Physicochemical properties and biological activities of polysaccharides from the peel of Dioscorea opposita Thunb. extracted by four different methods
Zuowei Zhao, Li Wang*, Yuan Ruan, Chunnan Wen, Menghuan Ge, Yanyan Qian, Bingji Ma*
Department of Traditional Chinese Medicine, Henan Agricultural University, Zhengzhou 450001, China
Keywords:Dioscorea opposita Thunb peel Polysaccharides Different extraction methods Physicochemical characteristics Biological activities
A B S T R A C T Polysaccharides are the important biologically active components found in the peel of Dioscorea opposita Thunb. (DTTP). The influences of 4 extraction methods, namely hot water extraction (W), acidic extraction(HA), hot-compressed water extraction (HCW) and enzyme-assisted extraction (EAE), on the yields,physicochemical properties, hypoglycemic and antioxidant activities of polysaccharides from DTTP were studied and compared. Among these DTTP polysaccharides, DTTP-HA was outstanding in several respects. DTTP-HA was the most water soluble; it had the highest total carbohydrate content (85.08%),the highest uronic acid (13.20%) and the highest thermal stability. DTTP-HA and DTTP-W possessed a triple-helix structure. These 4 kinds of polysaccharides have the same types of monosaccharides, but in different molar percentages. Extraction method had a significant impact on the microstructures of the extracted polysaccharides. DTT-HA exhibited irregular structure with many holes. Among the 4 extracted methods,the DTTP-HA and DTTP-W initially exhibited higher hypoglycemic and antioxidant activities. The better bioactivities of DTTP-HA may be related to the above factors. The findings indicated that acid extraction is an effective method to extract polysaccharides with high biological activities from DTTP.
1. Introduction
Dioscorea oppositaThunb. (Tiegun Shan Yao) is not only consumed as a tonic food but also used as an important invigorant in traditional Chinese medicine in most of China [1]. It contains many nutritional metabolites, such as polysaccharides, dopamine, amino acids, allantoin, organic acids, phytosterols, dioscin and essential amino acids [2]. These components have various biological activities,such as antioxidant, antidiabetic and antihypertensive [3]. Due to the short storage period of freshD. opposita, large quantities ofD. oppositaare processed into various forms such as soft drinks and powder. During this processing, a large amount ofD. oppositapeel is produced as a waste by-product. This represents a signif icant waste of a potential resource and causes environmental issues. Therefore,f inding a way to use peel ofD. oppositawould increase its value as a crop and reduce environmental problems.
Polysaccharides are important biomacromolecules widely found in plants, animals and microorganisms. It is well-known that they have biological activities, such as antioxidant, anti-hypertensive,antitumor, and antibacterial activities and neuroprotective effects [4].Plant-derived polysaccharides have attracted great interest among researchers because of their desirable activities and nontoxic nature [5].D. oppositaare typically peeled before processing; the waste is rich in dietary polysaccharides and may be an economically viable alternative resource [6]. However, there are very few studies about the peel ofD. oppositaand this limits its application in the food industry.
Various methods have been developed for the extraction of polysaccharides from plant material. The selection of extraction method because it influences the total carbohydrate content,monosaccharide components, molecular weight, and functional properties of the polysaccharides extracted [7,8]. However, there are very few studies about the impacts of different extraction methods on the yields, physicochemical properties and biological activities of polysaccharides from DTTP.
Therefore, the objectives of this study were to investigate the yield, total carbohydrate content, physicochemical characteristics,antioxidant and hypoglycemic activities of polysaccharides extracted from DTTP by 4 different extraction methods. These 4 methods were: hot water extraction (W), acidic extraction (HA),hot-compressed water extraction (HCW) and enzyme-assisted extraction (EAE). Extracted samples were characterized according to chemical composition, molecular weight distribution, monosaccharide components and surface structure. Finally, the antioxidant activities and hypoglycemic activities of polysaccharides from DTTPin vitrowere studied. The goal of the study was to find the best method for extracting polysaccharides from DTTP with high biological activity that could then be used in the food industry.
2. Materials and methods
2.1 Materials and reagents
The peel ofD. oppositawas obtained from Wuzhi county of Jiaozuo city (Henan Province, China). Dialysis membranes (molecular weight cut-off, 3 500 Da) were purchased in Shanghai Yuanye Bio-Technology Co., Ltd. (Shanghai, China). Monosaccharide standards were purchased from Sigma Chemical Co. (St. Louis,MO, USA). Acarbose was purchased from Shanghai Macklin Biochemical Co., Ltd. (Shanghai, China). PNPG and Dextrans with different molecular weight were purchased from Shanghai Bowman Biotechnology Co., Ltd. (Shanghai, China). DPPH was purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo,Japan).α-Amylase,α-glucosidase, cellulase (50 μ/mg) and papain(800 μ/mg) were purchased from Shanghai Yuanye Bio-Technology Co., Ltd. (Shanghai, China). HCl, NaOH, chloroform, acetone,trifluoroacetic acid (TFA), FeSO4, salicylic acid, H2O2, 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), and K3Fe (CN)6.All other chemicals and reagents were analytical grade.
2.2 Extraction methods
2.2.1 Acidic extraction
The acidic extraction was performed based on the previous reports [9]. Briefly, the powder (10 g) of peel fromD. oppositawas mixed with 200 mL of 0.1 mol/L HCl solution and the extraction was performed at 80 °C for 3 h (twice). The crude extract of DTTP was filtered and centrifuged (4 000 r/min, 5 min). Next, the filtrates were collected, neutralised, concentrated, and precipitated with 75%ethanol for 24 h at 4 °C. The precipitate was freeze-dried to obtain crude polysaccharides. The free proteins in the crude polysaccharides were removed using the Sevag reagent (chloroform:n-butyl alcohol = 4:1,V/V). After removing of the free proteins, the aqueous solution was dialysed against distilled water (molecular weight cut-off 3.5 kDa), concentrated, freeze-dried and the polysaccharide of peel fromD. opposita(DTTP-HA) extracted by hydrochloric acid was obtained.
The extraction flowchart is shown in Fig. 1.
2.2.2 Hot water extraction
The powder (10 g) of peel fromD. oppositaand 200 mL distilled water were placed in glass vials, performed at 80 °C for 3 h (twice).Then, the extracted solutions were combined, and the next steps were the same as Section 2.2.1. Finally, the polysaccharide of peel fromD. opposita(DTTP-W) extracted by hot water was obtained.
2.2.3 Hot-compressed water extraction
The powder (10 g) of peel fromD. oppositaand 200 mL distilled water were put into a pressure glass reactor (350 mL) and extracted for 3 h at 150 °C in an oil bath (twice). The mixture was centrifuged to obtain the supernatant. The next procedures were the same as the Section 2.2.1. Finally, the polysaccharide of peel fromD. opposita(DTTP-HCW) extracted by hot-compressed water was obtained.
