Energy status regulated umami compound metabolism in harvested shiitake mushrooms (Lentinus edodes) with spores triggered to release
Rongrong Xi Xuemei Zho, Gung Xin*, Libin SunHern Xu Zhenshn Hou Yunting Li Yfei Wng
a College of Food Science, Shenyang Agricultural University, Shenyang 110866, China
b Sichuan national inspection and Testing Co., Ltd., Luzhou 646000, China
Keywords:Shiitake mushrooms Spore release Energy metabolism Umami taste Transcriptome
A B S T R A C T The molecular mechanisms of energy status related to the umami taste of postharvest shiitake mushrooms during spore release remain poorly understood. In this study, the variations of energy status and umami taste of mushrooms were measured at 25 °C. At 24 h storage, slight spore prints of mushrooms were f irst pictured, respiration peaked. Signif icant ATP decrease and ADP increase were also observed as the initiation of postharvest senescence (P < 0.05). Meanwhile, the activities of phosphohexose isomerase, succinate dehydrogenase, glucose-6-phosphate dehydrogenase and cytochrome c oxidase and the contents of umami nucleotides and amino acids were maintained at higher levels in mushrooms with spore release. Notably, the equivalent umami concentration (EUC) was strongly correlated with energy levels (R = 0.80). Fifteen related gene expression levels in the energy metabolism pathway were downregulated. LecpdP1 and LeAK were signif icantly expressed in the conversion of ATP into AMP and played key roles in connecting the energy state and umami level. These results provided valuable insights on the umami taste associated with energy metabolism mechanism during postharvest mushroom spore release.
1. Introduction
Shiitake mushrooms (Lentinus edodes) are widely popular among consumers due to their nutritional value, unique taste and pleasant f lavor [1-4]. However, harvested mushrooms are susceptible to flavor loss, softening, browning, cap opening and spore release which symbolize mushroom senescence [5]. Mushroom flavor is not only pleasant to the senses of consumers but also responsible for spore release [6]. One mushroom can release billions of spores in a day [7]. The spores require osmotic transport across the membrane against a concentration gradient before ejection. Osmotic transport can be accomplished by a transmembrane protein pump (ATPase),which drives active transport by splitting adenosine 5’-triphosphate(ATP), or by a potential energy coupled transport pump using an electrochemical gradient [8].
Energy status is regarded as an important factor in postharvest ripening and senescence [9,10]. Yan et al. [11] showed that the umami components of mushrooms could be affected by regulating energy metabolism. Zhang et al. [12] found that a higher energy status could contribute to a higher umami taste of mushrooms during storage. 5′-Nucleotides and umami amino acids, including adenosine-5′-monophosphate (AMP), guanosine-5′-monophosphate (GMP),inosine-5′-monophosphate (IMP), xanthosine-5′-monophosphate(XMP), glutamic acid (Glu) and aspartic acid (Asp), are not only main contributors to the umami taste but also important energyyielding metabolites [13-15]. AMP was significantly related to equivalent umami concentration (EUC), which connects umami taste and energy [12]. Glu is a key regulator of the carbon-nitrogen balance, and catabolites can be directly utilized in the tricarboxylic acid (TCA) cycle to participate in energy metabolism in response to starvation [16]. Li et al. [9] speculated that the energy status in postharvest mushrooms plays key roles in regulating glutamate levels.However, limited information is available at the molecular level on the relationship between energy status and the umami level.
High-throughput transcriptomics provides a holistic approach to explore the complete transcriptome landscapes in biological systems [17]. Recently, genes in postharvest mushrooms have been characterized to investigate the regulatory mechanisms affecting maturation and senescence [18,19], but studies of the molecular mechanisms in energy changes during mushroom spore release and how energy status is related to the umami taste at the molecular level are still scant.. Therefore, the mushrooms stored at different periods were used for transcriptome sequencing (RNA-Seq) to identify differentially expressed genes (DEGs) related to energy and umami metabolism during spore release. The objective of this study was to reveal the connection between energy status and umami taste in the molecular mechanism during postharvest mushroom spore release.
2. Materials and methods
2.1 Mushroom materials
Shiitake mushrooms were obtained from a mushroom planting base located in Benxi city, Liaoning Province, China. Mushrooms free from mechanical damage, disease and cap opening were selected with uniform size and color. All harvested mushrooms were transported to the laboratory at Shenyang Agricultural University within 2 h.Mushrooms were placed in sealed bags (size: 17 cm × 51 cm,thickness: 32 μm, air permeability: 5 055.73 cm3/m2, moisture permeability: 13.09 g/m 24 h) every 0.5 kg and stored at (25 ± 1) °C for 72 h with 85%-90% relative humidity. Mushroom samples were taken every 12 h for analysis.
2.2 Respiratory rate and weight loss
The respiratory rate and weight loss of mushrooms were measured according to the method of Olaniyi et al. [20]. Mushrooms(0.2 kg) were placed in a closed container (4.4 L) and kept for 1.5 h.CO2concentration was measured using a gas analyzer (Yangguang yishida Technology Co., Beijing, China). Respiratory rate results were expressed as μg/(kg·s). The weight loss was expressed as the percentage (%) of mushroom samples compared to the initial weight.
2.3 Determination of free amino acids
The measurement of free amino acids contents was conducted according to our previous study [21]. Free amino acids contents were detected by an automatic amino acid analyzer (L-8900, Hitachi Ltd,Tokyo, Japan). Free amino acid concentrations were expressed as g of free amino acids per kg on a fresh weight basis (g/kg).
