用正电子湮没谱研究煅烧对SBA-15热稳定性的影响
李重阳 王飞跃 陈志权
用正电子湮没谱研究煅烧对SBA-15热稳定性的影响
李重阳1王飞跃1陈志权2
1(华北水利水电大学电力学院 郑州 450045)2(武汉大学物理科学与技术学院 武汉 430072)
多孔材料由于具有高比表面积、高渗透性、吸附性和可组装性等优异的物理化学性能,被广泛应用于气体吸附、电化学、药物输送、催化剂和催化剂载体等领域。多孔材料的应用性取决于其宏观性质,尤其是孔隙结构的多样性、孔径的可调性、热稳定性等对其大规模实际应用非常重要。利用正电子湮没谱学(Positron Annihilation Spectroscopy,PAS)观察了SBA-15的热稳定性。以正硅酸四乙酯为硅源,P123为结构导向剂,合成了原生有序介孔二氧化硅(SBA-15)和550 ℃煅烧后的有序介孔二氧化硅(SBA-15)。将上述两种SBA-15在100~1 000 ℃温度下煅烧处理,观察其孔隙结构的热稳定性变化。采用小角度X射线散射(Small Angle X-ray Scattering,SAXS)、扫描电子显微镜(Scanning Electron Microscopy,SEM)、高分辨透射电子显微镜(High-resolution Transmission Electron Microscope,HRTEM)、N2吸附/脱附(N2adsorption-desorption)和正电子湮没谱学(Positron Annihilation Spectroscopy,PAS)等方法研究了两种SBA-15有序孔隙结构的稳定性。正电子寿命测量结果表明:两种SBA-15均存在4种寿命组分,较长寿命3和4分别对应于o-Ps在材料中微孔和二维六角的较大孔中的湮灭结果。随着热处理温度的升高,这两种SBA-15中较长寿命4逐渐降低,其强度4也逐渐降低,但煅烧后SBA-15较长寿命及其强度在600 ℃后下降更为明显。同时,参数结果与正电子寿命的结果吻合较好,-曲线也表明煅烧后SBA-15孔隙类型在100~900 ℃间几乎未发生变化。结果表明:煅烧后SBA-15表现出较好的热稳定性,同时电子偶素(Ps)是一种非常灵敏的研究孔隙结构的探针。
SBA-15,孔隙结构,电子偶素,热稳定性
自20世纪90年代以来,多孔材料由于具有高比表面积、高渗透性、吸附性和可组装性等优异的物理化学性质,在气体吸附、电化学、药物传递、催化剂和催化剂载体等领域得到了广泛的应用[1‒ 2]。多孔材料的应用在很大程度上取决于其宏观性质,特别是其孔隙结构的多样性及孔径的可调节性。直到1992年,美孚公司的研究人员在碱性条件下使用阳离子表面活性剂和无机硅源,报道了一系列M41S(MCM-41、MCM-48和MCM-50),这是沸石从微米到介孔孔径的里程碑式发现[3‒7]。近年来,MCM-41、SBA-15、KIT-6等介孔材料已发展成为化学、物理、生物、医学等领域的研究热点之一[4, 8‒9]。SBA系列通常在酸性介质中合成,包括SBA-1、SBA-2、SBA-3、SBA-7、SBA-15、SBA-16等[10‒13]。以阳离子表面活性剂为模板剂,以聚环氧乙烷-聚环氧丙烷-聚环氧乙烷(PEO-PPO-PEO)为结构导向剂,可在强碱条件下合成了SBA-1、SBA-2、SBA-3、SBA-7,在强酸条件下合成了SBA-11、SBA-12、SBA-14、SBA-15、SBA-16。由于其表面含有丰富的硅羟基,介孔材料更容易掺杂一些具有催化活性的金属原子到其骨架中或在表面接枝特定的官能团,使其在大分子催化反应中表现出较好的转化率或选择性[14‒20]。