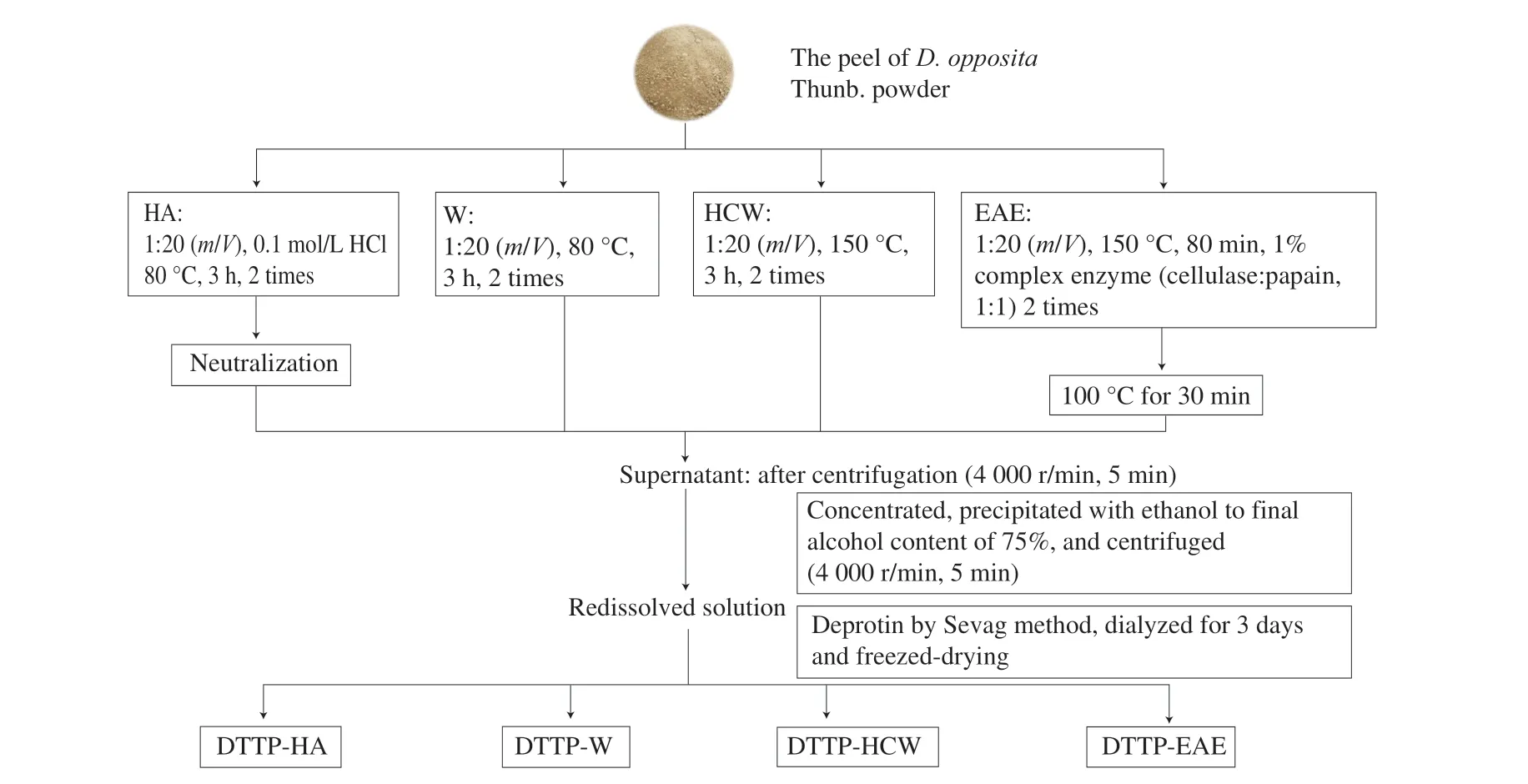
Fig. 1 The extraction flowchart of different extraction methods.
2.2.4 Enzyme-assisted extraction
The powder (10 g) of peel fromD. oppositawas mixed with 200 mL of distilled water (1:20,m/V). The enzyme complex applied to extract polysaccharides of DTTP contained cellulase and papain in equal proportions (mass ratio), and each enzyme accounted for 1% of the extracted powder. DTTP were extracted with the enzymatic complex at 50 °C for 80 min in the water bath and subsequently extracted at 100 °C for 30 min to deactivate the enzyme. After cooling, the filtrates were combined and concentrated. The next steps were according to Section 2.2.1, and the polysaccharide of peel fromD. opposita(DTTP-EAE) extracted by enzyme-assisted was obtained.
2.3 Analysis of chemical composition
The phenol-sulfuric acid method was used to determine the carbohydrate content of the above 4 polysaccharide samples, usingD-glucose as reference. The protein content in the four polysaccharide samples was analyzed by Coomassie brilliant blue staining with bovine serum albumin as a standard [10]. The uronic acid content was determined by them-hydroxyphenyl method withD-galacturonic acid as a standard [8]. The phenolic content was determined by Folin-Ciocalteu reagent method using gallic acid as the standard [11].
2.4 Determination of molecular weight and monosaccharide composition analysis
The molecular weights of the 4 polysaccharide samples were determined using DAWN HELEOS II (RID-20A). The separation was performed on series columns coupled of shodex OHpak SB-806M HQ and SB-804M HQ columns (300 mm × 7.8 mm, Shodex, Japan).The column was maintained at a temperature of 40 °C, eluted with 0.1 mol/L NaNO3at a flow rate of 0.5 mL/min.
Monosaccharide composition of the 4 polysaccharide samples was performed according to the previous reports [10]. Briefly, each sample was hydrolyzed with trifluoroacetic acid (TFA) at 121 °C for 2 h. The hydrolysates were dried under nitrogen, washed with methanol and dried again under nitrogen (2–3 times). Finally, each sample was filtered by a 0.22 μm filter and injected into the detection system (Thermo ICS5000+, Thermo Fisher Scientific, USA) equipped with a Dionex CarbopacPA-20 column at a flow rate of 0.5 mL/min.The column temperature was held at 30 °C and column mobile phase was 97.5% (H2O) and 2.5% 100 mmol/L (NaOH) in water.
2.5 UV spectroscopy analysis and FT-IR spectroscopic analyses
The UV-vis spectra of the 4 polysaccharide samples (1 mg/mL)were recorded in a spectrophotometer (TU-1810, PERSEE, Beijing,China) in the wavelength range of 200-800 nm at room temperature.
The FT-IR spectra of the 4 polysaccharide samples were identified using a Bruker-Vector 22 spectrometer (Bruker, Rheinstetten,Germany). The dried samples (1 mg) were mixed with spectroscopic KBr powers (1:100), ground and pressed into a pellet, and the IR spectra were recorded in the range of 400–4 000 cm-1.
2.6 Thermal analysis
The thermal characteristics of the 4 polysaccharide samples were determined using thermogravimetry (TG) and differential thermogravimetry (DTG) (Shimadzu, Japan). The samples(3–5 mg) were analyzed under a dynamic nitrogen atmosphere and the temperature was increased from 25 °C to 800 °C at a heating rate of 10 °C/min.
2.7 Scanning electron microscopy (SEM)
The freeze-dried sample was mounted on the metal platform and coated with a thin gold layer, and then examined using an SEM(JSM-7800 F, JEOL. Tokyo. Japan) [12]. The images were observed at a voltage of 3.0 kV and magnification of 1 000.
2.8 Congo red analysis
The Congo red analysis of the above 4 polysaccharide samples was determined according to previous reports with minor modifications [13]. In brief, the solution of sample (1.0 mL, 1 mg/mL)was mixed with an equal volume of Congo red solution (50 μmol/L)and then 1 mol/L NaOH solution was gradually added to the mixtures to establish a final NaOH concentration of 0, 0.05, 0.1, 0.15, 0.2, 0.25,0.3, 0.35, 0.4, 0.45 and 0.5 mol/L. After standing for 10 min at room temperature, the maximum absorption wavelength (λmax) was obtained over 400–700 nm with a UV visible spectrophotometer.
