Evaluation and selection of yeasts as potential aroma enhancers for the production of dry-cured ham
Xiaohui Gong, Ruifang Mi, Xi Chen,*, Qiujin Zhu*, Suyue Xiong, Biao Qi, Shouwei Wang
a School of Liquor & Food Engineering, Guizhou University / Guizhou Provincial Key Laboratory of Agricultural and Animal Products Storage and Processing, Guiyang 550025, China
b China Meat Research Center, Beijing 100068, China
Keywords:Yeast Flavor Dry-cured ham Meat model medium Debaryomyces hansenii
A B S T R A C T Yeasts play a critical role in the f lavor formation of dry-cured ham. In this study, 41 yeast isolates from the dry-cured ham at different processing stages were evaluated for their technological properties. Debaryomyces hansenii was the most dominant yeast and has been detected at each phase of dry-cured ham, followed by Candida zeylanoides which was mainly detected in salting phase. Yarrowia bubula and Yarrowia alimentaria were found at the f irst two-phase of dry-cured ham. All isolates of yeast showed enzymatic activities against milk protein and tributyrin, while only 4 strains displayed proteolytic activity on meat protein. Yeast strains were grown in a meat model medium and volatile compounds were identified. The result showed that inoculated yeast strains could promote the production of volatiles and there were significant differences among strains. D. hansenii S25 showed the highest production of volatile compounds, followed by the strain C. zeylanoides C4. D. hansenii S25 was the highest producer of alcohols showing the highest production of benzeneethanol and 3-(methylthio)-1-propanol. Based on OAV and PLS analysis, D. hansenii S25 was strongly correlated with overall f lavor and key volatile compounds of dry-cured ham, which could be selected as potential starter cultures.
1. Introduction
Dry-cured ham is a traditional meat product that is very popular around the world for its high nutrition and unique flavor [1,2].However, huge differences in the processing and production environment of dry-cured meat products produce a large disparity in terms of flavor and sensory quality [3,4]. In recent years, microbial starter culture has been paid more attention as a means of standardized production of dry-cured ham [5,6]. It is a common method for starter selection that screening isolates with the satisfactory performance from the autochthonous microbiota of fermented foods [7]. During the long fermentation process of dry-cured ham, a large number of microorganisms with dynamic changes colonized on the surface. Those microorganisms mainly consisted of some bacteria and yeasts that are tolerant to high-salt and low-moisture conditions and contributed signif icantly to the f lavor quality of dry-cured ham [8]. Among them,yeasts as the main fungus in the maturation stage of dry-cured hams that were considered to be closely related to the f lavor formation [9,10].
Several studies have shown that naturally fermented dry-cured hams have yeast populations ranging 5–7 (lg (CFU/g)) on muscle surface [11-13]. Yeasts isolated from dry-cured meat products includedDebaryomyceshansenii,Candidazeylanoides,Yarrowia lipolytica,Rhodotorulaslooffiae,Pichiatriangularis,Candida famata,Sporidiobolussalmonicolour[11,13-15]. Yeast population in dry-cured meat products seems to depend on the processing stage and conditions.D.hanseniiwas the most abundant species isolated from dry-cured ham and appeared largely at the ripening stage. At the end of drying, about 60%–99% of the yeasts in dry-cured meat products were identif ied asD.hansenii[12-14].C.zeylanoideswas mainly found in the fresh meats and early stage of the dry-cured meat products process, and it was decreased at the end of aging [11,14].C.famatawas isolated in small quantities and mainly found in muscle surface during the pre-fermentation of ham [13].
The positive contribution of yeasts to the sensory profile of drycured hams was principally attributed to their proteolytic and lipolytic activities, which promoted the production of precursors conducive to flavor development [16-18]. Corral et al. [19] reported thatD.hanseniiinoculation in dry-fermented sausages increased lipolysis and thus enhanced the production of ester compounds.D.hanseniihad also been shown to accelerate the degradation of myofibrils during the initial drying phase of fermented sausage and promoted the production of free amino acids at the end of fermentation [20]. In addition, yeasts had been reported to have some antioxidant activity,which facilitates the production of esters [21]. Therefore, yeasts isolated from dry-cured meat products could be explored as a potential starter culture. To avoid contamination by other microorganisms, selecting a medium that simulated the composition of dry-cured meat would be helpful to analyze the aroma production properties of yeast [22].
The diversity in volatile compounds generated by yeasts isolated from dry-cured meat products depend on specific species and strains [16,23,24]. Purriños et al. [25] reported that there were significant differences in the volatile profile of dry-cured meat products inoculated with three different species of yeast. Andrade et al. [26] found that the esters and sulfides vary considerably produced by the three fermented sausages inoculated with different biotypes(mitochondrial DNA (mtDNA) patterns) ofD.hansenii. Some studies suggested that wide differences in the isolation of yeast from dry-cured meat products were likely related to the origin of the strain [11,27].However, there is no research on the evaluation of aroma-producing performance of yeasts isolated from dry-cured ham at different processing stages.
This study aimed to evaluate the aroma production properties of yeast isolated from dry-cured ham at different processing stages to select yeast starters that could contribute to the standardized production of dry-cured ham. In this study, molecular identification,enzymatic characterization, and basic growth characteristics assessment of yeast isolated from dry-cured ham at different processing stages were performed. Based on screening, 2–7 strains of isolated yeast were selected in each stage for analysis of aroma production properties.
2. Materials and methods
2.1 Dry-cured ham sampling
Fresh pork hind legs (Landrace, weight, (13.07 ± 0.84) kg, pH(5.73 ± 0.12)) were selected for dry-cured ham. Firstly, the fresh legs were cooled at 75%–80% relative humidity (RH) and 0–2.5 °C, for 24 h. Then, a generous amount of sea salt was daubed (Jinmeitian Co., Ltd., Beijing, China) and rubbed repeatedly until the leg are completely covered. For the first salting, used about 4% sea salt and placed at 1.5–3.5 °C, 80%–85% RH for 7 days. After the moisture in the legs was released, the second salting was performed at 3.0%salt, 70%–80% RH for 15 days. Subsequently, pre-curing phase continued 15 days and curing phase continued 70 days at 60%–80%RH, 1.5–4.5 °C. After curing, cleaned the salt from the surface of the ham and hung it in drying chamber at 20 °C, 60%–75% RH for 7 days, gradually lowered the temperature to 15 °C. Finally, hams were gradually matured at 18–20 °C, 70%–85% RH and achieved a unique flavor. Samples were collected from fresh pork hind legs and the pivotal processing stage of salting phase (15 days), pre-curing phase (37 days), curing phase (90 days), drying phasayse (130 days),and maturing phase (200 days).
