Preparation and Characterization of Sepiolite Microfibers with High Aspect Ratio
XU Tong(续 通), HU Yelei(胡叶蕾), QIAN Di(钱 迪), ZHU Yuanzhao(朱元昭), ZHONG Yi(钟 毅), ZHANG Linping(张琳萍), 4, XU Hong(徐 红)*, MAO Zhiping(毛志平)
1 Key Laboratory of Science & Technology of Eco-Textile, Ministry of Education, Donghua University, Shanghai 201620, China2 National Dyeing and Finishing Engineering Technology Research Center, Donghua University, Shanghai 201620, China3 National Manufacturing Innovation Center of Advanced Dyeing and Finishing Technology, Taian 271000, China4 Key Laboratory of Bioorganic Phosphorus Chemistry & Chemical Biology, Ministry of Education, Department of Chemistry, Tsinghua University, Beijing 100084, China5 Sino-German Engineering College, Shanghai Technical Institute of Electronics & Information, Shanghai 201411, China
Abstract: It is difficult to access exfoliated sepiolite (Sep) fibers with high aspect ratio from Sep ore. The traditional method used to purify Sep ore also reduces its aspect ratio. In this study, impurities in the Sep ore were removed by acid activation followed by a cetyltrimethylammonium chloride (C16) treatment to organically modify the purified Sep by cation exchange. Then, the organically-modified Sep (O-Sep) was stripped and processed by an ultrasonic cell crusher to obtain Sep microfibers at a specific frequency for a given period. These Sep samples had relatively high aspect ratio, compared with the Sep fibers gotten by traditional method. Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) demonstrate the micro-morphology of exfoliated Sep samples in an intuitive way. Moreover, pure inorganic membrane prepared only with the exfoliated Sep fibers exhibited excellent flexibility, further demonstrating the excellent properties of Sep fibers with high aspect ratio.
Key words: sepiolite microfiber; high aspect ratio; inorganic membrane; flexibility
Introduction
Sepiolite (Sep, Mg8Si12O30(OH)4·(H2O)4·8H2O) material is a natural silicate in the transition state of layer chains composed of continuous silica-oxygen tetrahedra and discontinuous magnesium-oxygen octahedra[1-2]. The silica-oxygen tetrahedra and magnesium-oxygen octahedra share a common oxygen atom, and the positive tetravalent silicon in the crystal structure may be replaced by some trivalent metal ions, resulting in a negative charge on the entire crystal surface. To neutralize this negative charge, a certain amount of metal cation hydrate is adsorbed between the crystals[2-3]. The cation exchange capacity (CEC) is generally used to characterize the amount of the metal cation[4]. Because of the unique structure and morphology, Sep has high specific surface area[5-7], cation exchange capacity of 0.04-0.40 mol/kg[8], and micropores with good adsorptive properties[9]. Sep is an excellent reinforcement for polymer based nano-composites, with the fibrous morphology and surface characteristics[10-12]. To improve the performance of Sep/polymer composites, the problem of how to improve the compatibility between inorganic silicate and organic polymer[13]needs to be solved. The compatibility of fibrous inorganic material with polymers is significantly higher than that of granulated inorganic materials[14]. Therefore, a solution to this issue, that is, obtaining a kind of Sep microfibers with high aspect ratio to increase the compatibility of inorganic materials and organic polymers, is offered in this work.