2.4 Nucleotide-related compounds determination
The determination of ATP, ADP, AMP, GMP, IMP and XMP was carried out by high-performance liquid chromatography based on the modified method of Zhang et al. [12]. Mushroom samples (5.0 g)were homogenized with 20 mL of distilled water, boiled for 60 s, and then centrifuged at 4 500 ×gfor 15 min after cooling. The residues were extracted using 20 mL distilled water as described above.The supernatant was collected, and distilled water was added to a total volume of 50 mL. The solution was filtered through a 0.45 μm membrane filter before analysis.
A Waters 1525 HPLC system equipped with a UV detector(Waters Corporation, Shanghai, China) and LiChrospher RP-18 column (250 mm × 4.6 mm, 5 μm, Merck) at a flow rate of 1.0 mL/min were used for the determination. Nucleotide-related compounds in the samples were identified by comparison with the retention times of ATP, ADP, AMP, GMP, IMP, and XMP standards (Shanghai Yuanye Bio-Technology Co., Ltd., Shanghai, China). The concentrations were quantified based on the calibration curve, and data are presented as g of compound per kg on a fresh weight basis (g/kg).
2.5 Equivalent umami concentration assay
The EUC value (g MSG/100 g) reflects the concentration of MSG equivalent to the umami intensity given by a mixture of flavorfree amino acids and 5’-nucleotides, which is expressed using the following formula according to Yamaguchi et al. [22]:
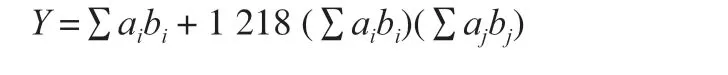
WhereYis the EUC of the mixture (g MSG/100 g);aiandajare the concentrations (g/100 g) of umami amino acids and 5’-nucleotides;biandbjare the relative umami concentration (RUC) of umami amino acids and 5’-nucleotides with respect to MSG (Glu = 1; Asp = 0.077;GMP = 2.3; IMP = 1; XMP = 0.61; AMP = 0.18); and 1 218 is a synergistic constant based on the concentration (g/100 g) used.
2.6 Assays of enzyme activities
Shiitake mushrooms (1.0 g) were homogenized in 10 mL of 50 mmol/L sodium phosphate buffer (pH 7.8). The samples were centrifuged (6 800 ×g, 4 °C, 10 min), and the supernatant was used for the following enzymatic assays.
Phosphoglucose isomerase (PGI) and succinate dehydrogenase(SDH) activities were determined following the method of Zan et al. [23]with some modifications. The crude enzyme (0.5 mL) was mixed with 1.0 mL of 3.9 g/L glucose-6-phosphate (dissolved with 50 mmol/L sodium phosphate buffer, pH 7.4) and incubated at 30 °C for 5 min. Then, 2.0 mL of 100 g/L trichloroacetic acid was added to the mixture, and the mixture was centrifuged at 1 900 ×gfor 10 min.Then, 1.0 mL supernatant, 3.0 mL hydrochloric acid and 1.0 mL 1.0 g/L resorcinol were mixed and kept in boiling water for 8 min.The absorbance of the mixture was immediately recorded at 520 nm after cooling. Fructose was used as a standard, and one unit (U) of PGI activity was defined as the amount of enzyme that liberated reducing sugars equivalent to 1.0 mg of fructose per minute. We assayed SDH activity by mixing the sample with 1.5 mL of sodium phosphate buffer (50 mmol/L, pH 7.4), 1.0 mL of 19.45 g/L sodium succinate,0.1 mL of 0.24 g/L 2,6-dichlorophenolindophenol and 0.2 mL of distilled water. The reaction started by adding crude extract solution (0.1 mL) and 9.0 g/L methyl sulfonyl phenazine. One unit of SDH activity was defined as an increase of 0.01 in absorbance per minute at 600 nm.
Glucose-6-phosphate dehydrogenase (G-6-PDH) and cytochrome c oxidase (CCO) activities were executed strictly by an enzymelinked immunosorbent assay (ELISA) kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the instructions and information provided by the manufacturer. One unit of G-6-PDH activity was defined by an increase in absorbance at 340 nm by 0.01 per minute. One unit of CCO activity was defined by an increase in absorbance at 450 nm by 0.01 per minute. All enzyme activities in this study were described as U/mg protein.
2.7 RNA extraction and transcriptome sequencing
RNA extraction, total RNA sample testing, mRNA enrichment,double-stranded cDNA synthesis, fragment selection and PCR enrichment, sequencing library generation and sequencing were carried out by Zhongke New Life Biotechnology Co., Ltd. (Shanghai,China) on an Illumina HiSeq platform. The sampling points were selected on mushrooms stored at 0, 24 and 60 h, and three replicates were set for each sample. DESeq2 was used for analysis, and the identification of DEGs was screened with |log2FC| ≥ 1 andP< 0.05 for further analysis. The Gene Ontology (GO) categories of DEGs were analyzed with an online tool (http://www.geneontology.org/),and KEGG (Kyoto Encyclopedia of Genes and Genomes) is the main public database about pathways [24]. Because of the annotation results and official classification, DEGs were classified into different groups according to functions and involved biological pathways.