2.2 Yeast isolation and culture-dependent analysis
Each ham sample (10 g) was placed in a sterile blender bag containing 90 mL sterile sodium chloride solution (0.85% NaCl) and homogenized. Appropriate dilutions were spread on yeast extract peptone dextrose (YPD) medium with 100 mg/L chloramphenicol and incubated at 28 °C for 48 h [28]. Repeat the count twice with the appropriate diluent. Then isolates were selected from the highest three dilutions according to different morphologies and purified by passaging three times on potato dextrose agar (PDA) medium. All the yeasts were maintained at –80 °C in YPD containing 20% (V/V)glycerol until analysis.
2.3 Identification of yeast strains
Genomic DNA of the yeast isolates was extracted using DNeasy Plant Mini Kit (Qiagen, Germany), following the product descriptions. Identification of the isolates was carried out by using the primer pairs ITS1 and ITS4 to amplify ITS region [29]. Yeast isolates were sequenced by ITS and 26S rDNA analysis. To determine species attribution, 26S rDNA sequences were obtained using primer pairs of NL1 and NL4 [30]. Sequencing of amplified products was achieved in Thermo Fisher Scientific Company. The obtained sequences were used in a similarity search by methods of the GenBank database, and only when the adequate identification level was ≥ 99% could the species be identified.
2.4 Evaluation of growth characteristics at different NaCl concentrations, pHs, nitrite and low temperatures of yeast isolates
All the isolates of yeast were propagated at 28 °C in liquid YPD medium for 3 days. Then, 30 mL of incubated yeast strains was taken in a 50 mL centrifuge tube, and centrifuged (4 °C) at 8 000 ×gfor 20 min to obtain the cell pellet, which was washed twice with 0.85% NaCl. The cell precipitates were re-suspended in the saline and adjusted to a concentration of 106CFU/mL. Each isolate was inoculated into liquid YPD medium (106CFU/mL) and characterized by growth in YPD added with 5%, 10% and 15% of NaCl, as well as growth on pH value adjusting 4.5 and 6.0. Effect of low temperature and nitrite on yeasts was observed in YPD at incubation temperature 15 °C and containing 150 mg/L NaNO2.The optical density (OD)value of each sample was measured at 600 nm after 5 days of incubation and the data of yeasts growth at different pH, NaCl concentrations, low temperature and nitrite were evaluated as Growth Index (GI) [31]. The represents the absorbance of the sample at OD600nmafter treatment under different conditions and represents the absorbance of the controls. The samples incubated at 28 °C in liquid YPD medium were used as controls.
2.5 Evaluation of lipolytic and proteolytic activities of yeast isolates
According to Buzzini et al. [32], the lipolytic activity of yeasts was determined on plate medium containing tributyrin ester, and the proteolytic activity against milk protein was detected on PDA medium containing 2% skimmed milk powder. Yeast was inoculated in the plate medium and incubated at 28 °C for 5 days. Then, the lipolytic and proteolytic activity was indicated by calculating HC value. HC value represents the ratio of the diameter of the transparent halo to the diameter of the colony. The ability to hydrolyze meat protein was tested on protein model culture medium with 71% (V/V)sarcoplasmic, 1% (m/V) glucose and 4% (m/V) NaCl [33]. The extraction of pork sarcoplasmic proteins was obtained according to the method of Park et al. [34] and slightly modified. Fresh lean pork samples homogenized with 0.2 mol/L phosphate buffer (1:3,m/V) pH 7.4 and centrifugated (10 000 ×gat 4 °C for 20 min).Protein concentration was determined by the BCA Protein Assay Kit(Solarbio Co., Ltd, Beijing, China). The concentration of sarcoplasmic protein was 10.4 mg/mL. Aliquots of 4 mL of the culture medium were dispensed into a 10 mL centrifuge tube, inoculated at 106CFU/mL of each yeast strain and incubated at 28 °C for 7 days in shaking. The hydrolysis of meat protein was detected by sodium dodecyl sulfate gel electrophoresis (SDS-PAGE). Proteins were denatured by boiling 40 μL of model supernatant mixed in a ratio of 4:1 with 5 × protein loading buffer (Solarbio Co., Ltd., Beijing, China). Twelve percent separation gel and 5% spacer gel were prepared and stained with Coomassie blue R-250.
2.6 Yeast growth in meat model culture medium
The meat model culture medium was prepared according to the method of Paik et al. [35], containing meat extract (Sigma-Aldrich,USA), 12 g/L; glucose, 10 g/L; NaCl, 20 g/L; MgSO4·7H2O, 0.15 g/L;glutamate, 0.5 g/L; NaNO2, 0.15 g/L; K2HPO4·3H2O, 2 g/L.The medium pH was adjusted to 6.0 with 0.5 mol/L HCl before autoclaving. The glucose solution sterilized by filtration and added to the autoclaved medium. Aliquots of 30 mL of the model mediums were placed into a 100 mL conical flask, inoculated at 106CFU/mL,then grown at 28 °C for 24 days. Two uninoculated samples were incubated in the same conditions and used as control.
2.7 Analysis of volatile compounds
After 24 days of incubation, the inoculated and uninoculated model medium were stored at –80 °C until flavor analysis was performed.Three-milliliter sample supernatants were obtained from the model medium and were transferred into in 10 mL headspace vial, and 0.082 mg/mL 2 μL 3-octanol was added as the internal standard substance. The headspace vials heating in water bath were at 40 °C for 20 min. Then, a 50/30 μm SPME fiber was inserted in head space of vials and extracted for 30 min. After extraction, the fiber adsorbed with volatiles was desorbed in the GC-MS 1310/TSQ8000 system(Thermo Fisher Scientific,USA) at 230 °C for 5 min. DB-WAX capillary column was used to separate the desorbed molecules. The temperature program was constant at 40 °C for 3 min, then increased to 50 °C at a rate of 10 °C/min, and then raised to 230 °C at 12 °C/min.Mass spectra were operated by electron ionization at 70 eV and the data were collected across the rangem/z35–400. The C5-C20n-alkanes (Sigma-Aldrich, USA) were used as standards to calculate the retention index (RI). Volatile substances were identified by mass spectrometry database (NIST11). The volatile compounds were expressed as milligram per kilogram of meat model medium.
2.8 Statistical analysis
Statistical analysis of the data of volatile compounds in the meat model medium was performed by one-way analysis of variance(ANOVA) with Duncan test by IBM SPSS Statistics 22.0. Heatmap was formed by Heml version 1.0, variable importance in projection(VIP) analysis was performed using SIMCA-p+11.lnk. The phylogenetic tree was constructed by the MEGA-11 software.