2.9 Antioxidant activities
2.9.1 Hydroxyl radical scavenging assay
Assessment of the scavenging ability of the above 4 polysaccharide samples on hydroxyl radical was performed according to the method determined as reported previously with a minor modification [14]. In brief, 400 μL of sample solution at various concentrations (0.05, 0.1, 0.5, 1, 2, 4, 6, 8, 10 mg/mL) was mixed with FeSO4(4.5 mmol/L, 400 μL), salicylic acid ethanol (4.5 mmol/L,400 μL). The reaction was initiated by the addition of H2O2(6 mmol/L, 800 μL), and this mixture was subsequently incubated at 37 °C for 30 min. Vitamin C (VC) served as positive control.The absorbance was detected at 510 nm. The scavenging rate was calculated with the following equation:
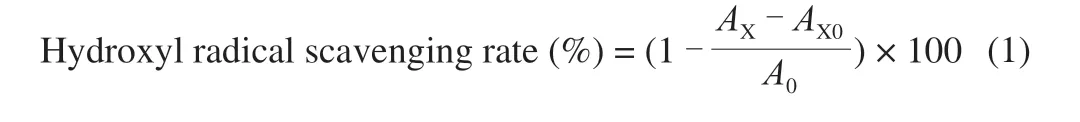
A0is the absorbance of the mixture without sample.AXis the absorbance of the sample solution group.AX0is the absorbance of the mixture without the H2O2solution.
2.9.2 DPPH radical scavenging assay
The DPPH radical scavenging activities of the above 4 polysaccharide samples were determined according to the method reported previously with a slight modification [14]. Briefly,500 μL sample solution at various concentrations (0.05, 0.1, 0.5, 1,2, 4, 6, 8, 10 mg/mL) of the 4 samples were mixed with 500 μL of freshly prepared DPPH solution (0.4 mmol/L in ethanol). The reaction was kept in a dark place for 30 min at room temperature, and then the absorbance was measured at 517 nm. VC was used as a positive control. The DPPH radical scavenging rate was calculated by the following formula:
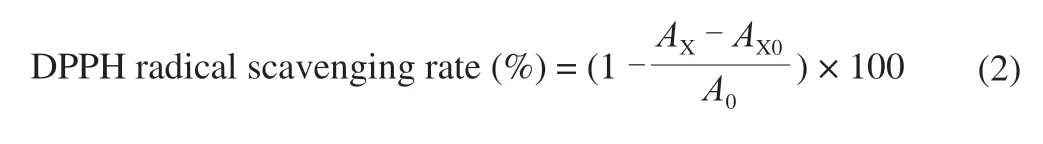
A0is the absorbance of the mixture without sample.AXis the absorbance of the sample solution group.AX0is the absorbance of the mixture without the DPPH solution.
2.9.3 ABTS radical scavenging assay
The ABTS radical scavenging of the above 4 polysaccharide samples was measured according to the reported method with some adjustments [15]. Briefly, the ABTS solution was adjusted with absolute ethanol to an absorbance of 0.70 ± 0.05 at 734 nm.Various concentrations (0.05, 0.1, 0.5, 1, 2, 4, 6, 8, 10 mg/mL) of each sample (0.2 mL) were mixed with 1.2 mL ABTS solution.Then the mixture was incubated at room temperature for 10 min; the absorbance was measured at 734 nm. VC was adopted as a positive control. The ABTS radical scavenging rate was calculated using the following equation:
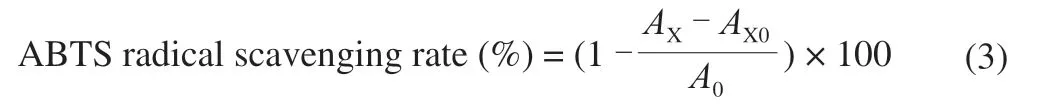
A0is the absorbance of the mixture without sample.AXis the absorbance of the sample solution group.AX0is the absorbance of the mixture without the ABTS solution.
2.9.4 Reducing power assay
The reducing powers of the above 4 polysaccharide samples were assessed as previously described with minor modifications [16]. In brief, 0.2 mL sample at various concentrations (0.05, 0.1, 0.5, 1, 2,4, 6, 8, 10 mg/mL) mixed with 0.5 mL phosphate buffer (0.2 mol/L,pH 6.8) and 0.1 mL 1% (m/V) K3Fe(CN)6. The mixture was incubated in a water bath at 50 °C for 20 min. When the samples cooled to the room temperature, 0.5 mL 10% trichloroacetic acid solution (TCA,m/V) was added. After the samples were centrifuged at 8 000 r/min,the supernatant (0.5 mL) was mixed with 0.5 mL distilled water and 0.1 mL 0.1% (m/V) FeCl3. VC was served as a positive control. The absorbance of the reaction mixture was measured at 700 nm.
2.10 Hypoglycemic activities
2.10.1 α-Glucosidase inhibitory activity
Theα-glucosidase inhibition activity of the 4 polysaccharide samples was evaluated by the method according to the previous study with slight modifications [17]. In brief, 200 μL of each sample at different concentrations (0.1, 0.5, 1, 2, 4, 6, 8, 10 mg/mL respectively)was mixed with 80 μL (0.2 U/mL, dissolved in 0.1 mol/L pH 6.8 phosphate buffer)α-glucosidase in 200 μL of phosphate buffer(0.1 mol/L, pH 6.8) solution, and the mixed solution was incubated at 37 °C for 15 min. After incubation, 120 μL of 10 mmol/L PNPG in 0.1 mol/L phosphate buffer (pH 6.8) solution was added. Then the mixtures were incubated at 37 ºC for 20 min, and the reactions were stopped by adding 400 μL of 0.2 mol/L Na2CO3solutions. Acarbose was used as positive control. The absorbance value was determined at 405 nm, and the inhibition rate ofα-glucosidase was calculated using the following equation:
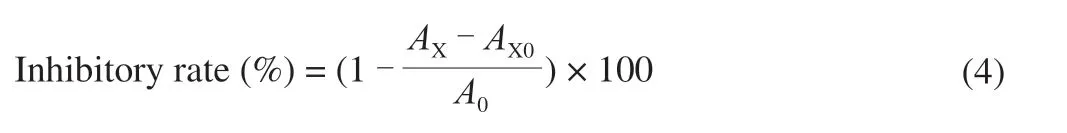
A0is the absorbance of the control (phosphate buffer instead of the sample).AXis the absorbance of the mixture containedα-glucosidase,starch solution and sample.AX0is the absorbance of the mixture contained sample and starch solution.