2.8 Quantitative real-time PCR (q-PCR) validation
To verify the sequencing results, seven genes were randomly selected, and quantitative polymerase chain reaction (q-PCR) was performed. Total RNA was extracted from mushrooms by using an Unlq-10 column TRIzol Total RNA Extraction Kit (Sangon Biotech.Co., Ltd.), and the reaction was carried out by the StepOne Real-Time PCR System. Relative expression levels were calculated based on the 2-△△Ctmethod [25]. All primers designed and used in this study are listed in Table S1.
2.9 Statistical analysis
All of the experiments in this study were carried out in triplicate(n= 3) and all results were presented as means ± standard deviation(SD). Duncan’s multiple-comparison test, one-way analysis of variance (ANOVA) and Pearson correlation analysis were performed using SPSS v24 (IBM Institute, USA). Findings withP< 0.05 were considered to be statistically significant.
3. Results and discussions
3.1 Storage quality indexes
The senescence of postharvest mushrooms during storage was shown as cap opening, stipe and gill growth, and sporulation [1,26].The typical appearance characteristics in shiitake mushroomstored at 25 °C induced the cap slightly opened at 12 h (Fig. 1A) with slight spore prints first pictured at 24 h. A large spore release was observed during 36 h to 60 h, and then spore release was completed during 60 h to 72 h. Respiration indicated the potential physiological aging process of fresh mushrooms, depending on the maturity of the sporophor [27]. The respiratory rate peaked significantly at 24 h(P< 0.05), which coincided with the period of spore first release.(Fig. 1B). Mushrooms had increasing respiration once removed from the nutrient substrate, and the respiration had to depend on the consumption of nutrientsin vivoto meet the energy supply [28].With spore release and cap opening in mushrooms, weight loss continuously increased (Fig. 1C). Mushrooms have a high respiration rate after harvest, resulting in weight loss and finally spoilage [9].Previous studies showed almost concurrent respiratory bursts upon the initiation of mushroom senescence [14,27]. Thus, the spore release of mushrooms was triggered at 24 h, corresponding with the phase of the respiration peak at the initiation of mushroom senescence.
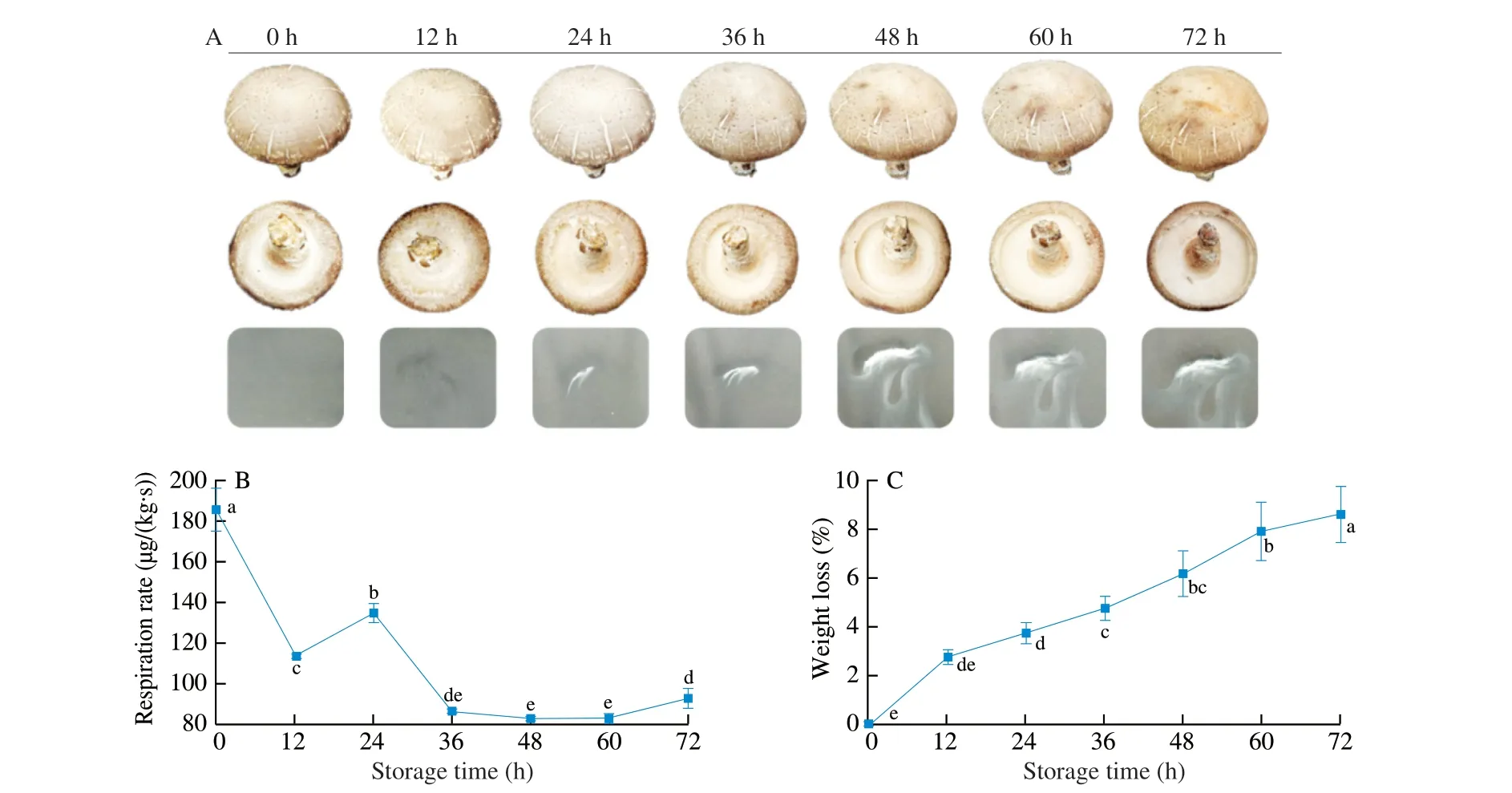
Fig. 1 Changes in appearance (A), respiration rate (B) and weight loss (C) during storage. Vertical bars represent the standard deviation. Different letters indicate significant difference during different storage (P < 0.05).