3. Results and discussion
3.1 Predominant yeasts in dry-cured ham detected by culture-dependent approach
In this study, the count of yeast in fresh pork hind leg was about 3.19 (lg (CFU/g)). During processing, the yeast density of each dry-cured ham sample ranged 4.27-8.02 (lg (CFU/g)) (Fig. 1).The yeast counts obtained from the dry-cured ham increased progressively from salting phase to curing phase, and then slowly descend until the amount of yeast was 5.99 (lg (CFU/g)) at the end of aging. These changes in yeast counts could be related to washing steps, temperature variation, and an adaptation period in salt content,water activity and available nutrients [12].
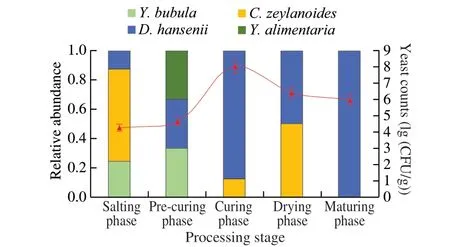
Fig. 1 Yeast species diversity and yeast counts in different processing stage(salting phase, pre-curing phase, curing phase, drying phase, maturing phase) of dry-cured ham. The yeast species diversity is displayed as relative abundances,calculated based on the number of isolates obtained per processing stage.
A total of 41 isolates was picked and obtained genetic information by ITS or 26S rDNA sequence. As shown in Table 1, four species of yeast were found, includingD.hansenii(30 isolates),C.zeylanoides(7 isolates),Y. bubula(3 isolates) andY.alimentaria(1 isolate).The phylogenetic trees were constructed based on the combination sequences of the 41 isolates and their closely related species,as shown in Fig. 2. Among the yeast isolates identified during processing,D.hanseniiwas the most abundant species followed byC.zeylanoide(Fig. 1). The result was in agreement with many reports thatD.hanseniiandC.zeylanoidehad been repeatedly isolated from European dry-cured ham [13,36,37].Y.alimentaria(homotypic synonym:Candidaalimentaria) has been isolated from dry-cured meat products [11,38], while it is also found in this study.The speciesY.Bubulahas been found in beef and minced pork range of Hungary, but as far as we know, this is the first time it was isolated from dry-cured meat products. However, another two frequently found species in dry-cured ham/meat products,Y.lipolyticaandC.deformans, were not observed in our study.They have been identified in other studies that aim to inquiry into yeast dynamics of the dry-cured ham from the region of Italian and Norwegian [13,14]. Unlike Asefa et al. [14] that picked isolates from all the dilutions obtained more diverse species (7 species),only the highest three dilutions were used when selecting strains in our study, thus 4 species were obtained probably the predominant yeast species in dry-cured ham samples.
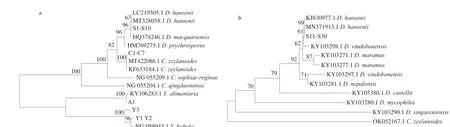
Fig. 2 The 26S rRNA gene (a) and ITS (b) based phylogenetic tree for yeast species (41 isolates) isolated from dry-cured ham at different process stage.Sequences of related species not generated in this study were obtained from GenBank. Numbers on the branches represent bootstrap values from 2 000 replicates.
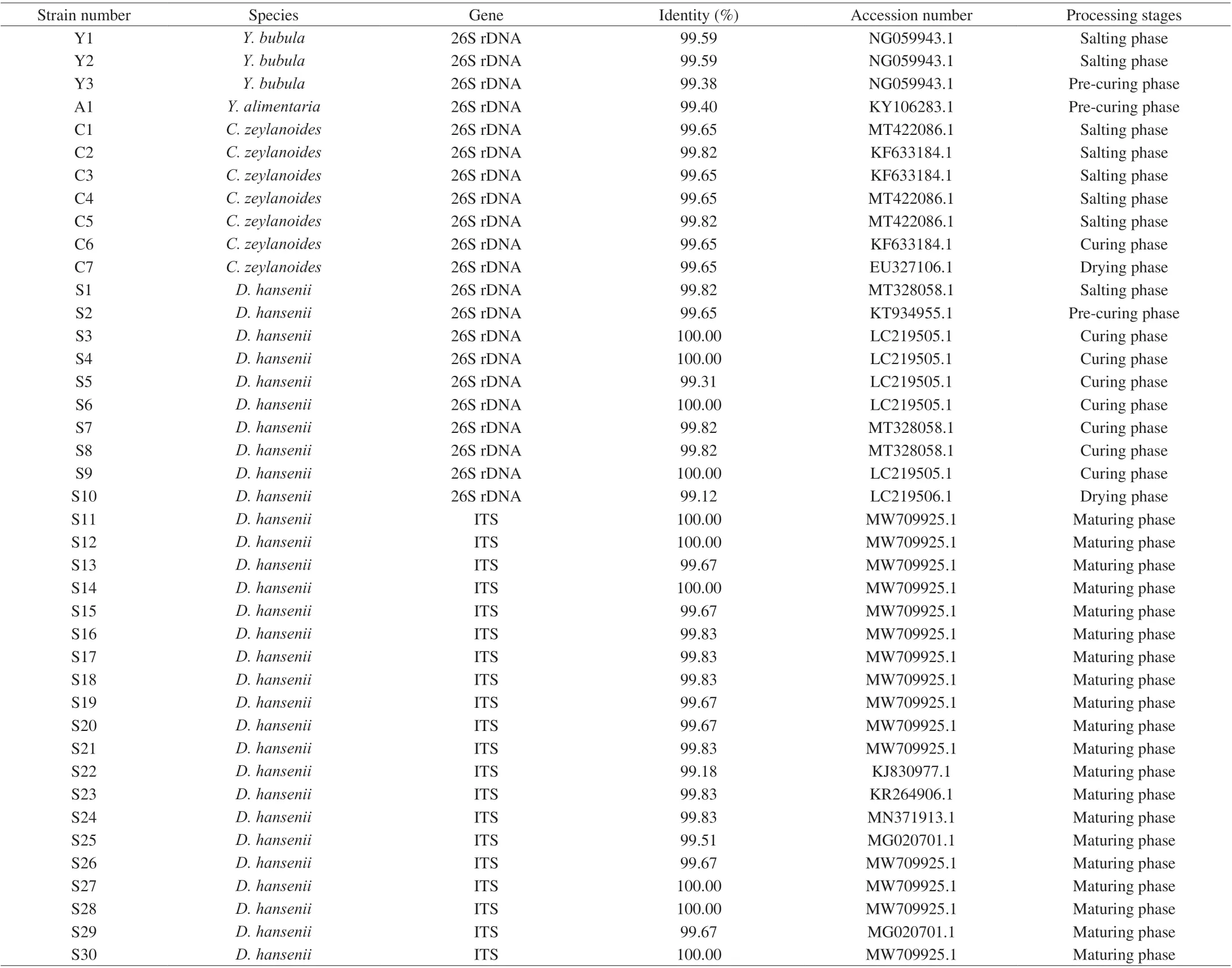
Table 1 Identities (%) of the sequenced genes (ITS or 26S rDNA) of yeast strains and the accession numbers of the entries with the highest identity.