In this study, Sep microfibers are obtained and characterized. Sep exists as aggregates bundles under normal circumstances because of the strong hydrogen bonding and the van der Waals interactions between the rods in the bundle[15]. Therefore, to obtain nano-sized Sep fibers with very high aspect ratio, the Sep ore must be purified and stripped. The Sep fibers gotten in this work with higher aspect ratio are different from those obtained by conventional stripping[7-8,16-19]. The experimental parameters for the purification, the organic modification, and the stripping of Sep fibers used in this study are based on previous research on silicate materials[20-21]. For example, cetyltrimethylammonium chloride (C16) has been identified as the most suitable material for the organic modification of Sep because quaternary ammonium cationic surfactants with a carbon number lower than 16 cannot significantly increase thed-spacing in silicates whereas the solubility of cationic surfactants with a carbon number greater than 16 is very poor and makes the organic-modification of Sep very difficult. Additionally, using an ultrasonic cell crusher is more effective to obtain Sep microfibers with high aspect ratio than mechanical stripping. In this work, a specific method is used to purify, organically modify, and strip Sep, to obtain Sep microfibers with relatively high aspect ratio, as illustrated in Fig.1, in whichLandDpresent the length and the diameter of the fiber, respectively.

Fig.1 Strategy used for the preparation of Sep microfibers
1 Materials and Methods
1.1 Preparation of the organically-modified Sep with high aspect ratio
The Sep ore was soaked in hydrochloric acid (HCl) at a concentration of 10 g/L for a period, separated by centrifugation, washed with deionized water, and dried at 100 ℃. C16 was used to modify the purified Sep by addition to Sep dispersions (10 g/L) at 10 times of the CEC at 50 ℃. Then, the mixture was stirred for 2 h at 1 500 r/min. After centrifugation atn= 800 r/min (FRC= 1.118×10-5×n2×r;FRCindicates relative centrifugal force, g;nindicates rotational speed, r/min;rindicates rotation radius, cm) for 3 min, the suspension was separated into upper phase (C16/water) and bottom sediments (organically-modified Sep, O-Sep). The O-Sep was washed with deionized water until there were no Cl-ions in the filtrate, then centrifugated at 800 r/min and dried at 60 ℃ to yield O-Sep. O-Sep was then dispersed in an ethanol/H2O (50/50, volume fraction) solution (100 mL) and processed by an ultrasonic cell crusher (SM 650A, Nanjing Sunma Instrument & Equipment Co., Ltd., China) with amplitude transformer bar (Ф=6 mm) at a frequency of 25 kHz for 30 min, as illustrated in Fig.2. In this way, the exfoliated O-Sep microfibers with high aspect ratio could be obtained.
1.2 Characterization
The Sep samples were characterized on a Fourier-transform infrared spectrometer (FTIR, Avatar 380, Thermo Group, United States) using the KBr powder compression method. The images of the surface and the elemental analysis results of the Sep samples were obtained on a scanning electron microscope (SU-3500, Hitachi Co., Ltd., Japan). X-ray diffraction(XRD) pattern obtained on Shimadzu XRD-6000 powder diffractometer (Japan) was used to investigate the microstructure of the Sep samples after the different physical or chemical modifications. The CEC of the Sep samples was measured using ammonium chloride-ethanol as reference. Exfoliated O-Sep with high aspect ratio was imaged with a transmission electron microscope (JEOL 2100F, Japan). The test samples for imaging were prepared by the steps of dispersion into ethanol, deposition on a copper mesh, and drying. The thermal stability of Sep and O-Sep was measured with a thermo-gravimetric analyzer (TGA, TG209F1, NETZSCH Instruments, Germany) in air at a temperature between 25 ℃ and 900 ℃ with a heating rate of 10 ℃/min.
2 Results and Discussion
2.1 Structure characterization
2.1.1TreatmentofSepore
Based on the previous work[20-21], Sep ore was processed by HCl first, then organic modified by C16, finally exfoliated by ultrasonic cell crusher, and a few experimental parameters were determined experimentally in this study.
