease of hormones, neurotransmitters and especially cate- cholamines (Luna-Acosta., 2017). In the clam, PO is copper-containing tyrosinase- type PO that can be inhibited by Ca2+, Mg2+, Cu2+, thio- urea, EDTA and DETC(Cong., 2005).In the scallop, PO is identified as a copper-con-taining laccase-type PO with a molecular mass of 555kDa and can be inhibited by sodium sulfite, EDTA and DETC(Jiang., 2011). In the Sydney rock oyster, two copper-containing tyrosinase-type POs areidentified with molecular masses of 219 and 192kDa re- spectively (Aladaileh., 2007).Moreover, hemocya- nin isolated from the gastropodsand(synonym of) have been de- monstrated to exhibit-diphenoloxidase activity (-diPO) (Idakieva., 2009).
Studying the biochemical characteristics of POs is very important for better understanding their roles in the in-vertebrates. With the aim of providing preliminary data on the POs characteristicsin the mollusks, three cultured spe- cies were selected for POs biochemical characteristics ana- lyses in the present research. Scallop, aba- loneand clam, two bivalves and one gastropod, are three important commercial marine mollusk species in China. In this stu- dy, their POs were isolated, and the substrate specificity, PO kinetics, and the effects of metal ions and other inhi- bitors on POs were investigated. The results can be the reference for further research of PO roles in the mollusks.
2 Materials and Methods
2.1 Experimental Animals
The adult scallop(5.8cm±0.44cm shell length),abalone(6.2cm±0.17cm shell length)and clam(4.2cm±0.21cm shell length) werepurchased from local farm in Qingdao, Shandong Province,China, and maintained in the aerated recirculating seawater system with sponge-filtering at 16℃±2℃ for two weeks. The water was renewed at a volume of 1/2 tank daily. The abalone was fed with(Taihua, China)and the scallop and clam were fed with(Ruikang, China) once a day.
Totally, about 400 individuals of each species were used for hemolymph collection.
2.2 Hemocyte Lysate Supernatant Preparation
About 100mL hemolymph was withdrawn from each ad- ductor muscle sinus using sterilized syringes. Then they were pooled and centrifuged at 700×for 10min at 4℃. The haemocyte pellets were collected and suspended inphosphate-buffered saline (PBS, 2.7mmolL−1KCl, 0.137molL−1NaCl, 1.47mmolL−1KH2PO4, 8.09mmolL−1Na2HPO4·12H2O, pH 7.6). The haemocyte suspensions were sonicated and the cell homogenates were then centrifuged at 15000×for 30min at 4℃. The resulting supernatants, representing hemocyte lysate supernatant (HLS), were col- lected and stored at −80℃for further purification.
2.3 Zymography and Isolation of POs
The HLS was subjected to linear-gradient native-PAGE (6%–20%) in Tris-Glycine buffer (0.025molL−1Tris, 0.2molL−1Glycine, pH 8.0) for 12h at 3W, using high mo- lecular weight markers ranging from 67 to 669kDa (GE Healthcare). After the electrophoresis, one lane of the gel was cut and stained with 1% (W/V) catechol to label the PO-containing bands. Based on the catechol staining band, the relevant unstained lanes of the gel were excised for PO- containing bands, which were successively sonicated in PBS and centrifuged at 17000×for 30min at 4℃. The col- lected fractions of the six PO bands were condensed and desalted separately, using centrifugal concentrators (Ami- con Ultra-15mL, Millipore), and then used as crude POs for the subsequent assay respectively.
2.4 PO Activity Assay
PO activity was measured spectrophotometrically by theformation of dopachromewith L-3,4-dihydroxyphenyla- lanine (L-DOPA) method. In brief, 100μL of the sample was added to 2.0mL L-DOPA (15mmolL−1 in 100mmolL−1Tris-HCl buffer, pH 8.0). Then the formation of dopa- chrome was measured with an UV-2100 Spectrophotome- ter (Unico, China) at 490nm every 3min for 30min. An increase of 0.001 absorbance value per min was consider- ed as one PO activity unit in the assay, expressed as 1U (49010−3min−1).
The protein concentrations were determined according to Bradford method using bovine serum album (BSA; Sig- ma, USA) as the protein standard.