Throughout processing, onlyD.hanseniiwas found at each phase. The speciesC.zeylanoideswas not isolated at maturing phase,probably replaced byD.hanseniias previously reported by Asefa and Mendonça [14,39].YarrowiabubulaandY.alimentariawere not found at curing phase, drying phase and maturing phase, indicating that their inadaptability to high salt environment and poor utilization of carbon source (glucose) [38,40].
3.2 Growth characteristics of yeast under different salt concentration, pH, low temperature and nitrite
Yeast strains were surveyed to evaluate their growth ability at specific conditions similar to dry-cured ham (the NaCl tolerance ability, growth at low temperature, growth at specific pH value, and nitrite tolerance ability). As NaCl is the important salting ingredient for dry-cured ham, salt tolerance was usually the criteria to select starter cultures [7,27]. Among 41 isolated strains,D.hanseniiS14 and S20 strains had the capability to grow in 15% NaCl (Table 2). And 46.6% ofD.hanseniiand 28.6% ofC.zeylanoidesshowed tolerance to 10% NaCl (70 > GI > 20). Ozturk et al. [11] also proved thatD.hansenii,C.zeylanoidesandY.alimentariagrow in the presence of 10% (m/V) NaCl.
Nitrite not only has antibacterial activity, but also facilitates color generation and flavor enhancement of dry-cured ham [41]. The results of this study indicated that 88% of strains were able to grow in YPD medium supplemented with 150 mg/L nitrites. In the presentstudy, all isolate strains could grow at pH 4.5 and 6.0. Studies have shown that dry-cured hams produced in different regions typically have a pH value among 5.38–5.97 [42]. As regards resistance to low temperature, at 15 °C the most of the isolates appeared optimal growth (GI > 70), otherwise five strains showed a partially inhibited(70 > GI > 20).
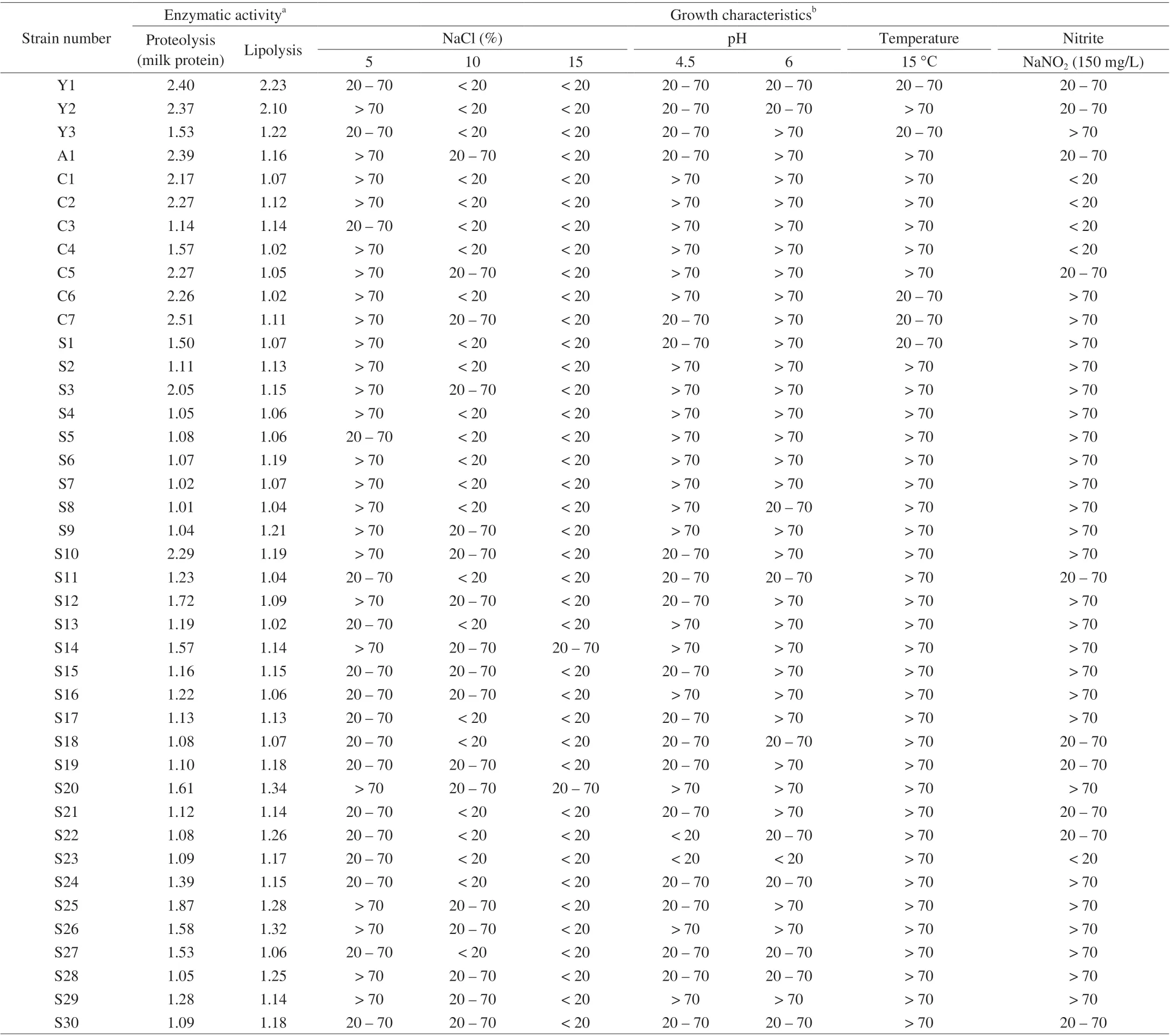
Table 2 The enzymatic activity and growth characteristics of isolated yeasts.