3.2 Energy status
Physiological disorders of mushrooms have been demonstrated to begin along with cell death after harvest due to an insufficient supply of energy [23,29]. As shown in Table 1, the decrease in ATP and AMP accompanied by the increase in ADP resulted in a decrease in EC. Mushrooms showed significantly higher ADP content and lower ATP content at 24 h storage (P< 0.05), which was consistent with the peak respiration rate. The change in ADP content was inverse to the change in ATP content, which was due to the transformation between ATP and ADP through phosphorylation and oxidative phosphorylation [30,31]. Once the senescence process was initiated,ATP dissipation was aggravated, while the ADP content was triggered to increase [32]. EC was a key factor in switching on senescence,and the decline in ATP levels was characteristic of initiation of deterioration [11,30,33]. As shown in Table 1, the variation trend of EC, ATP/ADP and ATP/AMP were consistent with the variation trend of ATP content, significantly decreased after 24 h storage, and then peaked at 36 h. Maldonado et al. [34] found changes in ATP/ADP ratios were related to mitochondrial function by enhancing (low ATP/ADP ratio) or inhibiting (high ATP/ADP ratio) Embden-Meyerhof-Parnas pathway (EMP) metabolism. Senescence is usually related to the ATP/AMP ratio, and changes indicate alterations in cellular macromolecules [32]. As mentioned above, mushroom senescence is closely related to energy status [35]. Studies have speculated that the spores of mushrooms with energy consumption would be liberated soon [12]. In this study, mushroom spore release at 24 h was accompanied by a significant decline in ATP content and ATP/ADP and ATP/AMP ratios and a significant increase in ADP content. Thus,energy consumption might promote spore release and accelerate mushroom senescence.
3.3 Energy-related enzymatic activities
Postharvest mushrooms maintain a high respiration rate and metabolize carbohydrates and organic acids to provide energy via pathways including EMP, TCA, pentose phosphate pathway(HMP) and mitochondrial electron transport chain pathway(mETC) [18,23,24]. ATP is the primary energy source for enzymatic activities, such as PGI, G-6-PDH, SDH and CCO, which are involved in oxidative phosphorylation and ATP synthesis [36]. As shown inTable 1, energy-related enzymes did not exhibit significant initial high activity. Initially, higher respiratory rate and energy levels might not be caused by enzymatic activities but would probably be caused by harvesting stress [37]. PGI is an important enzyme involved in the respiratory pathway and plays a key role in cellular energy metabolism [23]. The activity of PGI in mushrooms continuously increased during the first 24 h of storage. PGI activity of mushrooms stored at 24 h and 60 h had the highest levels. G-6-PDH is a key regulator enzyme that affects the activation of the HMP pathway directly, and high levels of HMP enhance the stress resistance of plants [38]. The G-6-PDH activity of mushrooms showed a notable increase at 24 h and peaked at 48 h (86.34 U/g pro). SDH is a key enzyme of the TCA pathway and catalyzes the oxidation of succinic acid reversibly into fumaric acid [37,39]. SDH activity presented a gradual increasing trend during the first 24 h and peaked at 36 h(0.12 U/g pro). CCO, a terminal enzyme in the mETC, plays an important role in energy production during oxidative phosphorylation [12,37].CCO activity exhibited change patterns similar to SDH activity and had relatively higher levels at 24 h and 36 h. Wang et al. [40] reported that enhanced activity of SDH and CCO were associated with higher consumption of ATP levels. All energy-related enzymes were significantly increased at 24 h (P< 0.05). The results indicated that spore first release accompanied energy consumption and that energy enzyme activities increased in mushrooms. Therefore, the storage quality of mushrooms could be maintained by delaying the time when spores began to release and decreasing respiration and energy-related enzymatic activities.