正电子在小颗粒细粉末中的湮没是基础而又特别有吸引力的研究课题[21‒26]。在金属中,由于其电子密度高,正电子注入或扩散到该材料表面后,由于简单的库伦引力作用快速发生湮没,不会形成电子偶素(Ps)[27‒29]。与金属不同,当正电子入射到小颗粒的纳米粉末中,如Al2O3、SiO2和MgO,相当一部分正电子会捕获低密度区域中的电子形成Ps,很大可能发生3γ自湮没,进而显示出较长的寿命成分。因此,多孔绝缘材料中,正电子湮没谱(Positron Annihilation Spectroscopy,PAS)关键特征是能够通过o-Ps的形成、扩散和湮没检测其孔洞信息。正电子从22NaCl源中发射时携带0~545 keV的能量,一旦进入凝聚态物质内部,因电离作用和原子核的强烈散射,e+将受到强烈排斥,发生非弹性碰撞后快速损失其动能,1~3 ps内迅速慢化到热能级(在室温下为0.025 eV)。热化后e+在固体中以无规则热运动的形式随机扩散,约100 nm后,与电子发生自由湮没或被空位型缺陷捕获后湮没。
如前所述,正电子从22Na源中发射时携带的能量较高,最高峰值为0.545 MeV,平均能量为0.220 MeV。这些高能的正电子在与周围的原子、分子碰撞过程中可以产生大量的电子-空穴对[30‒32]。当晶粒尺寸小于正电子的扩散长度,正电子将有机会扩散至晶粒的表面并在表面形成表面态Ps,或者在表面捕获一个电子后形成Ps[33‒36]。由于正电子和电子自旋方向的不同,Ps原子分为三重态o-Ps(=1,s=-1,0,+1)和单重态p-Ps(=0,s=0)。从自旋态的差异来看,o-Ps的形成概率约为p-Ps的3倍。在真空中,由于发射出3γ射线发生自湮灭,o-Ps寿命高达142 ns,而发射2γ射线的p-Ps寿命仅为125 ps。
正电子湮没谱学作为表征多孔材料孔隙结构的一种特色测量技术,已得到广泛的应用[23‒24,37‒40]。在具有二维六角柱状孔洞的SBA-15纳米颗粒中,正电子在其管道内先捕获一个电子形成Ps,进而发生湮没,且o-Ps原子寿命与孔隙结构有关[41]。当o-Ps局限于孔内时,o-Ps会从孔壁上拾取一个电子并发出2γ射线发生湮灭,称为拾取湮灭。因此,o-Ps的寿命将减少到几ns左右。根据Tao、Eldrup、Dull和Gowork等[42‒45]建立的寿命-孔半径间半经验模型:

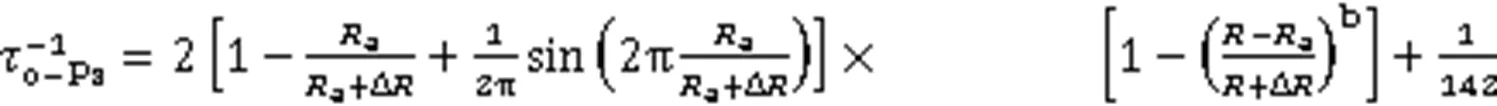

其中:

本文在酸性条件下制备了SBA-15,采用正电子寿命测量技术、小角X射线散射、扫描电子显微镜、高分辨透射电子显微镜和N2吸附/脱附测试等表征手段。同时,采用后热处理方式,通过分析热处理对SBA-15孔结构的影响,探究SBA-15的热稳定性。
1 实验部分
以两亲性三嵌段共聚物Pluronic P123(分子重量w= 5 800,EO20-PO70-EO20,Sigma Aldrich)为结构导向剂,正硅酸四乙酯(TEOS,C8H20O4Si)为硅源制备了SBA-15[4]。将2 g P123溶于11.823 g 37wt% HCl、60.026 g去离子水中,待P123完全溶解后,再加入4.271 g正硅酸四乙酯,35 ℃混合搅拌24 h,然后转移到Teflon-lined高压釜,在静态条件下100 ℃保持24 h。最后,经过滤、蒸馏水洗涤,并在100 ℃干燥24 h,命名为原生(As-Prepared)SBA-15。将部分原生SBA-15在550 ℃下空气中煅烧6 h,升温速率为1 ℃·min-1,称为煅烧(Calcined)SBA-15。将两种合成的SBA-15分别在玛瑙砂浆中手工研磨2 h后,在6 MPa的静压下压制5 min,制得厚度为1.5 mm、直径为15 mm的圆片状。然后在100~1 000 ℃温度下,以10 ℃·min-1的升温速率进行退火,研究其介孔结构的热稳定性。