2.5 Substrate Specificity and Kinetic Analysis
Kinetic parameters were determined using the Linewea- ver-Burk-plot method (Sajid-ur-Rehman., 2018). To 100μL of the crude PO solution, 2.0mL of different con- centrations of L-DOPA, catechol, hydroquinone, dopamine and tyrosine dissolved in 100mmolL−1Tris-HCl buffer (pH 8.0) was added, respectively. And the enzymatic ac- tivities were measured by the spectrophotometry at 490nm.
2.6 Effects of Divalent Metal Ions on POs
The effects of divalent metal ions on the activities of POs were assayed by mixing with divalent metal ions, inclu- ding Fe2+, Mg2+, Zn2+, Mn2+, Cu2+and Ca2+from FeCl2, MgSO4, ZnSO4, MnCl2, CuSO4and CaCl2(Songon, China).The crude POs were adjusted to the same UmL−1.A total of 100µL of each crude PO solution was incubated with 100μL of divalent metal ions at different concentrations for20min at 4℃, followed by the addition of 1.9mL of 15mmolL−1L-DOPA. The divalent metal ions and L-DOPA were both dissolved in 100mmolL−1Tris-HCl buffer (pH 8.0). In contrast, the control samples were performed by in-cubating the samples with 100μL of 100mmolL−1Tris-HCl buffer (pH 8.0) without divalent metal ions. Then the PO activities were measured spectrophotometrically at 490nm.
2.7 Effects of Inhibitors on POs
PO inhibition assay was performed by incubating 100µL of each crude PO solution with 100μL of PO inhibitors dissolved in 100mmolL−1Tris-HCl buffer (pH 8.0) at dif- ferent concentrations for 20min at 4℃. The PO inhibitors used in the assay include cysteine, ascorbic acid, sodium sulfite, citric acid, thiourea, sodium azide, ethylenediami- netetraacetic acid disodium (EDTA) and sodium diethyl- dithiocarbamate (DETC). The crude POs were adjusted to the same UmL−1. The control samples were performed by incubating the samples with 100μL of 100mmolL−1Tris- HCl buffer (pH 8.0) without the PO inhibitors. Then the solution was added with 1.9mL of 15mmolL−1L-DOPA and the PO activities were determined spectrophotometri- cally as described above.
2.8 Statistical Analysis
All of the experiments from section 2.5 to 2.7 were per- formed in triplicate, and the data from section 2.6 and 2.7 were presented as the means±standard deviations. Statis- tical analysis was performed using SPSS 11.5.
3 Results
3.1 Molecular Mass of POs
After the linear-gradient native PAGE,HLS andHLS showed only one band reacted to catechol, with a molecular mass of 576kDa and 228kDa respectively. However, in the HLS of, four bands reacted to catechol. The first three were with molecular masses of 391kDa, 206 kDa and 174kDa re-spectively, named as 391-PO, 206-PO and 174-PO. Thesmallest one was with a molecular mass lower than 67kDa, named as s-PO in this paper (Fig.1).

Fig.1 Linear-gradient native PAGE of HLS. Lane 1 was stain- ed with CBB, whereas lane 2 to lane 4 were stained with ca- techol. Lane 1, marker of protein; lane 2, C. farreri HLS; lane 3, H. discus hannai HLS; and lane 4, S. subcrenata HLS.
3.2 Substrate Specificity and PO Kinetics
Using the Lineweaver-Burk model,mvalues of thePO for catechol, L-DOPA, hydroquinone and do- pamine were 0.2, 0.61, 0.84 and 0.88mmolL−1respective- ly(Fig.2A), that ofPO were 0.57, 0.58, 3.57 and 1.6mmolL−1(Fig.2B), and in, that of 391-PO were 1.49, 0.77, 1.24 and 2.17mmolL−1(Fig.2C),that of 206-PO were 0.41, 0.65, 1.69 and 1.51mmolL−1(Fig.2D), that of 174-PO were 1.29, 0.55, 3.3 and 1.94m molL−1(Fig.2E), and that of s-PO were 0.52, 0.44, 10.50and 0.81mmolL−1(Fig.2F). However, no reaction with ty- rosine was detected using the POs obtained from the three mollusks.
3.3 Effects of Metal Ions on POs
In, Mg2+andCa2+stimulated while Fe2+inhi- bited the PO activity at all of the determined concentra- tions. Zn2+stimulated at concentrations of 5 and 10mmolL−1, but obviously inhibited at 50mmolL−1. Cu2+had sti- mulative effects at 5mmolL−1, and inhibitory effects at 20 and 30mmolL−1, which was opposite to Mn2+(Fig.3A).