After acid treatment, the total amount of exchangeable cation exchange on the surface of Sep would change to different degrees with different treatment parameters, and give different performances to Sep. Therefore, the CEC was used in this study to determine the experimental parameters of Sep acidification by HCl. As shown in Fig.3, when concentration of HCl is 3 mol/L, the CEC of Sep reaches above 1.0 mol/kg, and then the basic skeletal structure of Sep is destroyed due to excessive acidification[22-23]. But if the CEC is too low, the organic modification of Sep with cationic surfactants is limited. In our previous research work, we found that Sep had the best effect on the material properties and subsequent applications when the CEC of Sep was around 0.9 mol/kg. Therefore, the conditions for acidification of Sep were determined to be HCl concentration of 2 mol/L and acid-treatment time of 2.0 h.
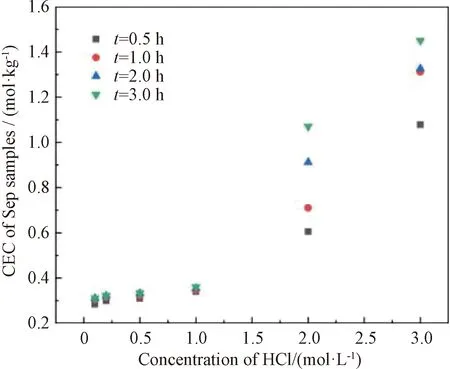
Fig.3 CEC of Sep as a function of HCl concentration
2.1.2Molecularstructureandcrystalstructure
The XRD spectra of the Sep ore, the purified Sep, and O-Sep are presented in Fig.4(b). All peaks of the impurities (including ettringite and calcite)[26-28]present in the spectra of the Sep ore, disappear in the spectra of purified Sep and O-Sep. Meanwhile, the characteristic peaks of Sep become more intense from the Sep ore to the purified Sep. The XRD data shows that the acid treatment successfully removed the impurities and did not damage the crystal structure of Sep. In addition, the CEC of the Sep ore is 0.347 mol/kg (tested in this study). After acid treatment, the CEC of the purified Sep increase to 0.917 mol/kg, which indicates that there are more active sites with a positive charge in the Sep crystal structure. Figure 4(b) shows the diffraction patterns of the purified Sep and O-Sep, especially the peak in the (110) plane of Sep. There is an intense diffraction reflection at 2θ= 12.26° with (110)d-spacing of 0.721 nm in the purified Sep and at 2θ= 12.08° with (110)d-spacing of 0.732 nm in O-Sep respectively. This indicates that the spacing between the unit cell of Sep increases after the organic modification of the purified Sep by C16, which agrees well with the FTIR results shown in Fig.4(a).