用小角X射线散射仪(X'Pert Pro,PANalytical,Netherlands)对Cu Kα辐射进行了小角度X射线散射(Small Angle X-ray Scattering,SAXS)测量,入射X射线为0.154 06 nm,工作电压和电流为40 kV、40 mA。扫描电子显微镜(Scanning Electron Microscope,SEM)(XL30(Philips),荷兰)和高分辨透射电子显微镜(High-Resolution Transmission Electron Microscopy,HRTEM)(JEOL JE-2010FEF(UHR),日本东京)用于观察其形貌,加速度电压为200 kV。为了验证合成的SBA-15的孔隙结构,利用JW-BK 100C气体吸附分析仪在77 K下进行N2吸附/脱附等温线测量,180 ℃预处理2 h。
22Na正电子源(1.85×106Bq)被包夹在两片相同的样品中形成三明治式的结构,随后放进真空样品室中,整个实验过程中真空度优于1.33×10-4Pa。为了同时收集正电子湮没寿命谱和多普勒展宽谱,寿命谱的两个探头与多普勒展宽谱的两个探头垂直交叉放置。为了尽可能多搜集o-Ps的3γ湮灭信号,将终止道(0.511 MeV湮灭γ射线)鉴别器上的低能级能窗设置得尽可能低。寿命谱的总计数为1.5×106,计数率为26,每个多普勒展宽谱的总计数大于1.5×107。
2 结果与讨论
2.1 SBA-15形貌及孔结构
图1为制备的SBA-15的小角X射线散射图。从图1可以看到,SBA-15在2分别为0.98°、1.78°和2.06°位置存在三个特征散射峰:(100)、(110)、(200),即P6mm结构的特征峰。该结果证实制备的SBA-15具有规则二维六角的孔洞排列。通过Bragg的公式,该样品的周期型间隔(孔径+孔壁)采用主散射峰位(100)计算得到:

通过小角X射线散射(Small Angle X-ray Scattering,SAXS)测量,我们能够得到SBA-15的周期型间隔,可估算出SBA-15的周期型间隔为9.01 nm,由周期型间隔减去孔壁得出SBA-15的孔径。从图2中SBA-15的SEM图可知,制备的SBA-15的颗粒分布均匀,均具有0.6 μm长和0.2 μm宽的珠状链条形貌。

图2 SBA-15的扫描电子显微镜
同时,高分辨透射电子显微镜(High-resolution transmission electron microscope,HRTEM)测试结果分别是垂直孔径方向和平行孔径方向证实了SBA-15中孔结构的高度有序性,即一条条排列紧密、高度有序的二维六角结构的孔洞管道,如图3所示。TEM结果表明:SBA-15的孔径约为7~9 nm,孔壁约为1.82 nm。结合SAXS测试中周期型间隔结果,可知SBA-15的孔径约为7.19 nm。