2.10.2 α-Amylase inhibitory activity
Theα-amylase inhibitory activity of 4 polysaccharide samples was conducted according to the reference with some modificationsin vitro[18]. Briefly, 50 μL of each sample at various concentrations(0.05, 0.1, 0.5, 1, 2, 4, 6, 8, 10 mg/mL respectively) was mixed with 50 μLα-amylase (20 U/mL, dissolved in 0.1 mol/L, pH 6.8 phosphate buffer) solution in 100 μL of phosphate buffer (0.1 mol/L, pH 6.8),and the mixed solution was preincubated at 37 °C for 10 min. Then,50 μL of soluble starch (2%,m/V) was added into the mixture and incubated at 37 °C for 5 min. Finally, the reaction was carried out at 100 °C for 5 min and stopped by adding 50 μL of 3,5-dinitrosalicylic acid (DNS). After cooling in ice-water. 100 μL of the mixed solution was diluted by adding 1.4 mL of deionized water. The absorbance value was determined at 540 nm. The inhibition rate ofα-amylase was calculated using the following equation:
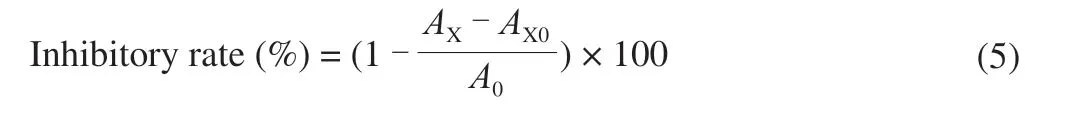
A0is the absorbance of the control (phosphate buffer instead of the sample).AXis the absorbance of the mixture containedα-amylase,starch solution and sample.AX0is the absorbance of the mixture contained sample and starch solution.
2.11 Statistical analyses
All data were shown as means standard deviation (SD). The experimental data are analyzed by SPSS software (19.0). Significant difference of the results was evaluated by One-way ANOVA test.P< 0.05 andP< 0.01 were considered to statistically significant differences.
3. Results and discussion
3.1 Yield and chemical compositions
The yields and chemical compositions of polysaccharides from DTTP extracted by 4 methods are shown in Table 1. As shown, the yields and physicochemical properties were significantly influenced by extraction methods. DTTP-HCW had the highest yield (19.55%),followed by DTTP-HA (15.94%), DTTP-EAE (7.61%), and DTTP-W(4.75%). The highest total carbohydrate content was obtained by acidic extraction, that is, in the DTTP-HA sample (85.08%), while the lowest was obtained by hot water extraction, that is, in the DTTP-W sample (46.37%). The reason could be that hydrogen ions from HCl could break the glucosidic bonds of cellulose and hemicellulose in cell walls, causing polysaccharides in the cell to be diffused into the solvent [19]. The total protein contents in the three extracted samples were low; DTTP-W had a relatively higher protein content. Uronic acid content of DTTP-HA (13.19%) was the highest, followed by DTTP-W (12.13%), DTTP-EAE (6.35%) and DTTP-HCW (1.83%).A few phenolic compounds were also detected, ranging from(0.26 ± 0.01)% to (1.14 ± 0.03)%, which indicated that DTTP polysaccharides might contain natural phenolic polysaccharide conjugates. In the UV spectra (Fig. 2A) the absorbance of DTTP-W was stronger than that of other samples in the region of 200 and 400 nm. DTTP-HA, DTTP-HCW and DTTP-EAE contained little nucleic acid and protein, according to the small absorptions peaks near 260 and 280 nm [5]; this pattern is consistent with the chemical analysis results (Table 1).

Table 1 Chemical composition of 4 polysaccharides obtained by different extraction methods.
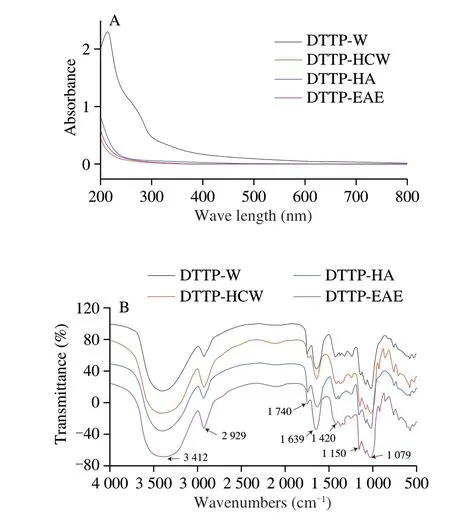
Fig. 2 UV spectra of 4 polysaccharides obtained by different extraction methods (A); FT-IR spectra of 4 polysaccharides obtained by different extraction methods (B).
3.2 Characterization of polysaccharides from DTTP
3.2.1 Molecular weight and monosaccharide composition
The monosaccharide composition of polysaccharides from DTTP extracted with the 4 methods is listed in Table 2. All of 4 DTTP polysaccharide samples consisted of mannose, rhamnose, arabinose,galacturonic acid, glucose, glucuronic acid, galactose and xylose.However, the molar ratios of these components in each sample differed. The major monosaccharides in DTTP-HCW, DTTP-HA and DTTP-EAE were glucose, galactose and mannose, while DTTP-W mainly consisted of arabinose, galactose and glucose. The types of monosaccharides were the same, but the molar percentages of monosaccharide components differed. Compared with HCW and EAE, the galactose and mannose contents of DTTP-HA were significantly higher than that in DTTP-HCW and DTTP-EAE. The reason may be that HA treatment induced hydrolytic breakage of polysaccharide chains, which disrupted intermolecular hydrogen bonds, thereby affecting monosaccharide structure. According to previous studies, polysaccharides containing uronic acid in high levels might have potent scavenging ability and antioxidant activities [20].The galacturonic acid contents of DTTP-W (5.8%) and DTTPHA (4.9%) were significantly higher than DTTP-HCW (2.5%) and DTTP-EAE (1.0%), which suggested that the DTTP-W and DTTPHA might have good bioactivity [21]. The HPLC chromatograms of monosaccharide composition are shown in Supplementary Fig. 1.
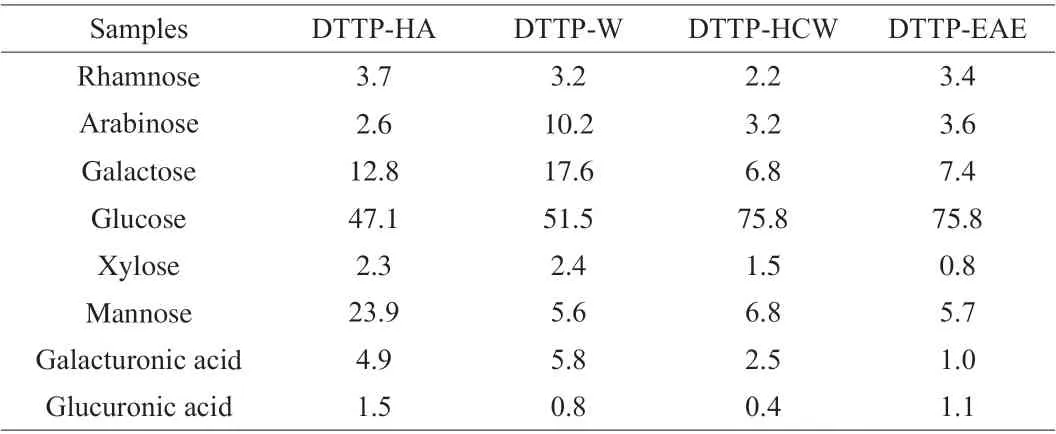
Table 2 Monosaccharide components (mol%) of 4 polysaccharides obtained by different extraction methods.