3.3 Proteolytic and lipolytic activities of yeast isolates
The typical flavor of dry-cured ham is mainly caused by proteolysis and lipolysis. It has been believed that proteolysis of drycured ham due to the action of endogenous enzymes and microbial enzymes [43-45]. Several studies have reported thatD.hanseniiandC.zeylanoideswith proteolytic and lipolytic activity could contribute to the flavor formation of dry-cured meat products by hydrolyzing protein and lipid [18,23,46]. In this study, all yeast strains showed proteolytic activity against milk protein (Table 2), while only 4 strains(D.hanseniiS2, S10, S14,Y.alimentariaA1) were able to partially hydrolyze all the bands except for 28 kDa band (Fig. S1). One study showed the ability ofS.cerevisiaeoriginating from dry-cured meat to degrade sarcoplasmic protein was demonstrated by electrophoresis, but it did not show enzyme activity on the medium containing casein [33].These observations would indicate that the difference of enzyme activity of the same strain was related to different evaluation methods.All yeast strains exhibited lipolytic activities on tributyrin agar, but only two strains ofY.bubulaisolated from dry-cured ham samples showed strong lipase activity. It is noteworthy thatC.zeylanoidesshowed weak lipolysis activity in our study, while the Mendoza et al. [47]found that it exhibited the highest lipolytic activity. These differences indicate that yeast enzymatic activities probably dependent on the strain. Furthermore, this is the first time that the species ofY.bubulawas reported could exhibit proteolytic and lipolytic activities isolated from dry-cured ham have been reported.
3.4 Analysis of volatile compounds produced by yeasts
The aroma-producing ability of yeast isolated from dry-cured ham in meat model medium were evaluated. According to the species and number of isolates at each processing stage, 21 yeast strains with good enzyme activities or salt tolerance were selected (Table 3). Strains Y1, Y2, Y3, C1, C2, C4, C5, C6, C7, S3, S12, S20, S25 and S26 showed strong or medium enzyme activities and strains A1, S2, S10 and S14 showed ability to hydrolyze meat protein. In addition, strains S9, S28 and S29 with weak enzyme activity but could grow at 10%NaCl also were selected. All strains were able to grow in the model medium, and yeast counts were performed prior to evaluate aromatic capability, with the number of viable yeasts in inoculated samples exceptY.bubulawere around 7 (lg (CFU/mL)) (data not shown). The number of yeasts in the model medium inoculated withY.bubulawas ranged 6.5–6.7 (lg (CFU/mL)) (data not shown).
A total of 58 volatile compounds were found in uninoculated control and inoculated groups (Table 4), including aldehydes (10),alcohols (19), ketones (3), esters (11), acids (3) and other N/S compounds (12). Most of the compounds detected have been previously found in dry-cured ham [48]. Twenty-two volatile compounds were identified in the uninoculated group (control sample). These volatile compounds were probably derived from the chemical degradation of amino acids, lipids and sugar in themeat model medium [49]. Among them, furfural, 3,5-ditertbutyl-4-hydroxybenzaldehyde, methyl hexadecanoate, 2-acetylthiazole and 3-(methylthio)-propanal) were found only in control groups. Furfural was an intermediate related to sugar degradation in Maillard reactions,and its formation has been related to the temperature, time, glucose concentration and low pH (< 7.0) in model medium [50]. However,with inoculated groups, it seems likely that yeasts consumed part of the glucose to inhibit the production of furfural. 3-(Methylthio)-propanal and 3,5-ditertbutyl-4-hydroxybenzaldehyde generation was related to the Strecker degradation of the amino acids in meat model medium [51]. These 5 volatile compounds have not been reported as necessary in the flavor development of dry-cured ham.
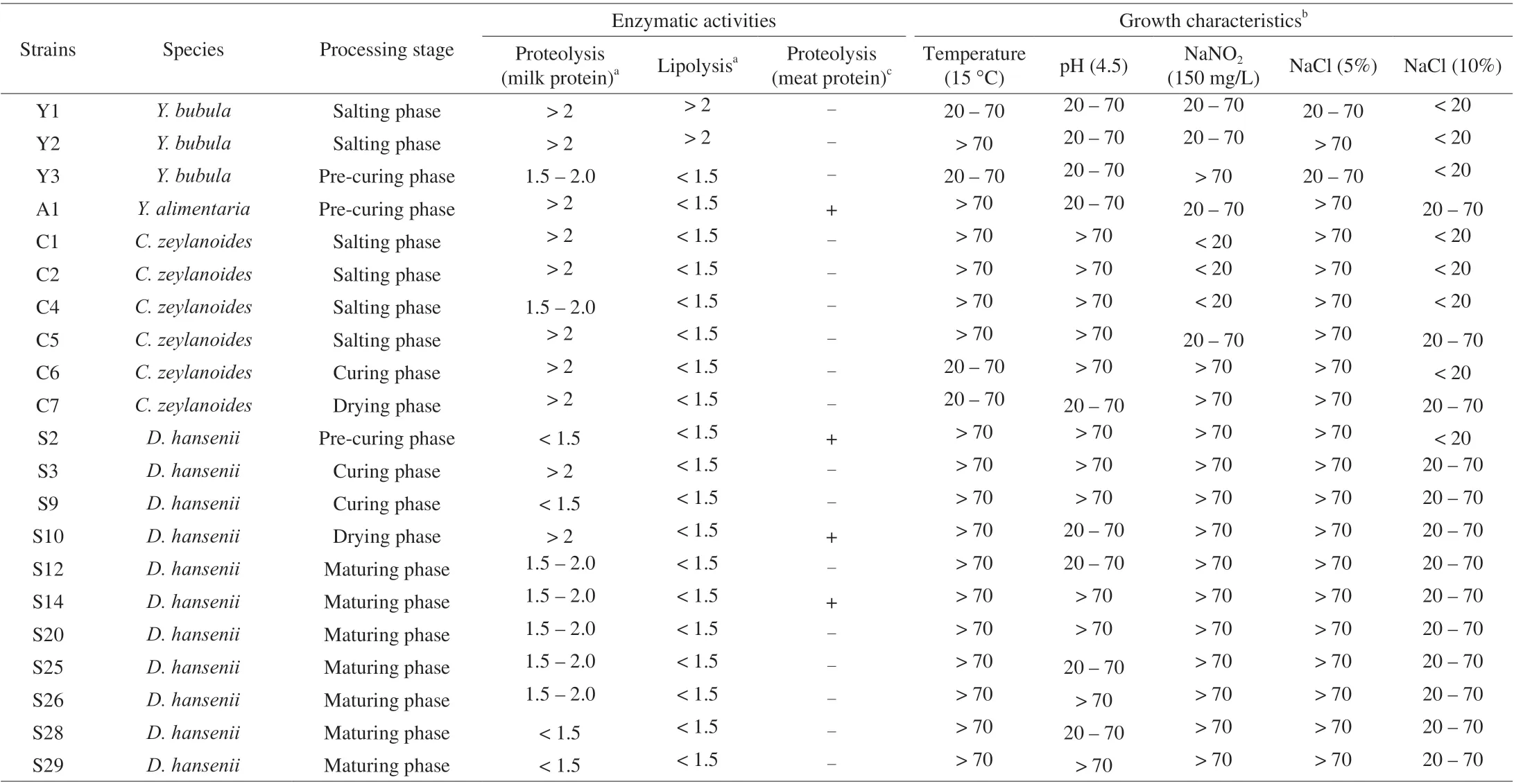
Table 3 The technological properties of selected 21 strains for volatile compounds analysis.