为了获得更详细的孔隙参数,特别是孔径分布信息,对合成的SBA-15在低温77 K下进行N2吸附/脱附测量,得到SBA-15的吸附/脱附等温线及其孔径分布。如图4所示,N2吸附/脱附等温线为典型的IV型曲线,具有H1滞后回线,在相对压力/0为0.6~0.9时发生明显的毛细凝结,这也是介孔尺寸的特征现象[46]。同时,利用Barrett-Joyner-Halenda(BJH)模型[47]从吸附分支计算样品的孔径分布曲线,其孔径分布相对较窄,最可几孔径约为10.0 nm,平均孔径约为7.5 nm,与SAXS和SEM结果基本一致。基于BET模型[48],合成的比表面积约为597.92 m2·g-1。

图3 SBA-15(100)方向的高分辨透射电子显微镜 (a) 100 nm,(b) 50 nm,(c) 20 nm

图4 SBA-15的N2吸附/脱附等温线和孔径分布图
2.2 正电子湮没谱学结果
合成SBA-15的峰归一化的正电子湮没寿命谱,如图5所示。结果表明,SBA-15中正电子寿命组分相对较长,表明样品中孔隙较大。由PATFIT程序[49]可知,两个较长寿命3(约6 ns)和4(约92 ns)是o-Ps在多孔材料中孔洞内湮没的结果[50]。众所周知,o-Ps的寿命与材料的孔径密切相关。两种o-Ps寿命成分表明SBA-15中存在两种不同类型的孔隙,分别具有较小和较大的开孔体积,即o-Ps在SBA-15材料中二维P6mm六角柱状介孔孔管道和管道-管道间的连接管内湮没的结果。对于本文SBA-15中二维六角的孔结构,根据已建立的寿命-半径的半经验模型、Tao-Eldrup及其拓展模型[42‒43]、基于长方体的Dull拓展模型[45]得到的孔径误差略大,而基于圆柱状的Goworek拓展模型[44]更为适用,可得出这两种孔隙宽度分别约为0.64 nm和7.20 nm,介孔孔径结果与SAXS、N2吸附/脱附等测量结果基本一致。这些结果同样表明制备SBA-15是成功的,同时也证实正电子湮没是表征孔结构的较好探针。
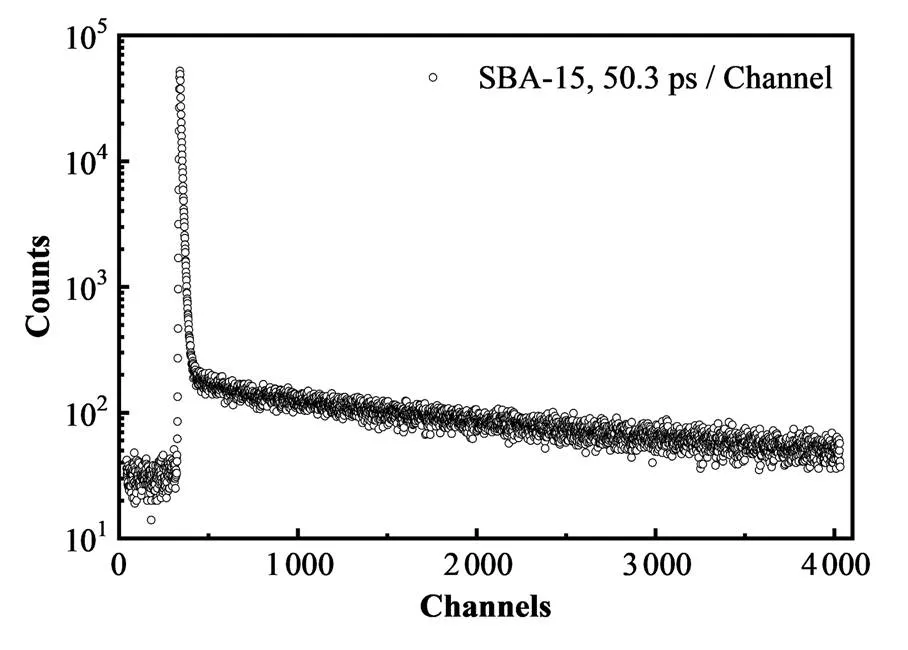
图5 合成SBA-15的归一化峰的正电子湮没寿命谱
图6显示了SBA-15中较长寿命3、4及其相应强度3、4随退火温度的变化。对于原生SBA-15,较短o-Ps寿命3保持在6 ns左右。同时,4、4和3变化明显,可分为3个阶段:在100~400 ℃阶段,4由91.8 ns急剧下降至54.6 ns,4由30%下降至27%。4的急剧下降可能与孔隙结构的破坏以及表面活性剂P123的分解有关;在400~900 ℃退火过程中,较长寿命4下降缓慢,4急剧下降;在第3阶段,4和4均再次急剧下降。寿命4的降低可能是由于较大孔隙(晶粒间未占据的空间)被破坏成微孔或这些孔隙的收缩所致,这也是4降低、3增加的原因。由于原生SBA-15未在550 ℃下煅烧,样品中含有P123聚合物模板剂在350 ℃左右可以完全分解。但是,10 ℃·min-1的升温速度使得P123模板不规则地分解,导致原生SBA-15孔隙结构被破坏。同时,通过PATFIT分析,部分较长o-Ps寿命被拟合为3成分,这可能是3增加的原因。对于在550 ℃下以1 ℃·min-1的速率煅烧5 h的煅烧后SBA-15样品,在退火后也进行了正电子湮灭寿命的测量。随着退火温度升高,3呈连续上升趋势。由于200 ℃的退火温度过低而不能破坏孔结构时,较长寿命成分4和其强度4基本保持不变。随着煅烧温度升高至1 000 ℃,较长寿命4逐渐降低至18 ns,但下降速度慢于原生SBA-15。另外,在100~600 ℃退火温度范围内,对应的强度4基本保持不变。但当退火温度高于600 ℃时,4从30%下降到5%,降幅较大。结果表明:煅烧后SBA-15的热稳定性较好,在较低的热处理温度下其热稳定性几乎未受到破坏。
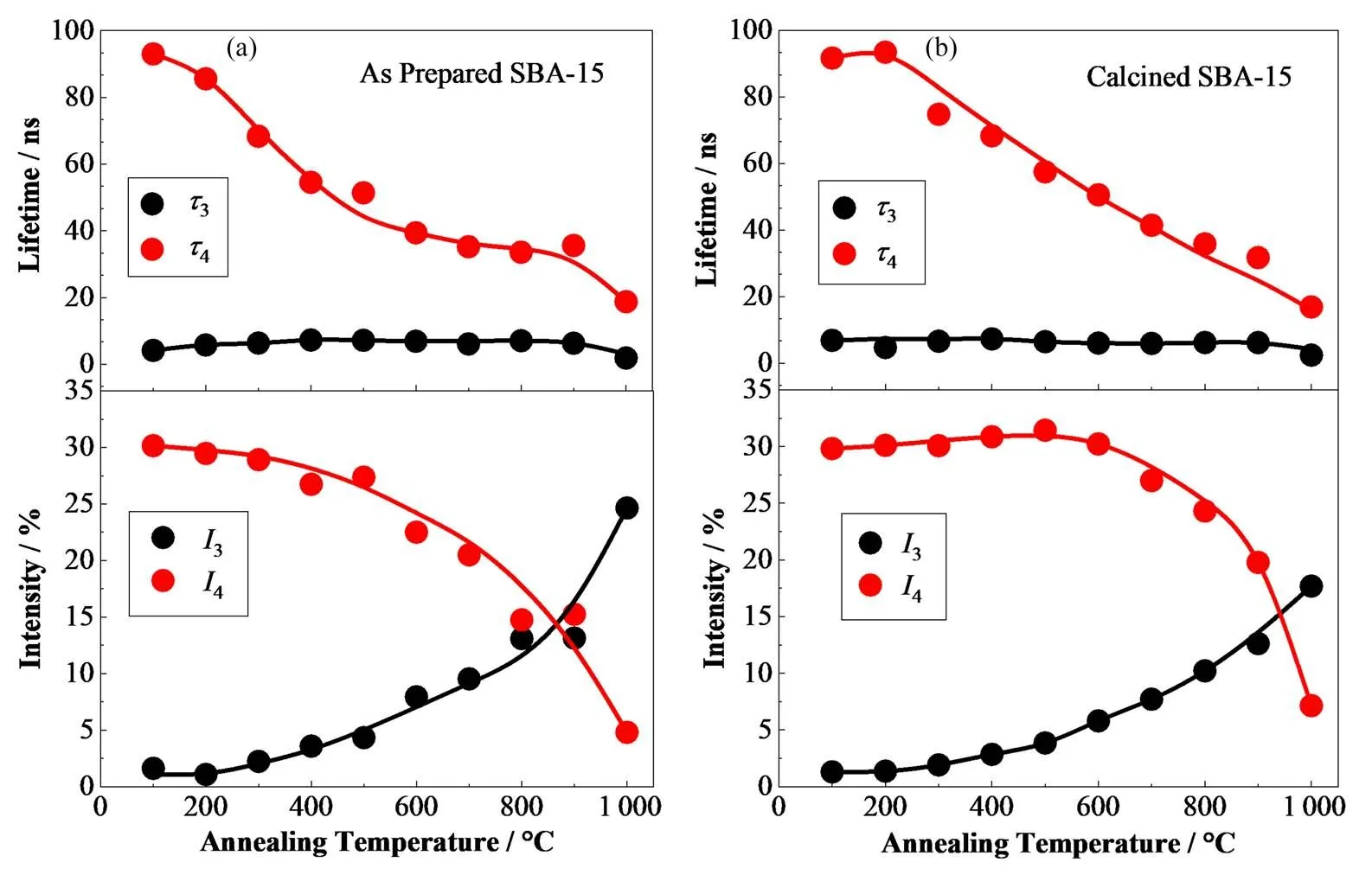