In, Mn2+stimulated while Cu2+, Zn2+, Fe2+and Ca2+inhibited the POactivity at all of the deter- mined concentrations, and Mg2+had inhibitory effect at 5mmolL−1and stimulative effect at 50mmolL−1(Fig.3B).
In, as for 391-PO, Mn2+stimulated while Cu2+, Fe2+and Ca2+inhibited the PO activity at all of the determined concentrations, and Zn2+showed stimulative effect at 5mmolL−1and inhibitory effect at 20, 30 and 50mmolL−1, which was opposite to Mg2+(Fig.3C). For 206- PO, Mn2+, Mg2+and Ca2+stimulated while Fe2+inhibitedat all of the determined concentrations, Zn2+and Cu2+had stimulative effects at 5mmolL−1and inhibitory effects at 50mmolL−1(Fig.3D). For 174-PO, Mn2+and Mg2+stimu- lated while Fe2+inhibitedat all of the determined concen- trations; Zn2+had a stimulative effect at 5mmolL−1, and Cu2+showed obvious inhibition at the concentrations of 5, 10 and 20mmolL−1(Fig.3E). For s-PO, Mn2+, Mg2+and Ca2+stimulated while Cu2+and Fe2+inhibitedPO activity at all of the determined concentrations, and Zn2+showed a stimulative effect at 5mmolL−1and inhibitory effect at 50mmolL−1(Fig.3F).
In general, all POs of the three species were inhibited by Fe2+at various determined concentrations. Except for scallopPO, other five POs were stimulated by Mn2+at various determined concentrations. Furthermore, Mg2+, Cu2+, Zn2+and Ca2+inhibited the PO in, relatively different from what they did inPO andPO.
3.4 Effects of Inhibitors on POs
When the inhibitors were adjusted to 50mmolL−1, cri- tric acid, ascorbic acid, DETC, cysteine and sodium sul- fite showed 100% inhibition to the activities of POs, while the inhibition of EDTA varies from 30% to 90%. Furth- more, the inhibition of critric acid and EDTA are dose-de- pendent. Additionally, sodium azide only showed slightly inhibition toPO and 391-PO, while thio- urea only showed slightly inhibition toPO, 391-PO and 174-PO (Fig.4).
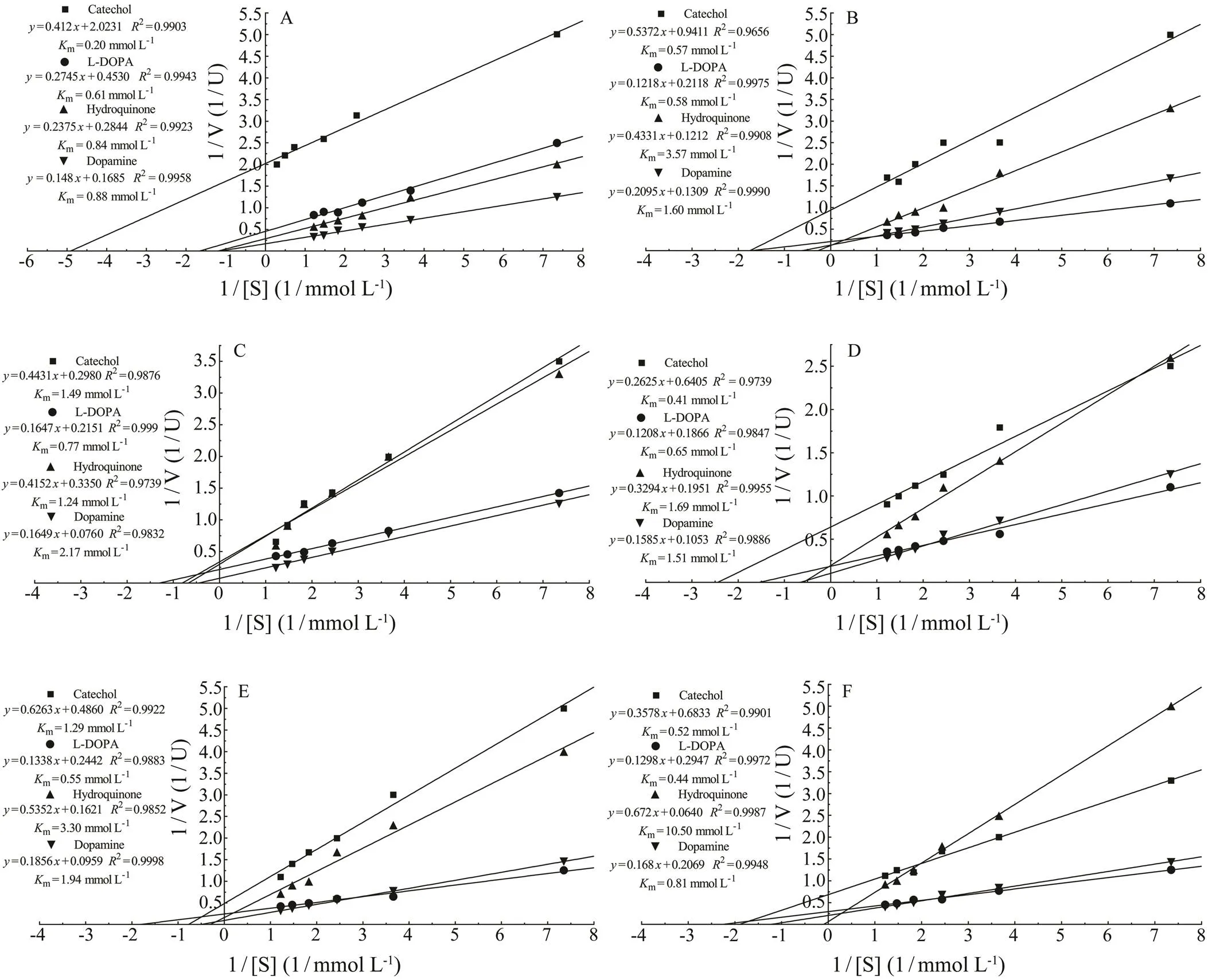
Fig.2 Kinetic analyses of isolated POs. The Km values were determined using L-DOPA, catechol, hydroquinone and do- pamine as substrates, and were calculated according to the Lineweaver-Burk model. A, C. farreri PO; B, H. discus hannai PO; C,391-PO; D, 206-PO; E, 174-PO; F, s-PO.
4 Discussion