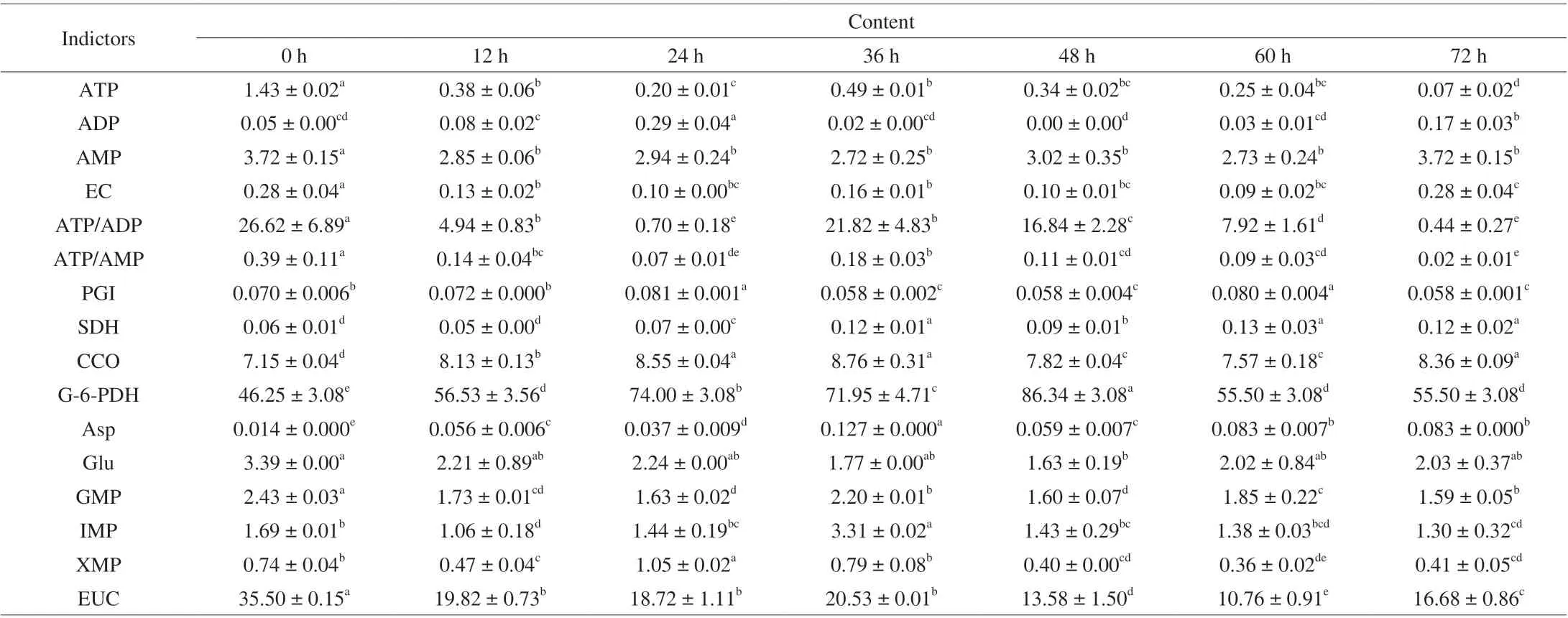
Table 1 Means of nucleotide-related compounds (g/kg), energy-related enzymatic activities (U/g pro), umami amino acids (g/kg), and EUC values (g MSG/100 g) of shiitake mushroom during storage.
3.4 Umami-taste amino acids, flavor 5′-nucleotides, and EUC analysis
GMP, IMP, XMP and AMP are regarded as flavor nucleotides in mushrooms [15]. As shown in Table 1, four-flavor nucleotide content constantly decreased during storage. ATP is converted into ADP and AMP by the removal of phosphoric acid groups, and AMP contributes to the umami taste [12]. Yan et al. [11] demonstrated that purine nucleotides are major energy carriers, and the cellular ATP level determined adenylate catabolism and the AMP deaminase-catalyzed AMP to IMP. The mushrooms had relatively higher umami nucleotide content at 24 h and 36 h, which could be due to the large consumption of ATP converted into umami nucleotides.
Asp and Glu were identified as the characteristic umami tastes,and Glu was the richest amino acid in mushrooms [41,42]. The content of Asp increased initially and peaked at 36 h. The Glu content, ranging 1.63-3.39 g/kg, remained at a relatively higher level at 24 h. The extent of Glu catabolism might be closely related to the carbon skeleton supply and energy status of plant cells [9].
According to Table 1, the EUC value ranged from 10.76 to 35.50 g MSG/100 g and significantly decreased with prolonged storage time (P< 0.05). The EUC value reached a peak at 36 h with no significant difference from the value at 24 h (P> 0.05). After 24 h of storage, the spores were triggered by release, and the umami compound content remained at a relatively higher level with a large amount of energy consumption.
3.5 Correlation analysis
The relationship between energy status and umami compounds was analyzed in this study (Fig. 2). The correlation coefficient test detected that ATP and AMP content was significantly related to GMP and Glu content (P< 0.05). Amino acids contribute to mitochondrial metabolism and ATP production, in which carbon skeletons are generally converted to precursors or intermediates of the TCA cycle [16].The correlation between umami-taste compound content and energy metabolism-related enzyme activity was also analyzed. Glu content was negatively correlated with G-6-PDH and SDH activities (P<0.05), while Asp content was positively correlated with SDH and CCO activities (P< 0.01). SDH was probably an important enzyme that affected umami taste in harvested mushrooms [12]. Notably,EC exhibited a strong positive correlation with EUC (P< 0.001).Accordingly, we suggested that the umami compound level was closely related to energy status in mushrooms during spore release.
3.6 DEGs involved in energy and umami metabolism pathways and q-PCR
In this study, we evaluated the changes in energy and umami metabolism pathways during shiitake mushroom spore release at the molecular level. Transcripts of stored mushrooms at 24 h and 60 h were compared with their initial expression level (CK). There were 1 080 (712 up- and 368 downregulated) and 1 053 (789 upand 264 downregulated) DEGs identified in CK vs. TA 24 and CK vs. TA 60, respectively (Fig. S1A, B). Notably, 352 upregulated and 126 downregulated DEGs both existed in CK vs. TA 24 and CK vs. TA 60 (Fig. S1C, D). According to the DEG-enriched KEGG pathways and the gene functional annotation, DEGencoded enzymes in energy, purine and amino acid metabolism pathways were identified. Therefore, energy, purine and amino acid metabolism pathways were activated with spore release. A total of 19 key genes related to the energy metabolism pathway,12 key genes of purine metabolism and 6 key genes of amino acid metabolism were selected, which were significantly expressed during mushroom spore release (Table S2).