Molecular weight is an important physicochemical parameter,closely related to biological activity of polysaccharides. Previous studies showed that the polysaccharide with lower molecular weight was likely to exhibit higher biological activity [22]. The molecular weights distribution of the 4 polysaccharide samples are listed in Table 3. The molecular weight of DTTP-W was the highest (514.7 kDa), which indicated that the polysaccharide molecule tended to aggregate under the long extraction condition period at high temperature. The molecular weight of DTTP-EAE(36.93 kDa) was smaller than that of DTTP-W (514.7 kDa), DTTP-HCW(318.2 kDa) and DTTP-HA (153.9 kDa), which might be due to the synergistic effect of complex enzymes (cellulase and papain) [21].The molecular weight of DTTP-HA was lower than that of DTTP-W and DTTP-HCW, which suggested breakage and degradation of the polysaccharide chains during the acid extraction process. In addition,the polydispersity indicesMw/Mnof the 4 polysaccharide samples were 4.1 (DTTP-HA), 3.6 (DTTP-W), 2.6 (DTTP-HCW) and 4.2(DTTP-EAE); in summary, theMw/Mnof DTTP-HCW was much lower than the other three. The separation of polysaccharides by different extraction methods led to a wide range ofMwdistributions [20].
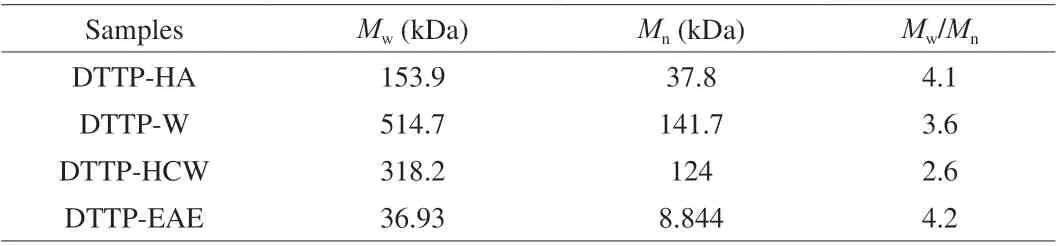
Table 3 Molecular characteristics of DTTP from the peel of D. opposita by different extraction methods.
3.2.2 FT-IR spectra of the obtained polysaccharides
The FT-IR spectroscopy is an effective method to analyze the type and vibration of functional groups in polysaccharides. Structural features of its components can be inferred from a spectrum [23].Fig. 2B shows the effect of extraction methods on the FT-IR spectra of DTTP polysaccharides. No visible differences could be observed,which indicated that the structures of the polysaccharides extracted by different extraction methods were similar. The strong absorption peaks at 3 200–3 600 cm–1indicated O—H stretching vibrations.Bands in the region of 3 000–2 800 cm–1represent C—H stretching and bending vibrations that includes CH, CH2, and CH3[24]. The peaks at approximately 1 740 and 1 630 cm–1were attributed to C=O stretching vibration and asymmetric stretching [15], revealing the existence of uronic acid [25]. The peaks at 1 150, 1 079, and 1 020 cm–1were dominated by the stretching vibrations of the C—O—H side groups and the C—O—C glycosidic vibration of a pyranose ring [26].All four samples showed weak absorption bands at 862–875 cm–1,which suggested a pyranose form of theβ-configuration [27].
Most of the signal peaks at 525–849 cm–1in the spectra of DTTP-HCW and DTTP-EAE disappeared in the DTTP-W and DTTP-HA,and the wavenumber curves were flat [28]. The FT-IR result was in accordance with the results of chemical analysis shown in Table 1.
3.2.3 Thermal analysis
Thermogravimetric analysis is one of the most important methods to understand the thermal stability of polysaccharides and analyze their components. Fig. 3 presents the thermal decomposition curves of the 4 polysaccharide samples of DTTP. The first stage occurred at a temperature of 30–120 °C with a slight decrease in weight, which might be due to the loss of free and bound water. Comparatively,the DTTP-W had the highest loss rate (13.5%), indicating that it had the maximum water-retention capacity. The second stage occurred at 200-550 °C, with a prominent decrease in weight, which was mainly attributed to changes in the functional groups, including depolymerization and decomposition of the polysaccharide structure.DTTP-HCW had the highest maximum mass loss rate (80.84%), and DTTP-W had the lowest maximum mass loss rate (74.12%), which indicated that DTTP-W had a relatively steady mass loss. Finally, the weights of the samples remained almost constant as the temperature increased > 530 °C.
Differential scanning calorimetry (DSC) is a thermal analysis technique that has been widely used in polysaccharides research. As shown in Figs. 3A–D, the DSC of 4 polysaccharide samples of DTTP showed a similar trend. The first broad peak might be attributed to water evaporation. The second decrease might be attributed to depolymerization and decomposition of the polysaccharide. However,some differences were observed in the degradation behavior of the 4 polysaccharide fractions. As shown in Figs. 3A–D, the degradation of the 4 polysaccharide fractions occurred between 200 °C and 550 °C. One peak temperature was observed in three of the DTG curves, while two peaks were observed for DTTP-W. The first peak at a relatively low temperature (263 °C) might be attributed to degradation and loss of low-molecular weight molecules, while the second peak (310 °C) might be related to the degradation of large-molecular weight molecules.
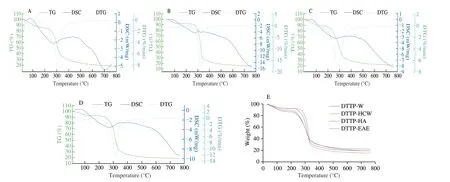
Fig. 3 The thermal analysis of the DTTP extracted by 4 different extraction methods. (A) DTTP-W; (B) DTTP-HCW; (C) DTTP-HA;(D) DTTP-EAE; (E) residual weight of the 4 DTTPs during heating from 30 °C to 763 °C.
After further heating throughout the whole tested temperature range (maximum temperature of 763 °C), the final residues were equal to 18.97% (DTTP-W), 15.72% (DTTP-HCW), 21.79% (DTTP-HA),and 18.79% (DTTP-EAE) of the corresponding initial weights(Fig. 3E). Variations in the structural and functional groups of polysaccharides affect their thermal stabilities [29]. According to the water content and values of residual char in the polysaccharide samples, DTTP-HA had the highest thermal stability. The differences of thermal stabilities and degradation behaviors of samples were attributed to compositional and structural variations among the 4 polysaccharide fractions [30].
3.2.4 Triple helix structure analysis
The triple-helix conformations of polysaccharides are related to the functional and biological activities, and these conformations can be examined by Congo red dye [31]. Polysaccharides containing a triple helical structure when combined with Congo red, display aλmaxred shift compared to the Congo red control. If strong alkali disrupts the triple-helical conformations of the biopolymers, the redshift of the Congo red-polysaccharide complex is weakened [32].As shown in Fig. 4,the effects of NaOH concentration on theλmaxof the Congo red-polysaccharide samples are different. DTTP-W and DTTP-HA displayed obvious red shifts inλmaxcompared to the Congo red blank in the entire tested range, suggesting these two polysaccharides possessed triple-helix structures. However, the red shifts in DTTP-HCW and DTTP-EAE were not significant indicating that these two polysaccharides did not possess triple-helical conformations. The stability of intramolecular and intermolecular hydrogen bonds will affect the triple-helical structure of a polysaccharide, and the fracture of the hydrogen bond will destroy the triple helix. The differences of red shifts of the samples could be attributed to differences in the hydrogen bond components of the samples [32]. These results suggested that DTTP-HA and DTTP-W might have good biological activities.