The function of POs has close relationship to the bio- chemical and enzymatic characteristics, and the characte- rization of POs has been reported in some seashells, such as the clam, the scallop, the Sydney rock oyster, and the Pacific oyster(Cong., 2005; Aladaileh., 2007; Jiang., 2011; Luna-Acosta., 2011). Here, we iso- lated POs from the hemocytes of scallop, aba- lone, and calm, and char- acterized POs based on the kinetic parameters, the effects of divalent metal ions and inhibitors on enzymatic activi- ties.
The results of zymography showed that only one PO was detected inand, while four POs were detected in theThePO (576kDa) had a larger molecular mass thanPO (228kDa) andPOs (391kDa, 206kDa, 174kDa, <67kDa).Among the reported marine mollusk POs, the molecular mass of PO is parallel between(576kDa) and(555kDa) (Xing., 2012),(228kDa) and(219kDa) (Aladaileh., 2007),391-PO (391kDa) and mussel(381kDa) (Renwrantz., 1996),s-PO (<67kDa) and Pacific oyster(<40kDa)(Luna-Acosta., 2011).Column chromatography is a preferred method for protein isola- tion and mass spectrometry should be important. However, homogenization was not achieved, which could be caused by the complicated generation of PO. Since PO is produced by the proPO system and the content is very low(Monwan., 2017). It is a relatively complicated pro- cess and different forms of POs may be produced, which need to be fully studied. We have also triedse- quencing.However, the N-terminal of the POs was block- ed. Additionally, conservation or specific genes for the lac- case type PO in the three species have not been studied thoroughly.
All of the POs obtained in this study were capable of oxidizing L-DOPA, catechol and hydroquinone effective- ly, but failed to oxidize tyrosine. Based on the classifica- tion of POs(Luna-Acosta., 2017), we suggested that the three POs are all laccase-type phenoloxidase, similar to that of silkworm(Yamazaki, 1972), oyster(Jordan and Deaton, 2005),(Jiang., 2011)and(Wang., 2018). Kinetic analysis indicated that thePO,PO, 206-PO, 174-PO and s-PO inhad higher affinity to L-DOPA and catechol than hy- droquinone and dopamine, while the 391-PO ofhad higher affinity to L-DOPA and hydroquinone than to catechol and dopamine.All of the POs had the highest affinity to L-DOPA. Considering the affinity from high to low, POs can be listed as s-PO, 174-PO,PO,PO, 206-PO and 391-PO.