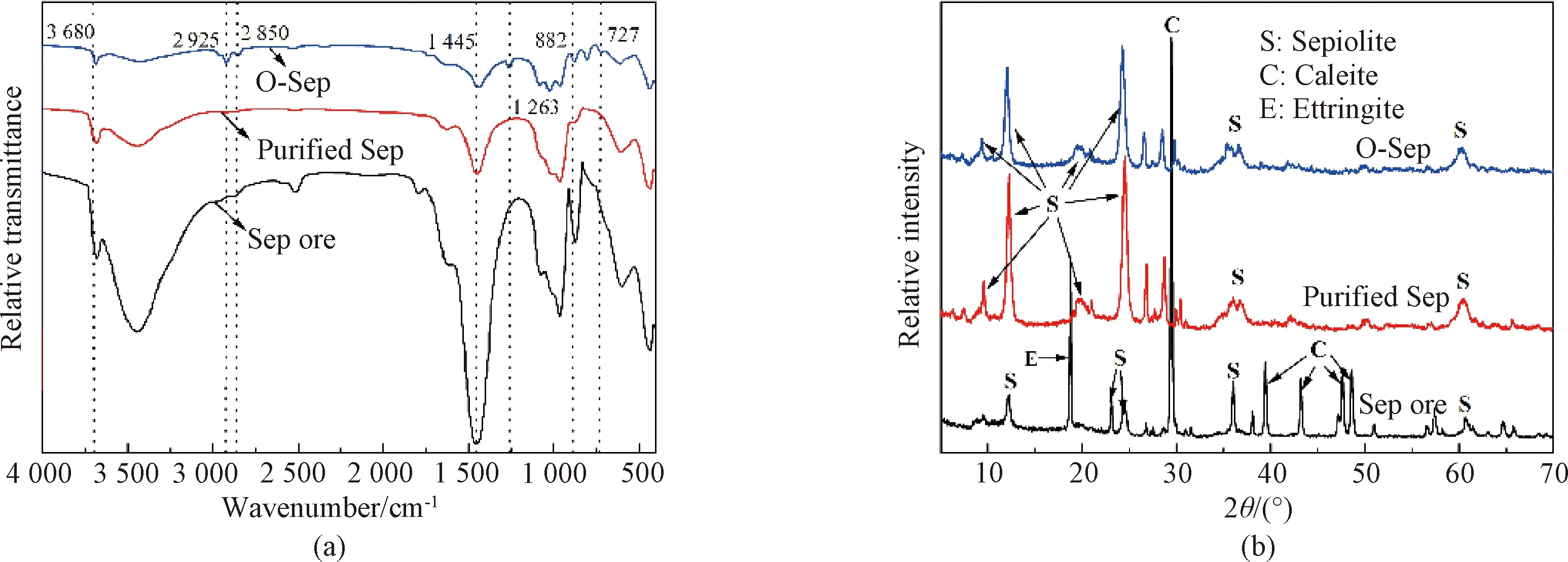
Fig.4 Structural analyses of Sep samples: (a) FTIR spectra; (b) XRD spectra
2.1.3Surfacemorphologyandelementalanalysis
Scannig electron microscopy(SEM) images and Energy dispersive spectroscopy(EDS) analyses are shown in Fig.5. The morphology and significant changes in the texture were observed from the SEM images. The SEM images of Sep indicate that Sep is composed of fibers with a needle-like shape. These fibers are assembled in bundles and form dense aggregates. After the acid treatment of Sep, there were almost no impurities in the Sep fiber matrix, seen from the EDS data in Figs. 5(a) and 5(b). After modified by C16, the distance between the O-Sep microfibers increased and a more regular arrangement was observed among the O-Sep fibers. C element appearing in the EDS of O-Sep could certificate the organic modification of Sep by C16. All SEM and EDS results were consistent with the results from XRD and FTIR.

Fig.5 SEM images and EDS analyses: (a) Sep ore; (b) purified Sep; (c) O-Sep
2.2 Properties of Sep samples
2.2.1Thermalproperty
The thermal degradation behaviors of the Sep ore, the purified Sep, and O-Sep are presented in Fig.6. The Sep ore underwent a high degradation between 611 ℃ and 722 ℃ because the ettringite and calcite decomposed at 611-722 ℃. The final weight loss of the Sep ore was about 40%. The purified Sep underwent a 3-step thermal degradation process within a range of 30-676 ℃. The mass loss of purified Sep at 30-100 ℃ corresponded to the removal of adsorbed water (step 1); then it was the crystal water loss from the voids in the Sep structure from 333 ℃ to 368 ℃ (step 2); and the structural water removal appeared from 553 ℃ to 676 ℃ (step 3). The final weight loss of the purified Sep and O-Sep were only 18.3% and 19.3%, respectively. The C16 adsorbed on the surface of O-Sep also underwent thermal degradation at 570 -737 ℃ because C16 and the Sep fibers were linked by ionic bonds that can be destroyed at high temperatures[29-32]. The thermal properties of the Sep samples imply that Sep can be used in Sep/polymer composites to enhance high temperature resistance.