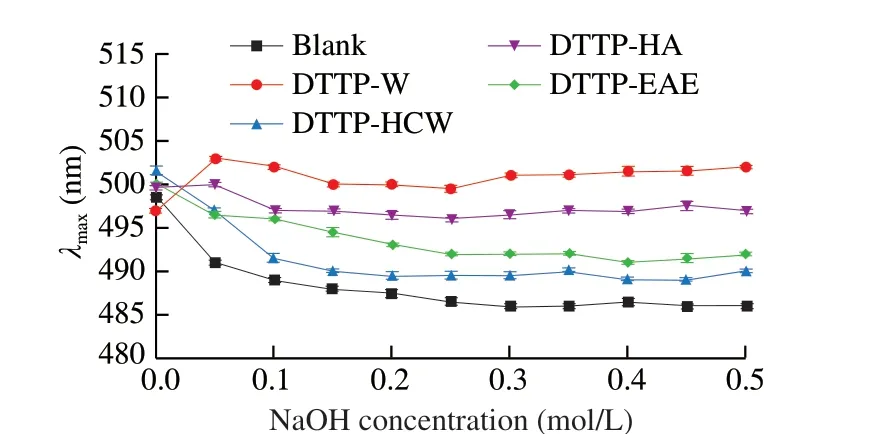
Fig. 4 The maximum absorption wavelengths of 4 Congo red-polysaccharide complexs and Congo red solution at various concentrations of NaOH.
3.2.5 SEM analysis
SEM was used here as a qualitative tool to determine the surface morphologies of the polysaccharides. The extraction, purification, and preparation conditions of polysaccharides may affect their structures,activities and surface morphologies [33]. The different morphologies of the four polysaccharide samples (DTTP-W, DTTP-HCW, DTTPHA, and DTTP-EAE) are shown in Fig. 5 at magnifications of 1000.Specifically, the surface of DTTP-W was smooth and sheet-like (Fig. 5A).The surface of DTTP-HCW was irregular (Fig. 5B); the fragment appearance might be attributed to the destruction of the polysaccharide structure under high temperature and pressure [34]. The surface of DTTP-HA was irregular with many holes (Fig. 5C). In contrast,DTTP-EAE exhibited a rough and uneven surface, and a loose structure with some small pores and cracks (Fig. 5D), which may be the disruption of the cell wall structure and disaggregation caused by the cellulase and papain. The results showed that nature of the extraction method had significant effects on the microstructures of the extracted polysaccharides.
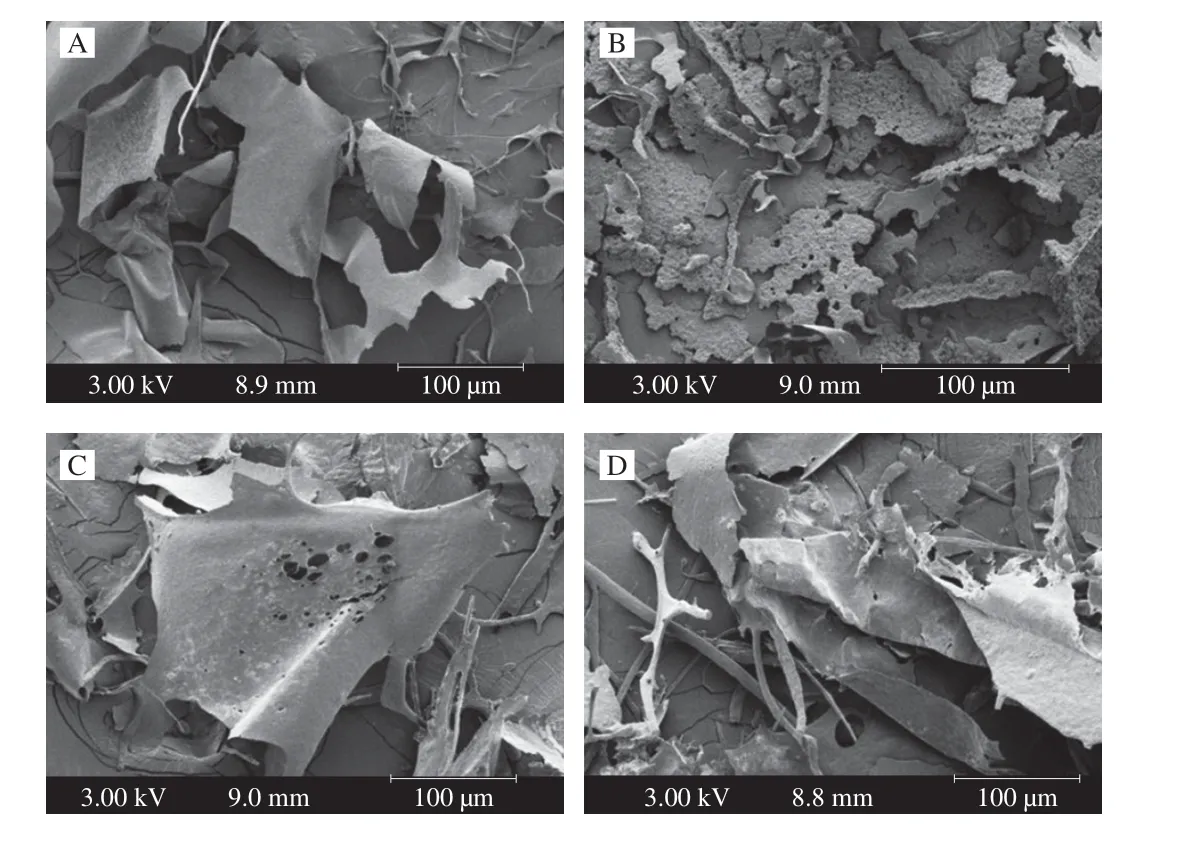
Fig. 5 SEM graphs (1 000 ×) of (A) DTTP-W, (B) DTTP-HCW,(C) DTTP-HA, and (D) DTTP-EAE.
3.3 Antioxidant activities
3.3.1 Hydroxyl radical scavenging assay
The hydroxyl radical is an extremely active free radical. In the human body, it can cause serious damages, even cell death, to adjacent biomolecules [35]. Hydroxyl radical scavenging capacities of the 4 polysaccharide samples are presented in Fig. 6A. All 4 polysaccharides showed a similar trend of increasing scavenging ability with increasing concentration in the tested range. For DTTP-W,small increases in concentration at the lower end of the range brought large increases in scavenging ability, while similar increases brought only small increases in scavenging ability for DTTP-HCW, DTTPHA and DTTP-EAE. At 10.0 mg/mL, the scavenging abilities of DTTP-W, DTTP-HCW, DTTP-HA, and DTTP-EAE on the hydroxyl radical were 91.19%, 63.23%, 61.34% and 65.69%, respectively. The highest scavenging ability of DTTP-W might be attributed to its high uronic acid content, as DTTP-W also had high uronic acid content.
3.3.2 DPPH radical scavenging assay
The DPPH assay is a simple method used here to test the antioxidant capacities of the 4 polysaccharide samples. Generally, the DPPH radical scavenging activities depends on hydrogen donating abilities [28]. As shown in Fig. 6B, in the tested concentration range, all samples showed increasing DPPH free radical scavenging activities with elevated concentrations. In the concentration range of 0.05-10.00 mg/mL, the scavenging abilities of the 4 samples that increase in concentration at the lower end of the range brought large increases in scavenging ability. At the concentration of 10.0 mg/mL, the scavenging effect increased to 90.82%, 87.61%,97.72% and 95.30% for DTTP-W, DTTP-HCW, DTTP-HA, and DTTP-EAE, which showed that the polysaccharides of DTTP had significant DPPH radical scavenging abilities. DTTP-HA showed the highest scavenging abilities among the 4 polysaccharide samples,which might be due to its high uronic acid content.