图6 原生SBA-15 (a)和煅烧后的SBA-15 (b)中τ3、τ4的强度I3、I4随热处理温度的变化
我们还对这两种SBA-15进行了多普勒展宽测量。从图7可以看出,参数的变化与各4的变化非常相似。对于原生SBA-15,参数先急剧下降至0.504,然后缓慢下降至0.497。但煅烧后SBA-15中参数的下降速率要低得多,在600 ℃以下几乎没有变化,与强度4的下降速率基本一致。由-相关曲线可知,除100 ℃煅烧的样品外,原生SBA-15的数据可被一条直线拟合,说明煅烧过程中塌陷的孔洞类型一致。而煅烧后SBA-15的-曲线表明除1 000 ℃外,100~900 ℃煅烧处理的样品孔洞类型一致。这些结果表明:550 ℃煅烧的SBA-15稳定性更好。

图7 原生SBA-15 (a)和煅烧后SBA-15 (b)中S参数随退火温度变化图和S-W曲线图
3 结语
综上所述,我们制备了两种SBA-15样品,分别为原生SBA-15和煅烧后SBA-15。TEM测试表明:它们都具有高度有序的二维六角的孔隙结构。对两种SBA-15在100~1 000 ℃的热处理条件下,以10 ℃·min-1的速率逐步破坏有序的二维六角的较大孔结构。正电子湮没测量结果得到的o-Ps寿命及其强度的变化以及参数和参数结果表明:550 ℃煅烧后SBA-15的热稳定性优于原生SBA-15。同时,该结果进一步证明了电子偶素可以作为表征多孔材料孔隙结构变化的灵敏探针。
作者贡献声明 李重阳:负责文章的制备样品、测试、数据处理,起草撰写及最终版本的修订;王飞跃:负责文章的细节修订、校对;陈志权:负责文章的修改和整体把握。
1 Larki A, Saghanezhad S J, Ghomi M. Recent advances of functionalized SBA-15 in the separation/preconcentration of various analytes: a review[J]. Microchemical Journal, 2021, 169: 106601. DOI: 10.1016/j.microc.2021.106601.
2 Shakeri M, Khatami Shal Z, van der Voort P. An overview of the challenges and progress of synthesis, characterization and applications of plugged SBA-15 materials for heterogeneous catalysis[J]. Materials (Basel, Switzerland), 2021, 14(17): 5082. DOI: 10.3390/ma14175082.
3 Kresge C T, Leonowicz M E, Roth W J,. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism[J]. Nature, 1992, 359(6397): 710–712. DOI: 10.1038/359710a0.
4 Zhao D, Feng J, Huo Q,. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores[J]. Science, 1998, 279(5350): 548–552. DOI: 10.1126/science.279.5350.548.