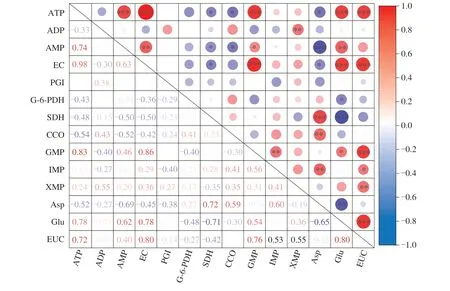
Fig. 2 Pearson correlation coefficient (R) matrix for energy status and umami compound content in shiitake mushroom during storage. Red and blue represent positive and negative correlations between factors. *, ** or *** indicate statistically significant correlations at P < 0.05, 0.01 or 0.001, respectively.
Correlations between energy level and energy metabolism pathway-related gene expression levels were strong, indicating that EMPR expression levels were highly consistent with the changes in energy levels (Fig. 3A). As shown in Fig. 4A,LeGALM,LeGADPH,LePGKandLePKgenes were significantly downregulated at 24 h,which led to a decrease in pyruvate content (Fig. 4A). Pyruvate is an important raw material of the TCA pathway. The production of energy is related mainly to mitochondrial reactions, such as the TCA and mETC pathways [43]. TheLeSDH,LeIDHandLeACLYgenes were downregulated at 24 h in the TCA pathway.LenuoA,LefbcH1,LefbcH2andLeCCOin the mETC pathway showed significantly downregulated expression at 24 h. Fifteen genes in energy metabolism were significantly downregulated, suggesting that large energy consumption was triggered to spore release. Zan et al. [23] reported that the maintenance ofvvSDHandvvCCOexpression levels could reduce energy loss and thus inhibit rapid quality deterioration and senescence in straw mushrooms. Previous studies revealed that melatonin suppressedBrSDH1/6andBrCOX5/6expression levels and effectively delayed leaf senescence and quality deterioration in Chinese flowering cabbage [44]. TheLeACLY,LenuoA,LeALDH1andLeALDH2genes were significantly downregulated at 60 h, which decreased ATP content. Hence, downregulating genes related to ATP synthesis could result in mushroom senescence.
As shown in Fig. 3B, high correlations existed between 5′-nucleotide contents and purine metabolism-related genes (PMRs).Fig. 3C showed that PMRs expression levels had a great correlation with energy status, indicating that there was a positive relationship between energy status and umami nucleotides, which was in keeping with our correlation analysis (Fig. 2). As shown in Fig. 4B,LeAKandLecpdP1were the key genes that modulated ATP conversion into AMP and were significantly upregulated at 24 h. Zhang et al. [12]indicated that AMP connected umami and the energy of mushrooms and that AMP content was significantly related to EUC. ATP and ADP are precursors of AMP, and AMP can be converted into IMP,XMP and GMP directly or indirectly.LecpdP2,LenrdJ1,LenuDandLeADKwere significantly downregulated at 24 h, which inhibited the decomposition of ATP, AMP, IMP and XMP. Therefore, the content of umami nucleotides was higher at 24 h, which was consistent with our previous findings (Table 1). Fig. 3D shows the correlation between the relative expression level of amino acid metabolismrelated genes and amino acid content. The correlation between the relative expression level of amino acid metabolism relatedgenes and energy status was shown in Fig. 3E. Fig. 4C showed that the TCA cycle was the intermediate pathway for the synthesis and decomposition of Glu and Asp in the amino acid metabolism pathway.LeGLTandLegudBexpression levels were significantly increased at 24 h, leading to the conversion of ketoglutarate into Glu in large quantities.LEGADupregulation resulted in an increase in the decomposition of Glu at 60 h. The significantly downregulated geneLEASNSat 24 h and 60 h was negatively correlated with the content of Asp, which led to an increase in the production of Asp. Genes in the purine and amino acid metabolism pathways were significantly expressed which showed that energy status was related to the umami taste at the molecular level. The above data suggested that umami compounds metabolism was regulated by energy status in mushrooms during storage.