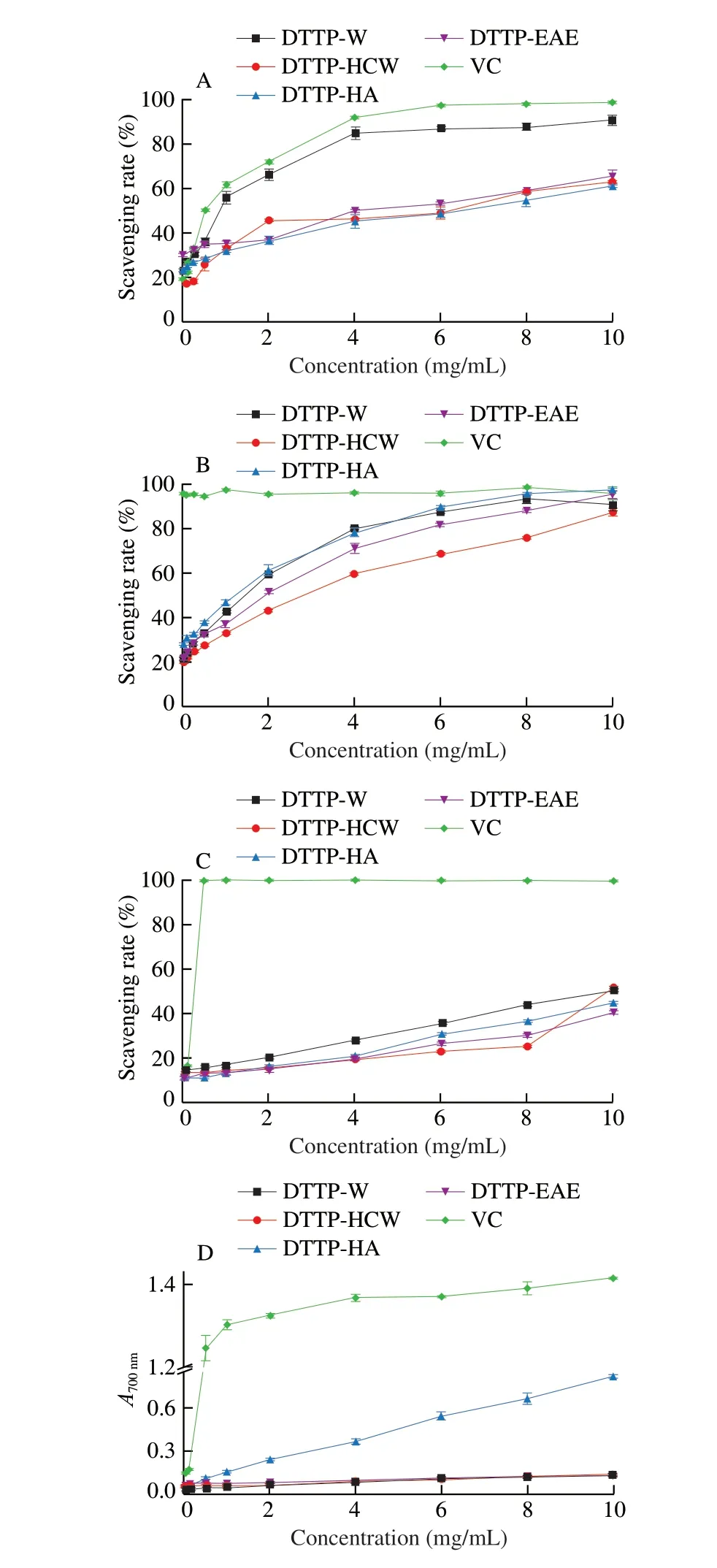
Fig. 6 Antioxidant activities of DTTP-W, DTTP-HCW, DTTP-HA,DTTP-EAE polysaccharides and VC. (A) Hydroxyl radicals scavenging activity; (B) DPPH radicals scavenging activity; (C) ABTS radical scavenging activity; (D) reducing power.
3.3.3 ABTS radical scavenging assay
ABTS radical assay is a simple method to determine the antioxidant capacities of polysaccharides. Antioxidants can provide hydrogen atoms or electrons to change the color of ABTS. As shown in Fig. 6C, the radical scavenging abilities of the four DTTP samples tested here increased with increased concentrations in the measurement range, showing that all had the potential ability to scavenge ABTS radicals. DTTP-W had a higher scavenging capacity for the ABTS radical at low tested concentrations, which might be attributed to its relatively stronger hydrogen supply capacity compared with the other three. It has been reported that the polysaccharides with uronic acid groups can react with the hydrogen atom of the anomeric carbon, thus contributing to scavenging the ABTS radical [17].DTTP-W showed a higher activity among the 4 polysaccharides,which might be due to its high galacturonic acid content.
3.3.4 Reducing power assay
Reducing power is necessarily associated with the presence of reductants; thus, reductants serve as indicators of antioxidant activities in natural compounds [16]. As showed in Fig. 6D, the reducing powers of the 4 DTTP samples exhibited similar trends of increased reducing power associated with increased concentration.It has been reported that the polysaccharides with lowerMwusually present more reducing and non-reducing ends on the molecules, thus contributing to the increase in reducing power [32]. At all the tested concentrations, the reducing power of DTTP-HA was stronger than that of other samples. At 10.0 mg/mL, the absorbances of DTTP-W,DTTP-HCW, DTTP-HA and DTTP-EAE were 0.142, 0.138, 0.813 and 0.132, respectively. This inconsistent result might be due to the influence of uronic acid, molecular weight and extraction methods.
3.4 Hypoglycemic activities
3.4.1 α-Amylase inhibition activity assay
α-Amylase inhibitory activity assay was always used as a method to find antidiabeticin vitro. The inhibitors ofα-amylase can restrain the rise of postprandial blood glucose by retarding the digestion of starch [34]. Fig. 7A presents the effects of DTTP-W, DTTP-HCW,DTTP-HA and DTTP-EAE onin vitroα-amylase inhibitory activities.The 4 samples had similar trends in inhibitory effects. As the concentration of polysaccharides of DTTP increased from 0.1 mg/mL to 10.0 mg/mL, theα-amylase inhibitory activities of all 4 samples increased in a dose-dependent manner. The inhibition rates of DTTP-HCW and DTTP-EAE were similar to each other in the concentration range of 0–2 mg/mL. The inhibition rate of DTTP-HA initially increased greatly in the concentration range, and then increased more incrementally; in contrast, the inhibition rate of DTTP-W increased incrementally throughout the range. At 10.0 mg/mL, the inhibition rates of DTTP-HA, DTTP-W, DTTP-HCW and DTTP-EAE were 82.01%, 65.78%, 61.83% and 58.58%,respectively. In particular, DTTP-HA showed the highestα-amylase inhibitory activity among the 4 samples. The results indicated that hydrochloric acid extraction produces polysaccharides from DTTP with the highestα-amylase inhibitory activity.