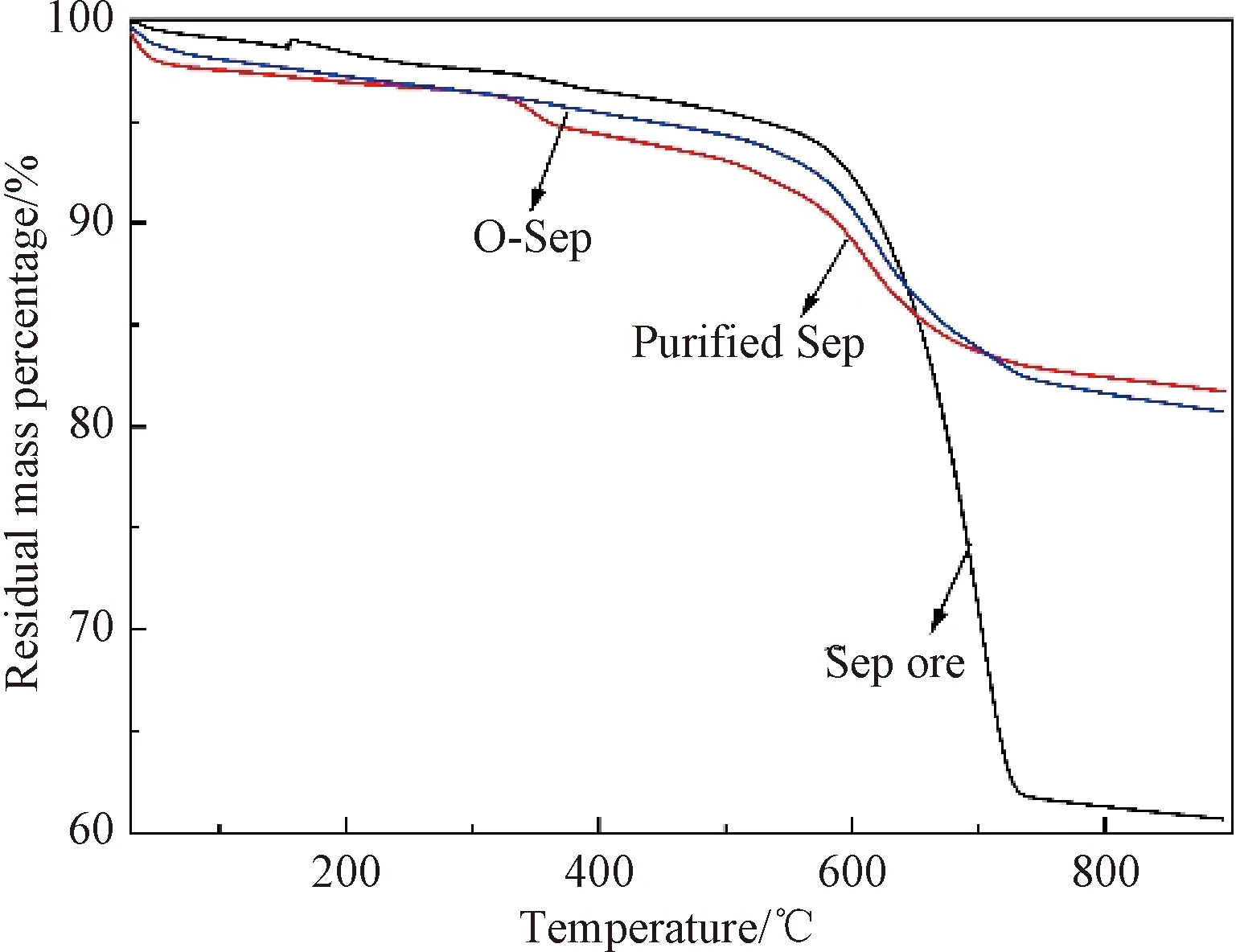
Fig.6 Thermogravimetric analyses of samples
2.2.2Micro-structureofSep
When O-Sep was treated by ultrasonic cell crusher, the exfoliation of Sep varied with different ultrasonic frequency and treatment time. Figure 7 shows the transmission electron microscopy(TEM) images of different Sep samples with different dispersed states.