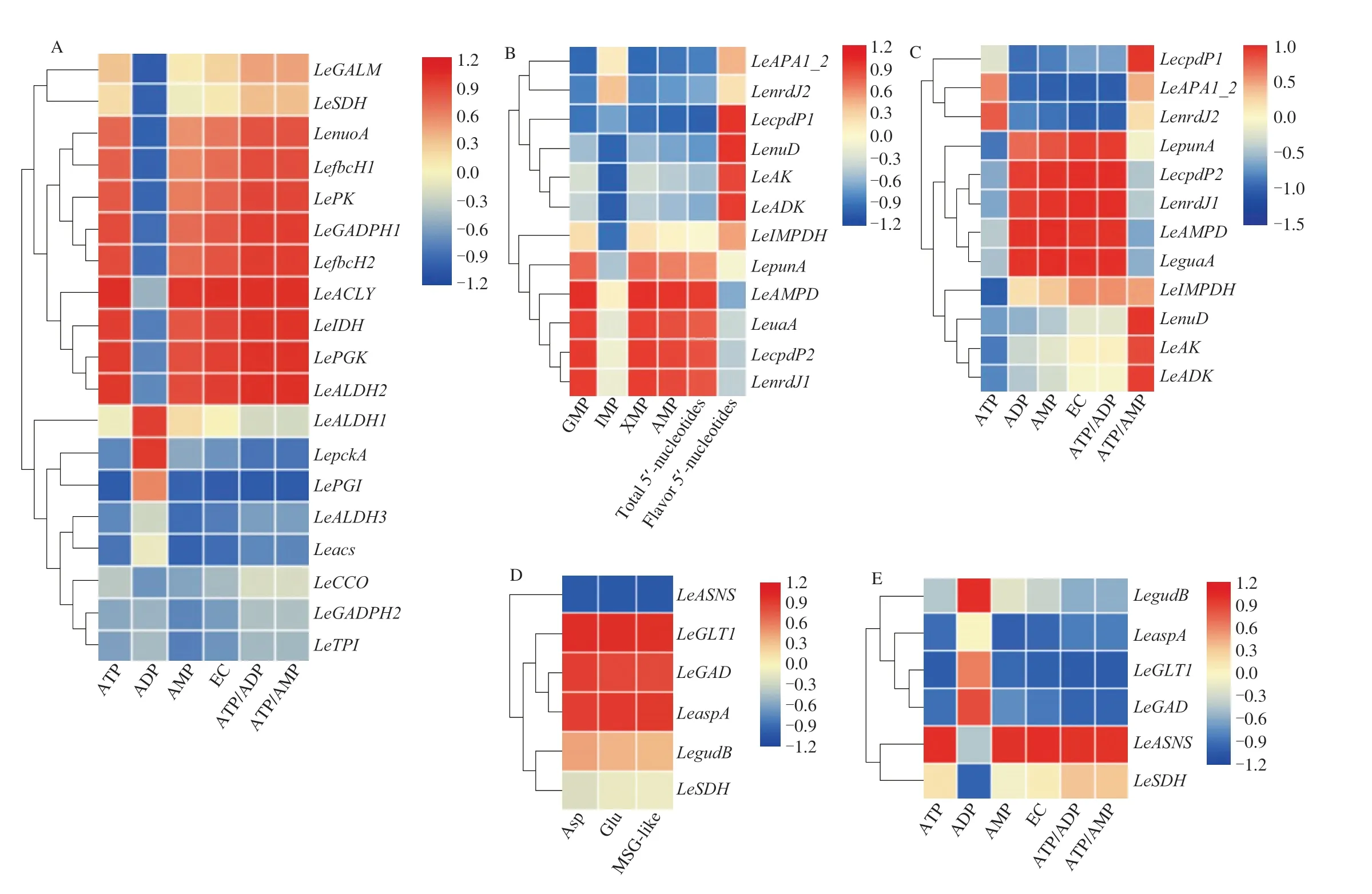
Fig. 3 Heat maps of correlation between relative expression levels (expressed as FPKM (fragments per kilobase of exon per million fragments mapped) of EMPRs and energy status (A), correlation between relative expression levels of PMRs and 5′-nucleotides content (B), correlation between relative expression levels of PMRs and energy status (C), correlation between relative expression level of amino acid metabolism related genes and amino acid content (D) and correlation between relative expression level of amino acid metabolism related genes and energy status (E).
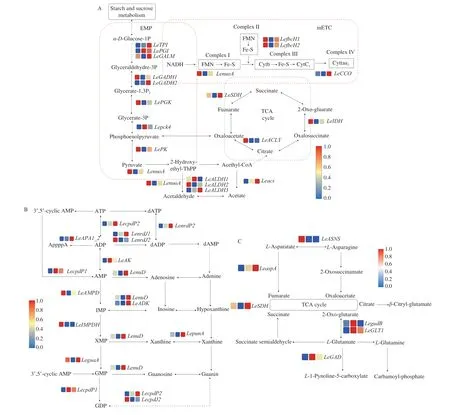
Fig. 4 Impacts of energy (A), purine (B) and amino acid (C) metabolism pathways in mushrooms during storage. Transcript levels, expressed as normalized FPKM, are presented at CK, TA 24, and TA 60 (boxes from left to right). dATP: Deoxyadenosine triphosphate, dADP: Deoxyadenosine diphosphate,GDP: Guanosine-5′-diphosphate, dAMP: Deoxyadenosine monophosphate, XTP: Xanthosine 5′-triphosphate.