Fig.3 Effects of 6 metal ions on the activity of isolated POs. The PO activity was assayed using L-DOPA (15mmolL−1) as a specific substrate. A, C. farreri PO; B, H. discus hannai PO; C,391-PO; D, 206-PO; E, 174-PO; F, s-PO.
The POs were inhibited by Fe2+at all determined con- centrations for the three species, which is also found in(Jiang., 2011)and clam(Jiang., 2012)ThePO was strongly sti- mulated in the presence of Ca2+and Mg2+, similar to(Xing., 2012),which may be due to the close relationship between the two species.In, Mn2+Cu2+, Zn2+and Ca2+inhibited the POactivity at all of the determined concentrations, absolutely different fromPO andPO. The possible reasons may be thatand the other two speciesbelong to two largely divergent classes. Furthermore, forPO and 206-PO, Cu2+had stimulative effects at 5mmolL−1, and inhibitory effects at high concentrations. Meanwhile, for other four POs, Cu2+had inhibitory effectsat all concentrations. As we have known, Cu2+is the activecenter of POsin most invertebrates. However, the extracopper ions, even at 5mmolL−1, may inhibit the enzyme ac-tivity, which is also found in(Jiang., 2012). Some studies speculated that metal ions may activate electrophile and nucleophile binding, thenrelease electrons to modulate PO activity or divalent ca-tions, which may change the secondary structure of certain peptides of PO to influence the activity (Feng., 2008; Zibaee., 2011). However, the detailed mechanisms behind this study require further investigation.
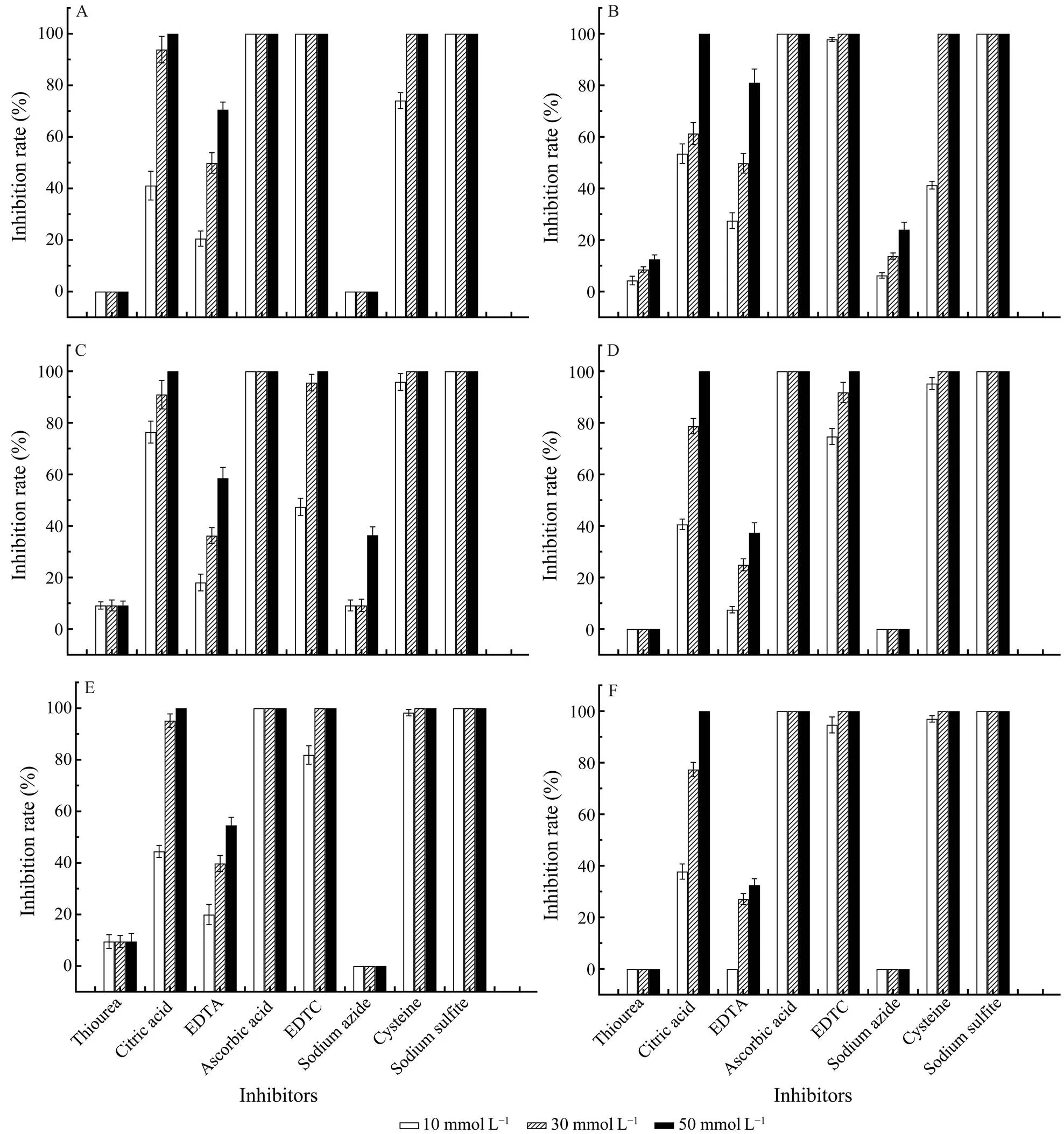
Fig.4 Effects of inhibitors on the activity of isolated POs. The PO activity was assayed using L-DOPA (15mmolL−1) as a specific substrate. A, C. farreri PO; B, H. discus hannai PO; C,391-PO; D, 206-PO; E, 174-PO; F, s-PO.