5 Beck J S, Vartuli J C, Roth W J,. A new family of mesoporous molecular sieves prepared with liquid crystal templates[J]. Journal of the American Chemical Society, 1992, 114(27): 10834–10843. DOI: 10.1021/ja00053a020.
6 Ryoo R, Jun S. Improvement of hydrothermal stability of MCM-41 using salt effects during the crystallization process[J]. The Journal of Physical Chemistry B, 1997, 101(3): 317–320. DOI: 10.1021/jp962500d.
7 Kim J M, Jun S, Ryoo R. Improvement of hydrothermal stability of mesoporous silica using salts: reinvestigation for time-dependent effects[J]. The Journal of Physical Chemistry B, 1999, 103(30): 6200–6205. DOI: 10.1021/jp990394k.
8 Fang K G, Ren J, Sun Y H. Synthesis and characterization of steam-stable Al-MCM-41[J]. Materials Chemistry and Physics, 2005, 90(1): 16–21. DOI: 10.1016/j.matchemphys.2004.05.018.
9 Zhao D Y, Huo Q S, Feng J L,. Nonionic triblock and star diblock copolymer and oligomeric surfactant syntheses of highly ordered, hydrothermally stable, mesoporous silica structures[J]. Journal of the American Chemical Society, 1998, 120(24): 6024–6036. DOI: 10.1021/ja974025i.
10 Saikia D, Deka J R, Wu C G,. pH responsive selective protein adsorption by carboxylic acid functionalized large pore mesoporous silica nanoparticles SBA-1[J]. Materials Science and Engineering: C, 2019, 94: 344–356. DOI: 10.1016/j.msec.2018.09.043.
11 Poonia E, Duhan S, Kumar K,. One pot hydrothermal synthesis of ordered mesoporous SnO2/SBA-16 nanocomposites[J]. Journal of Porous Materials, 2019, 26(2): 553–560. DOI: 10.1007/s10934-018-0651-y.
12 Zheng P, Hu D, Meng Q,. Influence of support acidity on the HDS performance over β-SBA-16 and Al-SBA-16 substrates: a combined experimental and theoretical study[J]. Energy & Fuels, 2019, 33(2): 1479–1488. DOI: 10.1021/acs.energyfuels.8b04133.
13 Li H C, Sakamoto Y, Li Y S,. Synthesis of carbon replicas of SBA-1 and SBA-7 mesoporous silicas[J]. Microporous and Mesoporous Materials, 2006, 95(1–3): 193–199. DOI: 10.1016/j.micromeso.2006.05.014.
14 Xiong H F, Zhang Y H, Wang S G,. Preparation and catalytic activity for Fischer–Tropsch synthesis of Ru nanoparticles confined in the channels of mesoporous SBA-15[J]. The Journal of Physical Chemistry C, 2008, 112(26): 9706–9709. DOI: 10.1021/jp800579v.
15 Wang H, Liu Y, Pinnavaia T J. Highly acidic mesostructured aluminosilicates assembled from surfactant-mediated zeolite hydrolysis products[J]. The Journal of Physical Chemistry B, 2006, 110(10): 4524–4526. DOI: 10.1021/jp056688p.
16 Yeasmin F, Mallik A K, Chisty A H,. Remarkable enhancement of thermal stability of epoxy resin through the incorporation of mesoporous silica micro-filler[J]. Heliyon, 2021, 7(1): e05959. DOI: 10.1016/j.heliyon. 2021.e05959.
17 Anyanwu J T, Wang Y R, Yang R T. Influence of water on amine loading for ordered mesoporous silica[J]. Chemical Engineering Science, 2021, 241: 116717. DOI: 10.1016/j.ces.2021.116717.
18 Mayanovic R A, Yan H, Brandt A D,. Mechanical and hydrothermal stability of mesoporous materials at extreme conditions[J]. Microporous and Mesoporous Materials, 2014, 195: 161–166. DOI: 10.1016/j.micromeso.2014.04.027.
19 Herbert R, Wang D, Schomäcker R,. Stabilization of mesoporous silica SBA-15 by surface functionalization[J]. Chemphyschem: a European Journal of Chemical Physics and Physical Chemistry, 2009, 10(13): 2230–2233. DOI: 10.1002/cphc.200900311.
20 Cui Z M, Hao J, Cao C Y,. Having it both ways: delicate hierarchical structure and robust mechanical stability on micro/nanomaterials with mesoporous silica coating[J]. Journal of Porous Materials, 2017, 24(1): 103–108. DOI: 10.1007/s10934-016-0242-8.
21 Brandt W. Positron dynamics in solids[J]. Applied Physics, 1974, 5(1): 1–23. DOI: 10.1007/BF01193389.
22 Gidley D W, Peng H G, Vallery R S. Positron annihilation as a method to characterize porous materials[J]. Annual Review of Materials Research, 2006, 36: 49–79. DOI: 10.1146/annurev.matsci.36.111904.135144.