Fig.7 TEM images of samples: (a) Sep ore; (b) purified Sep; (c) stripped Sep microfibers; (d) commercial Sep microfibers obtained by traditional method
Sep ore has a poor dispensability because of the van der Waals and the hydrogen-bonding interactions between the rod-like crystals, which seriously hinders its practical applications in various fields. In this study, an ultrasonic grinding method was used to strip O-Sep. Thus, the interaction between the rod-like crystals of O-Sep was weakened based on the increasedd-spacing in the Sep crystals after the organic modification by C16. When the ultrasound probe was inserted into the O-Sep dispersion and acted on the crystal structure, the energy produced by the ultrasound probe promoted the stripping of the O-Sep microfibers. Figure 7(c) shows that the stripped O-Sep microfibers had higher aspect ratio, about dozens of times more than that of commercial Sep, and most Sep microfibers existed as single fibers. As shown in Fig.7(d), commercial Sep microfibers obtained by traditional method(mechanical crushing) had very small aspect ratio, which was difficult to enhance the special features of the Sep/polymer composites.
2.2.3StructureandflexibilityofSepmembrane
To study the effect of the Sep microfibers with different aspect ratios on the material properties, the Sep microfibers were assembled into inorganic membrane. Figure 8(a) illustrates the interaction between the Sep microfibers with high aspect ratio. There are van der Waals interactions at the surface of Sep microfibers[33-34]. In addition, the attraction between the fibers was enhanced by the large specific surface area of the Sep microfibers[35]. Therefore, the Sep membrane had an excellent flexibility. As shown in Fig.8(b), the membrane showed a slight crease but no damage even if the membrane was folded twice in half, which verified its softness. While the membrane prepared with low aspect ratio Sep was divided into several pieces when folded, as shown in Fig.8(c). Figure 8(d) shows the deformation of the fracture of the membrane prepared with high aspect ratio Sep, indicating that the membrane was ductile even under freezing conditions. This is another evidence of the flexibility of the high aspect ratio Sep membrane. In contrast, there was a smaller contact area and a weaker interaction between the Sep fibers with a low aspect ratio. Therefore, it is impossible to prepare a flexibility inorganic membrane with such kind of Sep microfibers. Figure 8(e) shows the SEM image of the cross-section of Sep membrane in Fig.8 (c). It can be seen from Fig.8(e) that the cross-sectional fracture profile of the membrane is regular, demonstrating the brittleness of the membrane.
3 Conclusions
Obtaining pure Sep microfibers with relatively high aspect ratio is the key to extend their applications. In this work, an acid treatment and an ultrasound-assisted stripping treatment were used to remove the impurities from the Sep ore and get Sep microfibers with relatively high aspect ratio. In addition, an organic modification was proposed as a relatively simple alternative for stripping the Sep bundles. Finally, Sep microfibers with an aspect ratio about dozens of times more than that of commercial Sep were obtained. Moreover, the crystal structure of the Sep microfibers was not damaged by the modification. In summary, the combined ultrasound and chemical method can disaggregate the crystal bundles of Sep whereas each method fails separately. Such individualized Sep microfibers with excellent fiber dispersion can be used to produce nano-composites, improving fire retardant and mechanical performance of the materials.
 Journal of Donghua University(English Edition)2022年6期
Journal of Donghua University(English Edition)2022年6期
- Journal of Donghua University(English Edition)的其它文章
- Conductive Polyacrylonitrile Fiber Prepared by Copper Plating with L-Ascorbic Acid as Reducing Agent
- Fabrication and Characterization of Yarn-Based Temperature Sensor for Respiratory Monitoring
- Anti-wrinkle Finishing of Cotton Fabrics with Pyromellitic Acid Enhanced by Polyol Extenders
- Solution Blowing of Palygorskite-Based Nanofibers for Methylene Blue Adsorption
- Active Absorption of Perforated Plate Based on Airflow
- Customer Churn Prediction Model Based on User Behavior Sequences