The common antioxidants including citric acid, ascor- bic acid, cysteine, and sodium sulfitecan strongly inhibit the activities of all of the POs obtained in this research.EDTA and DETC-a divalent cation and specific copper chelator can also inhibit the activities of POs.Meanwhile, sodium azide only inhibited the activities ofPO and 391-PO, while thiourea only showed inhibi- tion toPO, 391-PO and 174-PO. These results indicated that the construction of protein differed obviously among some POs.On the other hand,PO, 206-PO and s-PO were relatively similar to each otherandPO was similar to 391-PO. Although POs are divided into three types according to the substrate specificity, the inhibitors do not have high specificity for the inhibition of the three types of POs.The mechanism of inhibiting POs of antioxidants, including citric acid, as- corbic acid, cysteine, sodium sulfite and thiourea, is caus- ing a strong reduction reaction. EDTA and DETC can che- late Cu2+in the PO molecule to change the active confor- mation of the enzyme, thereby producing inhibition effects,while sodium azide exerts an inhibitory effect through oxi- dation (Luna-Acosta., 2017).
In conclusion, POs were isolated from three cultured mol- lusk species, including scallop, abaloneand clamThebiochemical characteristics of the separated enzymes were studied. Further study with gene cloning and monoclonal antibody production are expected to deeply investigate the function of POs.
Acknowledgements
This research was supported by the Qingdao National La-boratory for Marine Science and Technology (No. QNLM 2016ORP0307), the National Key Research and Develop- ment Program of China (No. YFD0900504), the National Basic Research Program of China (No. 2012CB114405), and the Taishan Scholar Program of Shandong Province.
Aladaileh, S., Rodney, P., Nair, S. V., and Raftos, D.A., 2007. Characterization of phenoloxidase activity in Sydney rock oys- ters ().,148 (4): 470-480, DOI: 10.1016/j.cbpb.2007.07.089.
Anjugam, M., Vaseeharan, B., Iswarya, A., Amala, M., Govin- darajan, M., Alharbi, N. S.,., 2017. A study on β-glucan binding protein (β-GBP) and its involvement in phenoloxi- dase cascade in Indian white shrimp.,92: 1-11, DOI: 10.1016/j.molimm.2017.09.013.
Antonio, L. G., Maeda, M. A. N., Vargas, A. F., Ascencio, V. F., and Robles, M. M., 2003. Phenoloxidase activity in larval and juvenile homogenates and adult plasma and haemocytes of bi- valve molluscs., 15 (4): 275- 282, DOI: 10.1016/S1050-4648(02)00165-1.
Bharathi, N., and Ramalingam, K., 1983. Electrophoretic study of the enzyme phenoloxidase from the enzyme gland in the foot of., 70 (2): 123-128, DOI: 10.1016/0022-0981(83)90126-0.
Bris, C. L., Richard, G., Paillard, C., Lambert, C., Seguineau, C., Gauthier, O.,., 2015. Immune responses of phenoloxidase and superoxide dismutase in the manila clamchallenged with–Part I:Spatio-tem- poral evolution of enzymes’ activities post-infection., 42 (1): 16-24, DOI: 10.1016/j.fsi.2014.10.021.
Cong, R., Sun, W., Liu, G., and Fan, T., 2005. Purification and characterization of phenoloxidase from clam., 18 (1): 61-70, DOI: 10.1016/j.fsi.2004.06.001.