23 Rienäcker B, Gigl T, Nebbia G,. Absolute fraction of emitted Ps determined by geant4-supported analysis of gamma spectra[J]. Physical Review A, 2020, 102(6): 062212. DOI: 10.1103/physreva.102.062212.
24 Čížek J, Melikhova O, Hruška P,. Positronium formation in nanostructured metals[J]. Acta Physica Polonica A, 2017, 132(5): 1579–1584. DOI: 10.12693/aphyspola.132.1579.
25 Cassidy D B, Mills A P. Enhanced ps-ps interactions due to quantum confinement[J]. Physical Review Letters, 2011, 107(21): 213401. DOI: 10.1103/physrevlett. 107.213401.
26 Chiari L, Ohnuki C, Fujinami M. A positronium-based systematic study of the physico-chemical properties of zeolite pores[J]. Radiation Physics and Chemistry, 2021, 184: 109441. DOI: 10.1016/j.radphyschem.2021.109441.
27 Tuomisto F, Makkonen I. Defect identification in semiconductors with positron annihilation: experiment and theory[J]. Reviews of Modern Physics, 2013, 85(4): 1583–1631. DOI: 10.1103/revmodphys.85.1583.
28 Romero A H, Allan D C, Amadon B,. ABINIT: overview and focus on selected capabilities[J]. The Journal of Chemical Physics, 2020, 152(12): 124102. DOI: 10.1063/1.5144261.
29 Korhonen E, Tuomisto F, Gogova D,. Electrical compensation by Ga vacancies in Ga2O3thin films[J]. Applied Physics Letters, 2015, 106(24): 242103. DOI: 10.1063/1.4922814.
30 Golovchak R, Wang S J, Jain H,. Positron annihilation lifetime spectroscopy of nano/macroporous bioactive glasses[J]. Journal of Materials Research, 2012, 27(19): 2561–2567. DOI: 10.1557/jmr.2012.252.
31 Mariazzi S, Toniutti L, Patel N,. Formation and escaping of positronium in porous SiO2films at low temperature[J]. Applied Surface Science, 2008, 255(1): 191–193. DOI: 10.1016/j.apsusc.2008.05.207.
32 Bonnelle C, Jonnard P. Dynamics of charge trapping by electron-irradiated alumina[J]. Physical Review B, 2010, 82(7): 075132. DOI: 10.1103/physrevb.82.075132.
33 Nagai Y, Nagashima Y, Hyodo T. Lifetime of delocalized positronium in α–SiO2[J]. Physical Review B, 1999, 60(11): 7677–7679. DOI: 10.1103/physrevb.60.7677.
34 Hyodo T, Nakayama T, Saito H,. The quenching of ortho-positronium[J]. Physica Status Solidi C, 2009, 6(11): 2497–2502. DOI: 10.1002/pssc.200982118.
35 Zhang H J, Chen Z Q, Wang S J. Monolayer dispersion of NiO in NiO/Al2O3catalysts probed by positronium atom[J]. The Journal of Chemical Physics, 2012, 136(3): 034701. DOI: 10.1063/1.3676259.
36 Sudarshan K, Patil P N, Goswami A,. Positronium chemistry of the Ps quenchers adsorbed on to the mesoporous resin[J]. Physica Status Solidi: C, 2009, 6(11): 2552–2555. DOI: 10.1002/pssc.200982082.
37 Kim T W, Ryoo R, Gierszal K P,. Characterization of mesoporous carbons synthesized with SBA-16 silica template[J]. Journal of Materials Chemistry, 2005, 15(15): 1560. DOI: 10.1039/b417804a.
38 Zhang H J, Chen Z Q, Wang S J,. Spin conversion of positronium in NiO/Al2O3catalysts observed by coincidence Doppler broadening technique[J]. Physical Review B, 2010, 82(3): 035439. DOI: 10.1103/physrevb.82.035439.
39 Sagara A, Yabe H, Chen X,. Pore structure analysis of ionic liquid-templated porous silica using positron annihilation lifetime spectroscopy[J]. Microporous and Mesoporous Materials, 2020, 295: 109964. DOI: 10.1016/j.micromeso.2019.109964.
40 王少阶, 陈志权, 王波. 应用正电子谱学[M]. 武汉: 湖北科学技术出版社, 2008.
WANG Shaojie, CHEN Zhiquan, WANG Bo. Applied positron spectroscopy[M]. Wuhan: Hubei Science & Technology Press, 2008.
41 Boujibar O, Souikny A, Ghamouss F,. CO2capture using N-containing nanoporous activated carbon obtained from argan fruit shells[J]. Journal of Environmental Chemical Engineering, 2018, 6(2): 1995–2002. DOI: 10.1016/j.jece.2018.03.005.