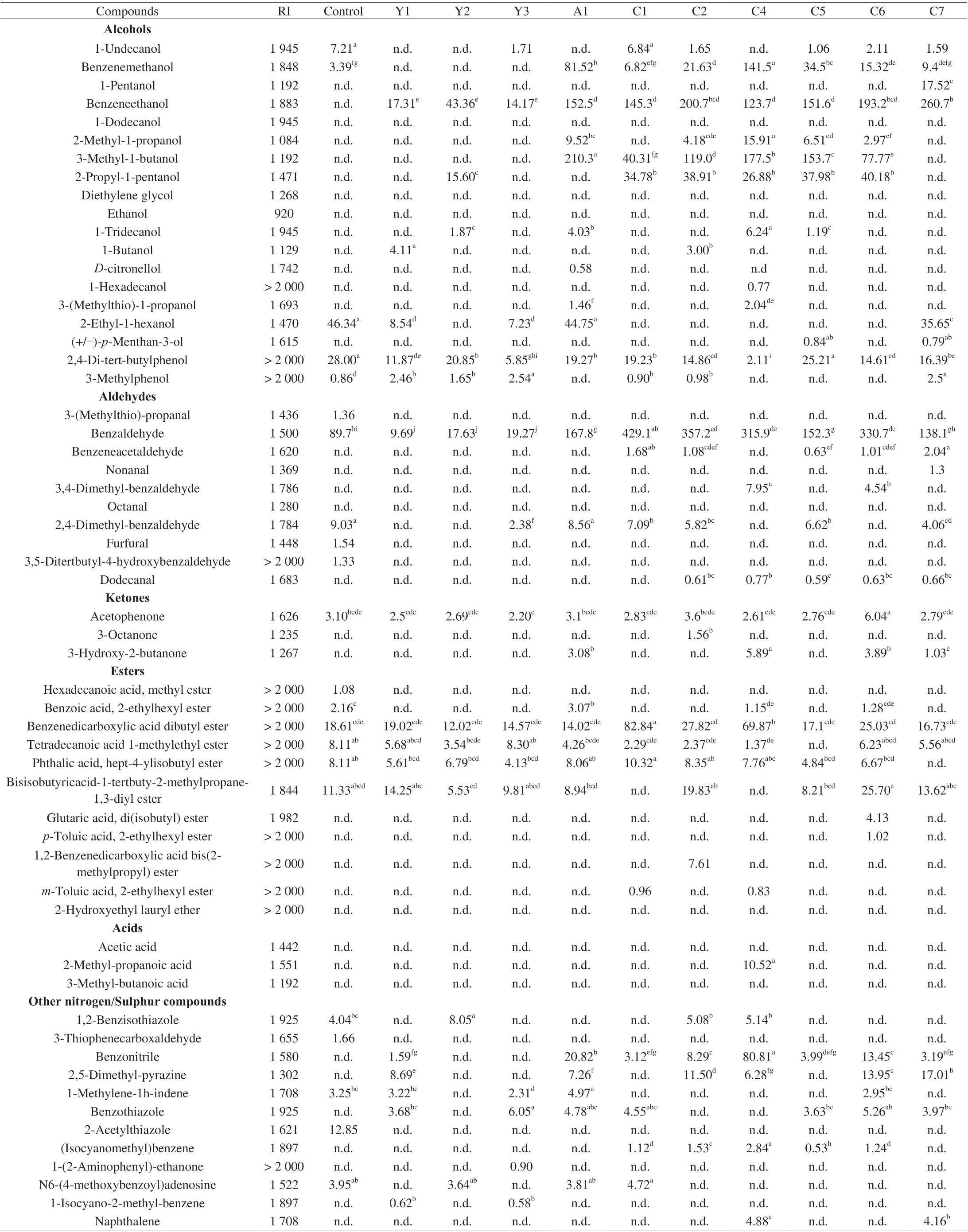
Table 4 Volatile compounds identified in meat model medium by yeast strains isolated from dry-cured hamA.
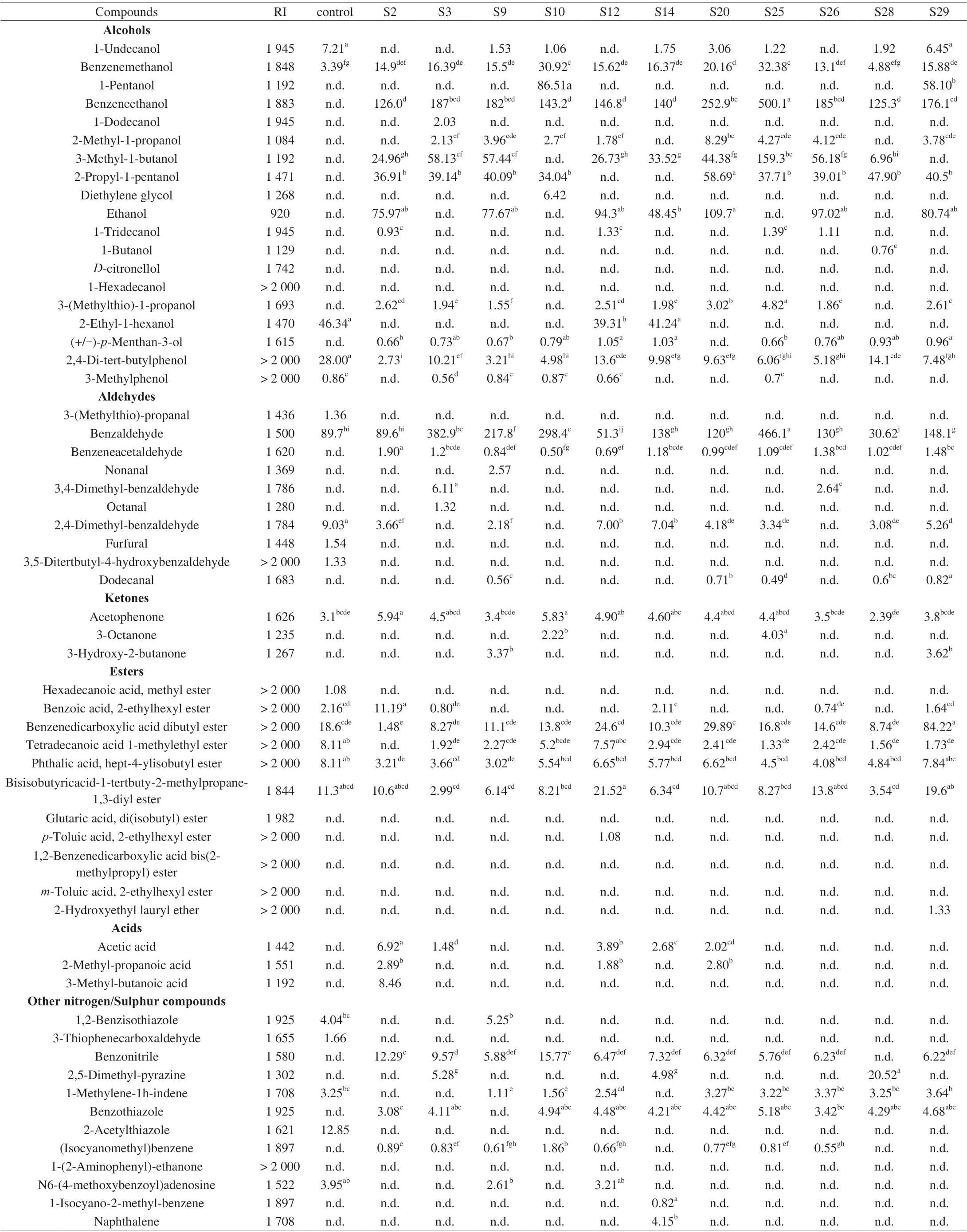
Table 4 (Continued)
Comparison of the quantification of the volatile compounds by the yeast strains in meat model medium revealed important differences.As shown in Fig. 3, the generation of total volatile compounds in inoculated groups was significantly higher than control group, except forY.bubulaY1, Y2, Y3 andD.hanseniiS28. In inoculated groups,D.hanseniiS25 strain isolated from dry-cured ham at maturing phase showed the highest content of volatile compounds, followed by the strainC.zeylanoidesC4 strain isolated from dry-cured ham at salting phase. Similar results have been reported that yeast inoculated remarkably alter the total area of volatile compounds [25]. The specieY.bubulaproduced significantly lower volatile compounds than other inoculated groups, which was might be related to the lower growth activity ofY.bubulaY1, Y2 and Y3 in the model medium. At the same time, the catalase activity of yeast probably lead to a decrease in lipids and amino acids oxidation products [52], resulting in lower volatile compounds ofY.bubulathan control group (the results were not significant).
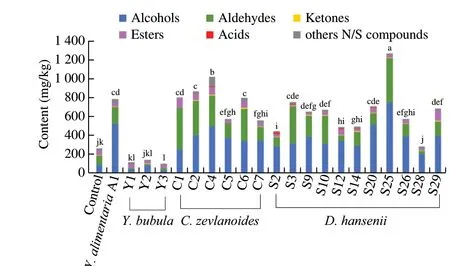
Fig. 3 Content of alcohols, aldehydes, ketones, esters, acids and other N/S compounds in uninoculated model medium (control) and meats model medium inoculated with different species of yeasts. Different letters indicate significant differences (P < 0.05) of total compounds between groups.
In inoculated groups, the most abundant compounds were alcohols and aldehydes (Table 4). These compounds were considered to be important components in the characteristic flavor of dry-cured meat products [48,53], which demonstrated the importance of yeasts in the flavor composition of this product. With regard to alcohol compounds, the most abundant volatile was benzeneethanol, followed by 3-methyl-1-butanol, ethanol, benzenemethanol and 2-propyl-1-pentanol. The results were in agreement with Mi et al. [54] that similar alcohol compounds were produced in the same meat model medium inoculated with yeast strains. StrainD.hanseniiS25 was the highest producer of alcohols showing the highest content of benzeneethanol, derived from the reduction of benzeneacetaldehyde degraded byL-phenylalanine, and 3-(methylthio)-1-propanol, derived from the degradation of the amino acidL-methionine [51,55]. Ethanol was detected only in some inoculatedD.hanseniigroups could be due to presence inDebaryomycesspp. of some pyruvate descarboxylases and alcohol dehydrogenases [56].Y.alimentariaA1 showed the highest production