Feng, C., Song, Q., Lü, W., and Lu, J., 2008. Purification and cha-racterization of hemolymph prophenoloxidase from(Lepidoptera: Pyralidae) larvae., 151 (2): 139-146, DOI: 10.1016/j.cbpb.2008.05.012.
Gu, Q., Zhou, S., Zhou, Y., Huang, J., Shi, M., and Chen, X., 2019.A trypsin inhibitor-like protein secreted byteratocytes inhibits hemolymph prophenoloxidase activation of., 116: 41- 48, DOI: 10.1016/j.jinsphys.2019.04.009.
Idakieva, K., Siddiqui, N. I., Meersman, F., De Maeyer, M., Cha-karska, I., and Gielens, C., 2009. Influence of limited proteo- lysis, detergent treatment and lyophilization on the phenolo- xidase activity ofhemocyanin., 45 (2): 181-187, DOI: 10.1016/j.ijbiomac.2009.04.022.
Jiang, J., Xing, J., and Zhan, W., 2012. Purification and charac- terization of laccase-type phenoloxidase from the clam., 43 (2): 294-298(in Chinese with English abstract).
Jiang, J., Xing, J., Sheng, X., and Zhan, W., 2011. Characteriza- tion of Phenoloxidase from the Bay Scallop., 30 (2): 273-277, DOI: 10.2983/035.030.0212.
Jordan, P. J., and Deaton, L., 2005. Characterization of phenolo- xidase fromhemocytes and the effect ofon phenoloxidase activity in the hemo- lymph ofand., 24: 477-482.
Liu, G., Yang, L., Fan, T., and Cong, R., 2006. Purification and characterization of phenoloxidase from crab., 20 (1): 47-57, DOI: 10.1016/j.fsi.2005.03.012.
Luna, A., Breitwieser, M., Renault, T., and Thomas, H., 2017. Re- cent findings on phenoloxidases in bivalves., 122: 5-16, DOI: 10.1016/j.marpolbul.2017.06.031.
Luna, A., Thomas, H., Amari, M., Rosenfeld, E., Bustamante, P., and Fruitier, I., 2011. Differential tissue distribution and spe- cificity of phenoloxidases from the Pacific oyster., 159: 220-226, DOI: 10.1016/j.cbpb.2011.04.009.
Ma, T. H. T., Benzie, J. A. H., He, J. G., Sun, C. B., and Chan, S. F., 2014. PmPPAF is a pro-phenoloxidase activating factor involved in innate immunity response of the shrimp., 44: 163- 172, DOI: 10.1016/j.dci.2013.12.007.
Monwan, W., Amparyup, P., and Tassanakajon, A., 2017. A snake-like serine proteinase (PmSnake) activates prophenoloxidase-activating system in black tiger shrimp., 67: 229-238, DOI: 10.1016/j.dci.2016.09.016.
Muñoz, P., Meseguer, J., and Esteban, M., Á., 2006. Phenoloxi- dase activity in three commercial bivalve species. Changes due to natural infestation with., 20 (1): 12-19, DOI: 10.1016/j.fsi.2005.02.002.
Palmer, C. V., Bythell, J. C., and Willis, B. L., 2011. A compara- tive study of phenoloxidase activity in diseased and bleached colonies of the coral., 35 (10): 1098-1101, DOI: 10.1016/j.dci.2011.04.001.
Panigrahi, A., Sivakumar, M. R., Sundaram, M., Saravanan, A., Das, R. R., Katneni, V. K.,., 2020. Comparative study on phenoloxidase activity of bio floc-reared Pacific white shrimpand Indian white shrimpon graded protein diet.,518: 734654, DOI: 10.1016/j.aquaculture.2019.734654.
Renwrantz, L., Schmalmack, W., Redel, R., Friebel, B., and Schneeweiß, H., 1996. Conversion of phenoloxidase and pero-xidase indicators in individual haemocytes ofspecimens and isolation of phenoloxidase from haemocyte ex-tract., 165: 647-658.
Rosenberg, G., 2014. A new critical estimate of named species- level diversity of the recent mollusca., 32 (2): 308-322, DOI: 10.4003/006.032.0204.