42 Tao S J. Positronium annihilation in molecular substances[J]. The Journal of Chemical Physics, 1972, 56(11): 5499–5510. DOI: 10.1063/1.1677067.
43 Eldrup M, Lightbody D, Sherwood J N. The temperature dependence of positron lifetimes in solid pivalic acid[J]. Chemical Physics, 1981, 63(1–2): 51–58. DOI: 10. 1016/0301-0104(81)80307-2.
44 Goworek T, Ciesielski K, Jasińska B,. Positronium states in the pores of silica gel[J]. Chemical Physics, 1998, 230(2–3): 305–315. DOI: 10.1016/S0301-0104(98)00068-8.
45 Dull T L, Frieze W E, Gidley D W,. Determination of pore size in mesoporous thin films from the annihilation lifetime of positronium[J]. The Journal of Physical Chemistry B, 2001, 105(20): 4657–4662. DOI: 10.1021/jp004182v.
46 Thommes M, Kaneko K, Neimark A V,. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report)[J]. Pure and Applied Chemistry, 2015, 87(9–10): 1051–1069. DOI: 10.1515/pac-2014-1117.
47 Barrett E P, Joyner L G, Halenda P P. The determination of pore volume and area distributions in porous substances. I. computations from nitrogen isotherms[J]. Journal of the American Chemical Society, 1951, 73(1): 373–380. DOI: 10.1021/ja01145a126.
48 Brunauer S, Emmett P H, Teller E. Adsorption of gases in multimolecular layers[J]. Journal of the American Chemical Society, 1938, 60(2): 309–319. DOI: 10.1021/ja01269a023.
49 Davis M E. Ordered porous materials for emerging applications[J]. ChemInform, 2002, 33(40): 245. DOI: 10.1002/chin.200240245.
50 Paulin R, Ambrosino G. Annihilation libre de l'ortho-positronium formé dans certaines poudres de grande surface spécifique[J]. Le Journal de Physique, 1968, 29(4): 263–270.
Effect of calcination on the thermal stability of SBA-15 studied by positron annihilation spectroscopy
LI Chongyang1WANG Feiyue1CHEN Zhiquan2
1()2()
Since the 1990s, porous materials have been widely used in the fields of gas adsorption, electrochemistry, drug delivery, catalyst and catalyst carrier because of their excellent physical and chemical properties such as high specific surface area, high permeability, adsorption and assemblability. The application of porous materials largely depends on their macroscopic properties, especially the diversity of pore structure and the adjustability of pore size, especially, the thermal stability of SBA-15 is very important for its large-scale practical application.This study aims to observe the thermal stability of SBA-15 by positron annihilation spectroscopy (PAS). [Methods] First of all, ordered mesoporous silica SBA-15 were synthesized by using TEOS (tetraethyl orthosilicate) as the silicon source and P123 as the structure-directing agent. Part of the obtained SBA-15 was further calcined at 550 ℃. Then, the above two kinds of SBA-15 were heated at 100~1 000 ℃ to check the thermal stability of their pore structure. Small angle X-ray scattering (SAXS), scanning electron microscopy (SEM),high-resolution transmission electron microscopy (HRTEM), N2adsorption/desorption and positron annihilation spectroscopy (PAS) measurements were used to study the ordered pore structure of SBA-15.Positron lifetime measurements reveal that four life components exist in both SBA-15 whilst the two longer lifetimes3and4correspond to the annihilation of o-Ps in the micropores and large pores of the material, respectively. With the increase of heat treatment temperature, the longest lifetime4of these two kinds of synthesized silica decrease gradually, as well as the corresponding intensity4. However, the longer life and intensity of calcined SBA-15 decrease obviously after 600 ℃. Meanwhile, theparameter of calcined SBA-15 is in good agreement with the results of o-Ps lifetime, and the-curve also shows that the pore types of calcined SBA-15 nearly are unchanged during in the heat treatment process at the temperature range of 100~900 ℃.All the results indicate that the synthesized SBA-15 calcined at 550 ℃ exhibits relatively better thermal stability, and positronium (Ps) is a very sensitive probe to study pore structure.
SBA-15, Pore structure, Positronium, Thermal stability
Supported by National Natural Science Foundation of China (No.11665017), Project of Central Plains Science and Technology Innovation Leading Talents of Henan Province (No.224200510022)
LI Chongyang, female, born in 1988, graduated from Wuhan University with a doctoral degree in 2016, focusing on positron annihilation spectroscopy
CHEN Zhiquan, E-mail: chenzq@whu.edu.cn
2022-02-20,
2022-03-25
O59,TL99
10.11889/j.0253-3219.2022.hjs.45.060201
国家自然科学基金(No.11665017)、中原科技创新领军人才项目(No.224200510022)资助
李重阳,女,1988年出生,2016年于武汉大学获博士学位,专业领域为正电子湮没谱学
陈志权,E-mail:chenzq@whu.edu.cn
2022-02-20,
2022-03-25