of 3-methyl-1-butanol which was possibly due to its strong proteolytic activity on meat protein. Studies have shown that the free amino acid leucine from protein hydrolysis was considered to be the precursor of 3-methyl-1-butanol and 2-methyl-1-propanol [54,57]. However, threeD.hanseniistrains (S2, S10, S14)that could degrade meat protein did not show this superiority. These phenomenons would demonstrate that volatile compounds was not only closely related to the composition of meat model, but it was also dependent on strain [58]. In addition, the conversion of amino acids into fusel alcohols through the three-step “Ehrlich pathway” pathway was an important reason for the largest number of alcohols (32.8%of total volatile compounds) in inoculated groups. In this pathway,enzymes encoded in the genome of yeast strains have been reported to modify amino acids for deaminated, decarboxylated and finally reduced to their respective alcohol derivatives [59].
In respect to aldehyde compounds, benzaldehyde was detected in the highest concentrations in theD.hanseniiS25 followed byC.zeylanoidesC1 andD.hanseniiS3. And benzeneacetaldehyde,dodecanal, nonanal and octanal were detected only in groups inoculated withD.hanseniiandC.zeylanoides. Aldehydes play an important role in the aroma of the dry-cured ham because their perception threshold was generally lower than other volatile compounds [48]. These compounds were derived from the degradation of amino acids and unsaturated fatty acids [60]. In inoculated groups,benzeneacetaldehyde and benzaldehyde were probably generated from phenylalanine by non-enzymatic Strecker degradation or microbial metabolism [61]. Dodecanal, nonanal and octanal coming from the oxidative degradation of lipid were commonly detected in dry-cured ham profiles [48]. Studies have suggested that yeasts have proteolytic and lipolytic activities, which released the free amino acids or free fatty acids as substrates to enhance the production of branched and linear aldehydes [19,23].
Ester compounds were detected in all groups. The strain ofD.hanseniiS29 was the highest producer of ester compounds followed byC.zeylanoidesC1. Several studies have reported thatD.hanseniiandC.zeylanoidesinoculation increased the production of ester compounds [58,62]. Small amounts of acids components (acetic acid, 2-methyl-propanoic acid, 3-methyl-butanoic acid) were detected only in some inoculated groups. Among other N/S compounds,benzonitrile, (isocyanomethyl)benzene and naphthalene were detected in the highest concentrations in theC.zeylanoidesC4 inoculated model medium. Although these compounds are not typical volatile components,they may be increasing the flavor diversity of dry-cured ham.