Sajid, R., Saeed, A., Saddique, G., Ali, C. P., Ali, L. F., Abbas, Q.,., 2018. Synthesis of sulfadiazinyl acyl/aryl thiourea derivatives as calf intestinal alkaline phosphatase inhibitors, pharmacokinetic properties, lead optimization, Lineweaver-Burk plot evaluation and binding analysis., 26 (12): 3707-3715, DOI: 10.1016/j.bmc.2018.06.002.
Stączek, S., Zdybicka-barabas, A., Pleszczyńska, M., Wiater, A., and Cytryńska, M., 2020. Aspergillus niger α-1,3-glucan acts as a virulence factor by inhibiting the insect phenoloxidase system., 171: 1-5, DOI: 10.1016/j.jip.2020.107341.
Tassanakajon, A., Rimphanitchayakit, V., Visetnan, S., Ampa- ryup, P., Somboonwiwat, K., Charoensapsri, W.,., 2018. Shrimp humoral responses against pathogens: Antimicrobial peptides and melanization., 80: 81-93, DOI: 10.1016/j.dci.2017.05.009.
Thomas, G. H., Gagnaire, B., Bado, N. A., Bouilly, K., Lapègue, S., and Renault, T., 2009. Detection of phenoloxidase activity in early stages of the Pacific oyster(Thun- berg)., 33: 653- 659, DOI: doi.org/10.1016/j.dci.2008.11.011.
Wang, L., Qiu, L., Zhou, Z., and Song, L., 2013. Research pro- gress on the mollusc immunity in China., 39: 2-10, DOI: 10.1016/j.dci.2012.06.014.
Wang, Z., Hu, R., Ye, X., Huang, J., Chen, X., and Shi, M., 2018. Laccase 1 gene from(PxLac1) and its functions in humoral immune response., 107: 197-203, DOI: 10.1016/j.jinsphys.2018.04.001.
Xing, J., Jiang, J., and Zhan, W., 2012. Phenoloxidase in the scallop: Purification and antibacterial activity of its reaction products generated.,32 (1): 89-93, DOI: 10.1016/j.fsi.2011.10.025.
Xing, J., Lin, T., and Zhan, W., 2008. Variations of enzyme acti- vities in the haemocytes of scallopafter in- fection with the acute virus necrobiotic virus (AVNV)., 25 (6): 847-852, DOI: 10.1016/j.fsi.2008.09.008.
Yamazaki, H. I., 1972. Cuticular phenoloxidase from the silk- worm,: Properties, solubilization, and purifica- tion., 2: 431-444, DOI: 10.1016/0020-1790(72)90023-6.
Yang, B., Pu, F., Li, L., You, W., Ke, C., and Feng, D., 2017. Functional analysis of a tyrosinase gene involved in early lar- val shell biogenesis inand its response to ocean acidification., 206: 8- 15, DOI: 10.1016/j.cbpb.2017.01.006.
Yang, L., Wang, Z., Zuo, H., Geng, R., Guo, Z., Niu, S.,., 2021. The LARK protein is involved in antiviral and antibac- terial responses in shrimp by regulating humoral immunity., 114: 103826, DOI: 10.1016/j.dci.2020.103826.
Yao, T., Zhao, M., He, J., Han, T., Peng, W., and Zhang, H., 2019. Gene expression and phenoloxidase activities of hemo- cyanin isoforms in response to pathogen infections in abalone., 129: 538-551, DOI: 10.1016/j.ijbiomac.2019.02.013.
Zibaee, A., Bandani, A. R., and Malagoli, D., 2011. Purification and characterization of phenoloxidase from the hemocytes of(Hemiptera : Scutelleridae)., 158 (1): 117-123, DOI: 10.1016/j.cbpb.2010.10.006.
October 19, 2020;
January 4, 2021;
March 23, 2021
© Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2022
. Tel: 0086-532-82031580
E-mail: xingjing@ouc.edu.cn
(Edited by Qiu Yantao)
 Journal of Ocean University of China2022年2期
Journal of Ocean University of China2022年2期
- Journal of Ocean University of China的其它文章
- Study of the Wind Conditions in the South China Sea and Its Adjacent Sea Area
- A Spatiotemporal Interactive Processing Bias Correction Method for Operational Ocean Wave Forecasts
- Characteristics Analysis and Risk Assessment of Extreme Water Levels Based on 60-Year Observation Data in Xiamen, China
- Underwater Target Detection Based on Reinforcement Learning and Ant Colony Optimization
- Polar Sea Ice Identification and Classification Based on HY-2A/SCAT Data
- Thermo-Rheological Structure and Passive Continental Margin Rifting in the Qiongdongnan Basin,South China Sea, China