3.4.2 α-Glucosidase inhibition activity assay
One explanation for the success of using natural polysaccharides to treat diabetes is the ability of these polysaccharides to inhibitα-glycosidase enzymes in the bowel, which can slow down the decomposition of carbohydrates, reduce postprandial hyperglycemia [36].As shown in Fig. 7B, the 4 samples showed varying degrees of inhibitory activities againstα-glucosidase in a concentrationdependent manner. Acarbose was used as the positive control.Interestingly, the 4 samples exhibited similar inhibitory trends,increasing significantly with small increases in concentration at the lower end of the range, and then increasing less dramatically as the sample concentrations increased from 0.1 mg/mL to 10.0 mg/mL. DTTP-HA had the maximum inhibition rate in the range of experimental concentrations. At 10.0 mg/mL, theα-glycosidase inhibitory activities of DTTP-W, DTTP-HCW, DTTP-HA and DTTP-EAE were found to be 38.13%, 33.04%, 41.60% and 25.46%,respectively. The results indicated that hydrochloric acid extraction was the most efficient in preparing polysaccharides of DTTP with highα-glucosidase inhibitory activity.
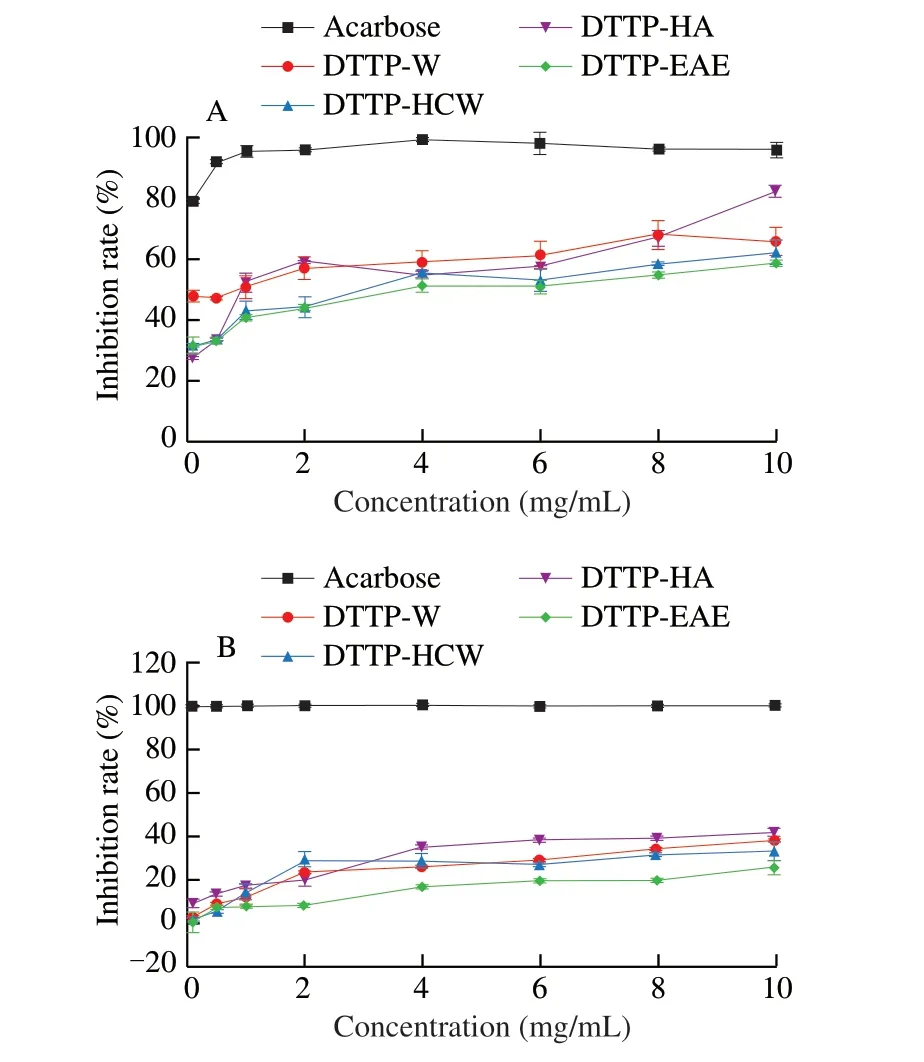
Fig. 7 Inhibitory effects of DTTP-W, DTTP-HCW, DTTP-HA, DTTP-EAE on the (A) α-amylase activity and (B) α-glycosidase.
α-Glucosidase andα-amylase are two key enzymes in the human body. The inhibitory effect of polysaccharides onα-glucosidase andα-amylase are non-competitive interaction, which could limit its activities [31]. DTTP-HA had the highest inhibitory activities of bothα-glucosidase andα-amylase among 4 polysaccharides. The reason could be attributed to its high content of carbohydrate, uronic acid and low molecular weight [37].
The water solubility, carbohydrate content and protein content could affect the bioactivities of the DTTP-HA and DTTP-W. DTTP-HA is more water soluble than DTTP-W. More carbohydrate was obtained by acidic extraction, i.e., in DTTP-HA (85.08%), while less carbohydrate extracted by hot water, i.e., DTTP-W (46.37%). Hot water treatment extracted more protein, as shown in the DTTP-W(3.68%), while acidic treatment extracted much less, i.e., DTTP-HA(0.43%). The better bioactivities of DTTP-HA may be related to the above factors. A clear structure-activity relationship among the polysaccharides of DTTP needs further, thorough study. The results suggest that the polysaccharides of DTTP have strongα-glucosidase andα-amylase inhibitory activities and it could be explored for use in hypoglycemic drugs.
4. Conclusion
In this study, hot water, hot-compressed water, acid and enzymes were used to extract polysaccharides (DTTP-W, DTTP-HCW, DTTP-HA and DTTP-EAE) from the peel ofDioscorea opposite. The results showed that the yields, physicochemical properties, and hypoglycemic and antioxidant activities of polysaccharides from DTTP obtained by different extraction methods were significantly different. DTT-HCW and DTT-HA had the highest yield, while DTTP-HA had the highest total carbohydrate (85.08%) and uronic acid (13.2%) content. The results of monosaccharide analysis showed that the 4 polysaccharide samples were composed mainly of glucose, mannose and galactose.Scanning electron microscopy showed that DTT-HA had an irregular,porous structure. Thermal analysis results indicated that DTTP-HA had the highest thermal stability among the 4 samples. Triple-helix structure analysis results revealed that DTTP-HA possessed a triplehelix structure. The highest antioxidant activities andα-amylase inhibitory activities of the DTTP-HA among the four samples might be due to its high uronic acid content and low molecular weight.Finally, the results indicated that acid was the best extraction method to obtain polysaccharides from DTTP in high yield with strong antioxidant activity for applications in the food industry.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors gratefully acknowledge the financial support provided by Zhengzhou 1125 Talents Gathering Project (2018) and the Doctor startup fund of Henan Agricultural University (30500431).
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://doi.org/10.1016/j.fshw.2022.07.031.
- 食品科学与人类健康(英文)的其它文章
- Wine, beer and Chinese Baijiu in relation to cardiovascular health:the impact of moderate drinking
- Comparative analysis of physicochemical properties, ginsenosides content and α-amylase inhibitory effects in white ginseng and red ginsen
- Monitoring and identif ication of spoilage-related microorganisms in braised chicken with modif ied atmosphere packaging during refrigerated storage
- Effect of cooking processes on tilapia aroma and potential umami perception
- Formation mechanisms of ethyl acetate and organic acids in Kluyveromyces marxianus L1-1 in Chinese acid rice soup
- Volatile prof ile and multivariant analysis of Sanhuang chicken breast in combination with Chinese 5-spice blend and garam masala