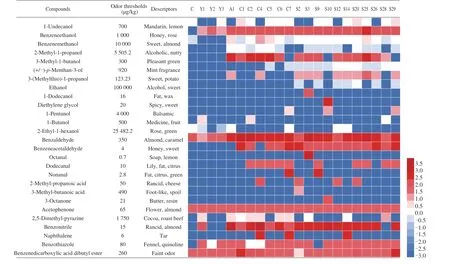
Fig. 4 Heatmap showed the OAV of volatile flavor compounds produced by 21 strains of yeast in the fermented sausage model-medium and the color intensity of the OAV value is expressed as a logarithm. The odor thresholds were queried from the book Compilations of Odour Threshold Values in Air Water and Other Media 2th Edition.
The odor activity value (OAV) represents the overall contribution of volatile compounds to flavor of the products and it was calculated as the ratio of the concentration of volatile compounds to the odor threshold [63]. It is generally believed that the volatile compounds of OAV > 1 (lg OAV > 0) were the main contributors which contributed significantly to the overall flavor. As shown in Fig. 4,a total of 27 main volatile compounds (lg OAV > 0) were detected in control and inoculated groups. Remarkably, 5, 20 and 27 of the main volatiles (OAV > 1) were observed in control,C.zeylanoidesandD.hanseniigroups, respectively. This indicated that inoculated yeast strains had a significant affection on the generation of the main volatile compounds. Furthermore, the date observed that the lg OAV of benzeneethanol (honey and rose) [48], 3-methyl-1-butanol (pleasant green), benzaldehyde (almond and caramel aroma), benzonitrile (almond and rancid dour) [64], naphthalene (tar and camphorwood smell), nonanal (fat and citrus flavor), octanal(soap and lemon notes), benzeneacetaldehyde (honey and sweet flavor), and benzenedicarboxylic acid dibutyl ester (faint odor) [65]were higher than 2.5. Such high OAV values indicated that these compounds made a significant contribution to aromas.D.hanseniiS25 showed the highest lg OAV values for benzeneethanol (> 2.5)and benzaldehyde (> 3), whileC.zeylanoidesC4 showed the highest lg OAV values for benzonitrile (> 3.5) and naphthalene (> 2.5). Study reported that excessive rancid flavor was the typical sensory defect of dry-cured ham, which severely affected consumers’ acceptability [66].At the same time,C.zeylanoidesC4 also showed the highest lg OAV values for 2-methyl-propanoic acid (> 2.3), which was considered to be the main acid compound in spoilage ham [67]. Therefore,C.zeylanoidesC4 should be avoided in starter culture.
PLS model was performed to predict the VIP values to estimate the relationship between the yeast strains and key volatile compounds conferred. TheQ2andR2of the PLS models were 0.976 and 0.989, suggesting the models fitted well with our data.As shown in Fig. 5, eight key volatile compounds including benzaldehyde, benzeneethanol, 3-methyl-1-butanol, 2-methyl-1-propanol, benzenemethanol, 3-(methylthio)-1-propanol, benzonitrile and 2-methyl-propanoic acid with VIP > 1 were identified by PLS,which were significantly affected by yeast strains and played a critical role in the volatile compound formation of meat model with different yeast strains. These volatile compounds were described as important volatile compounds to dry-cured ham, except for benzonitrile [48]. Considering the key volatile compounds with lg OAV > 2.5 and VIP > 1,D.hanseniiS25 was the highest producer which accounted for 100% of the 4 compounds: benzeneethanol,3-methyl-1-butanol, benzonitrile and benzaldehyde. Therefore,D.hanseniiS25, a yeast strain isolated from the maturing phase of dry-cured ham, was strongly correlated with key volatile compounds,which could have an important aromatic impact on dry-cured ham.
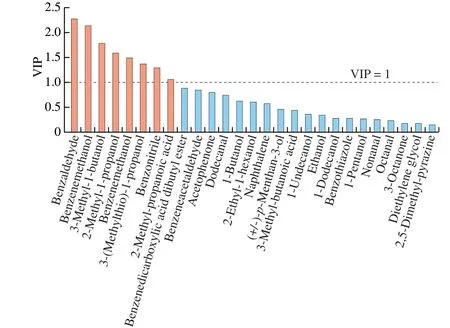
Fig. 5 The VIP values of main volatile compounds (lg OAV > 1) were calculated from the PLS regression model.
4. Conclusion
The technological characteristics of yeast isolated from different processing stages of dry-cured ham were examined.D.hanseniiwas the most predominant yeast species isolated from dry-cured ham,followed byC.zeylanoides,Y.bubulaandY.alimentaria. The results indicated that a total of 41 yeast strains showed NaCl tolerance, nitrite tolerance, lipolytic and proteolytic activities. Among isolate strains,D.hanseniiS25 isolated from the maturing phase of dry-cured ham showed the highest production of alcohol and aldehyde compounds.According to the OAV and LPS analysis,D.hanseniiS25 was closely correlated with 4 volatile compounds (lg OAV > 2.5 and VIP > 1),and a strong correlation was observed betweenD.hanseniiS25 and the formation of flavor in dry-cured ham. Therefore, this strain could be selected as potential starter cultures for dry-cured ham.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors acknowledge the financial support of Guizhou Province Science and Technology Plan Project(QKHZC[2020]1Y152), the Guizhou High-level Innovative Talent Training Project (Qianke Cooperation Platform Talent number [2016]5662), Guizhou Science and Technology Innovation Talent Team of Ecological Characteristic Meat Products(QKHPTRC[2020]5004).
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://doi.org/10.1016/j.fshw.2022.07.022.
- 食品科学与人类健康(英文)的其它文章
- Wine, beer and Chinese Baijiu in relation to cardiovascular health:the impact of moderate drinking
- Comparative analysis of physicochemical properties, ginsenosides content and α-amylase inhibitory effects in white ginseng and red ginsen
- Monitoring and identif ication of spoilage-related microorganisms in braised chicken with modif ied atmosphere packaging during refrigerated storage
- Effect of cooking processes on tilapia aroma and potential umami perception
- Formation mechanisms of ethyl acetate and organic acids in Kluyveromyces marxianus L1-1 in Chinese acid rice soup
- Volatile prof ile and multivariant analysis of Sanhuang chicken breast in combination with Chinese 5-spice blend and garam masala