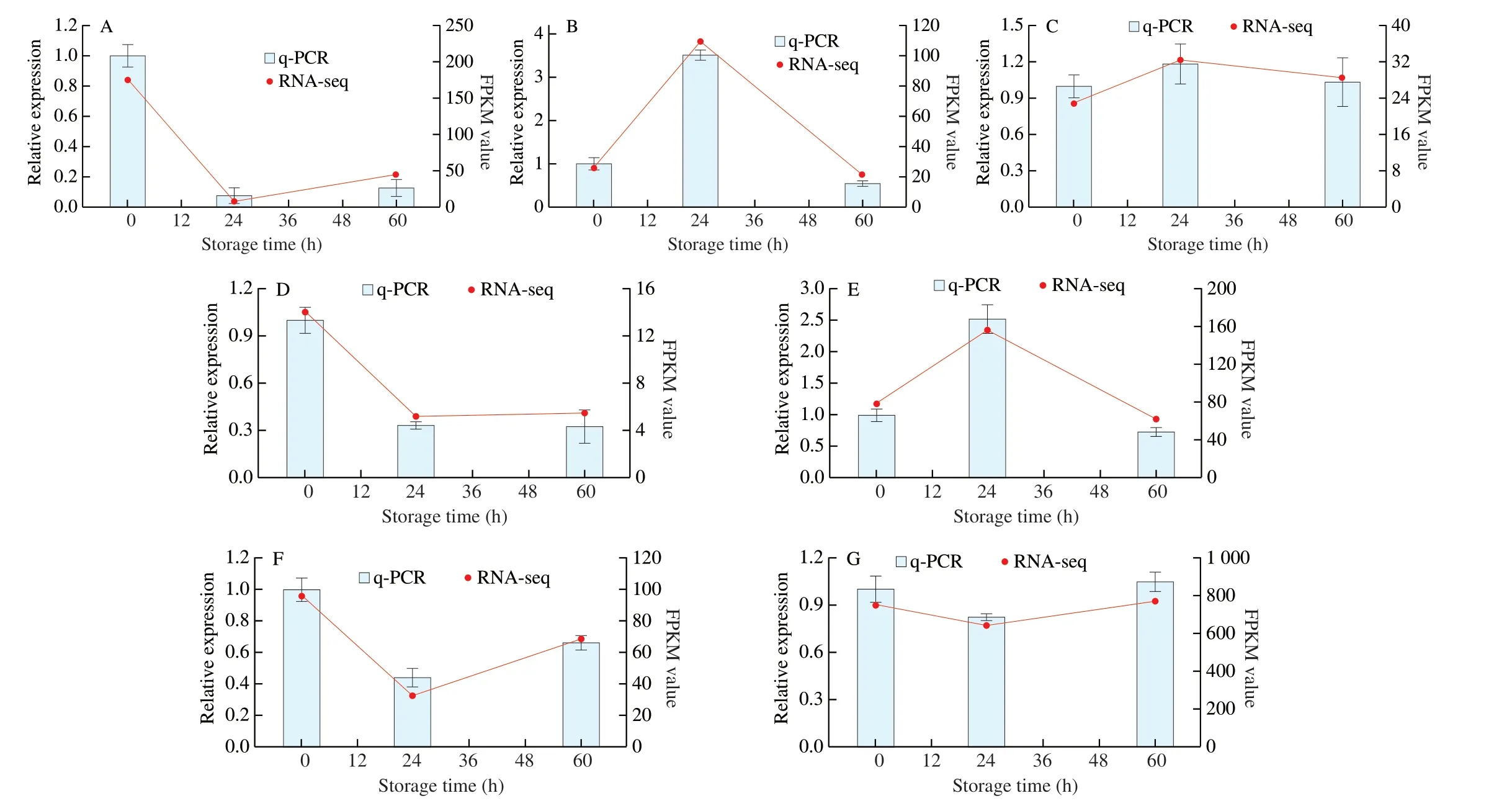
Fig. 5 Expression patterns of selected genes identified by RNA-Seq were validated by q-PCR. (A) TR16801_c0_g1, (B) TR2902_c0_g1, (C) TR349_c0_g2,(D) TR3373_c0_g1, (E) TR5624_c1_g1, (F) TR3922_c0_g1, (G) TR617_c0_g3.
Seven genes were randomly selected for q-PCR to verify the accuracy of RNA-Seq. As shown in Fig. 5, the expression levels of these genes were consistent with the RNA-Seq data. Therefore, the transcriptome data were reliable.
4. Conclusions
In summary, slight spore prints of mushrooms were pictured at 24 h during 25 °C storage, accompanied by a respiratory peak, a significant ATP decrease and an increase in energy-related enzymatic activity, suggesting that large amounts of energy were consumed to trigger spore release upon the initiation of postharvest mushroom senescence. Meanwhile, the contents of umami nucleotides and amino acids were relatively higher in mushrooms with spore release, and the EUC was significantly correlated with the energy state by correlation analysis (R= 0.80). Fifteen genes in energy metabolism were significantly downregulated with spore release.LecpdP1andLeAKwere key genes in connecting energy state and umami taste, which were responsible for the conversion of ATP into AMP in the purine metabolism pathway. These new findings suggested the molecular mechanisms of change in energy status during spore release and the relationship between energy status and umami taste of shiitake mushrooms. Future research on the other factors that affect spore release may provide some clues.
Conflicts of interest
The authors declare that they have no competing financial interests.
Acknowledgements
This work was supported by Liaoning Province Science and Technology Planning Project (2021JH5/10400011&2020JH2/10200013); the Central Guidance on Local Science and Technology Development Project of Liaoning Province(2021JH6/10500133) and Shenyang Agricultural University, high-end talent introduction fund (SYAU20160003).
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://doi.org/10.1016/j.fshw.2022.07.020.
- 食品科学与人类健康(英文)的其它文章
- Wine, beer and Chinese Baijiu in relation to cardiovascular health:the impact of moderate drinking
- Comparative analysis of physicochemical properties, ginsenosides content and α-amylase inhibitory effects in white ginseng and red ginsen
- Monitoring and identif ication of spoilage-related microorganisms in braised chicken with modif ied atmosphere packaging during refrigerated storage
- Effect of cooking processes on tilapia aroma and potential umami perception
- Formation mechanisms of ethyl acetate and organic acids in Kluyveromyces marxianus L1-1 in Chinese acid rice soup
- Volatile prof ile and multivariant analysis of Sanhuang chicken breast in combination with Chinese 5-spice blend and garam masala

