The formation and temperature stability of microemulsion emulsified by polyoxyethylene ether surfactant
Hixi Zhng, Jihui Xi, Chengung Ding, Bin Xu, Shoulong Wng, Zongxu Wu,Weimin Zho*
a Department of Chemical Engineering and Safety, Binzhou University, Binzhou, 256603, Shandong Province, People's Republic of China
b School of Chemistry and Chemical Engineering, Yangzhou University, Yangzhou, 225002, People's Republic of China
c Binzhou Dayou New Energy Development Company Limited, Binzhou, 256600, Shandong Province, People's Republic of China
Keywords:End-to-end distance DPD W/O microemulsion Mean interfacial tension
A B S T R A C T The effect of polyoxyethylene chain length on the formation and temperature stability of W/O microemulsion emulsified by polyoxyethylene ether surfactant is investigated by dissipative particle dynamics simulation (DPD)on the mesoscopic level.The results show that W/O microemulsions can be formed by three polyoxyethylene chain length ether nonionic surfactants with decreasing the water-oil ratio equal to increasing the oil phase content.However, W/O microemulsion is most easily formed by diethylene glycol monododecyl ether (H1T2)with the smallest oxyethylene base.
1.Introduction
Polyethylene ether nonionic surfactants are obtained from the addition reaction of fatty alcohol and ethylene oxide,also known as alcohol ethoxylate or alcohol ether [1-3], etc.The molecular structure of polyethylene ether nonionic surfactant is as follows: R-(CH2CH2O)n-H.Among these, n refers to the number of ethylene oxide, that is, the number of oxygen vinyl groups contained in the molecule,which directly affects the hydrophilic and hydrophobic properties of surfactant molecules [4,5].In addition to good emulsification properties, such surfactants also have good biodegradation properties [6-8], and have an essential application in the daily chemical industry, biopharmaceutical and material preparation[9,10].
The microemulsion is a transparent or translucent heterogeneous dispersed system [11-13] containing two or more wholly insoluble or partially soluble liquids emulsified by surfactant molecules [14-16].Microemulsions can be classified into three types: oil in water (O/W)microemulsion, water in oil (W/O) microemulsion and bicontinuous phase.Due to the unique structural properties of microemulsion droplets,microemulsions have been extensively applicated in oil recovery[17,18],delivery systems [8,19,20], reactions [21-23] and bacterial detection[24,25].Among them, water in oil (W/O) microemulsion, the aqueous phase is used as the dispersed phase [26,27].The W/O microemulsion droplets are extremely unstable due to the aqueous phase are prone to sediment,aggregate and coagulate[28-30].The formation and stability of the microemulsions are directly affected by the emulsification ability of the surfactant molecules.Chalmers et al.[3] investigated non-ionic surfactants containing polyethylene oxide (PEO) chains and water mixtures that span the phase diagram starting from randomly distributed arrangements by MD simulations using two molecular dynamics force fields.Coutinho et al.[2] observed that CiEjsurfactants exhibit a rich phase behavior in water and can self-assemble to form various 3-D structures with a controllable morphology by coarse-grained molecular dynamics simulations.They find the system has a variety of applications across different industrial segments.In view of this, the kind of polyoxyethylene ether nonionic surfactant with excellent performance is used as an emulsifier in this paper.
At present,the related research on the microemulsion mainly adopts experimental methods[31,32].Dissipative particle dynamics simulation method (DPD) can obtain the mesoscopic properties between microscopic and macroscopic properties,and more relevant information about the formation and stability mechanism of microemulsion [33-35].Therefore,DPD simulation method was used to investigate the influence of polyoxyethylene ether nonionic surfactants with different ethylene oxide numbers on the formation and stability of W/O microemulsion.The results show that the number of ethylene oxide directly affects the emulsifying ability of polyoxyethylene ether nonionic surfactant molecules.The water-oil ratio forming W/O microemulsion is different and affects the temperature stability of the microemulsion.
2.Simulation methods and conditions
The dissipative particle dynamics simulation method was proposed by Kolelman and Hoogerbrugge [33] in 1992 to simulate the thermodynamic behavior of complex fluids on a mesoscopic scale.In the DPD simulations,the molecule is replaced by a coarse-grained pearl necklace model, where each bead represents one group of multiple atoms.The beads are connected by a spring-harmonic oscillator, and the motion of the beads conforms to the Newton equation of motion.The forces on each bead include: conservative force, i.e., flexible repulsion force along the connecting line between two beads; dissipative force, i.e., the relative velocity between two beads;and random force,i.e.,energy supply for the system in the simulation process.Three resultant forces exerted on the beads maintain the bead momentum conservation.There is the interaction between beads, which is determined by interaction parameters(repulsion parameters).
The molecular and coarse-grained bead models in this study are:water molecule-W1,oil molecule(dodecane)-O2,and polyethylene ether nonionic surfactants-HmTn.In which, m represents the number of hydrophilic groups in the surfactant molecules;n represents the number of hydrophobic groups in the surfactant molecules.To investigate the influence of oxyethylene base, the surfactant molecules and coarse granulation models studied in this paper are diethylene glycol monododecyl ether-H1T2, decylethylene glycol monododecyl ether-H5T2, and twentyethylene glycol monododecyl ether-H10T2, respectively, as shown in Fig.1.
DPD units are adopted for the simulated particle mass, temperature and box length.The temperature unit is kBT, and the box unit length is RC.In this simulation,the box size is 20×20×20R3C.Furthermore,the beads in the simulation have fixed density and elastic constants,and the simulation time was controlled at 15,000 steps.The selection of this simulation time can ensure that the system reaches a stable state as shown in Fig.2.
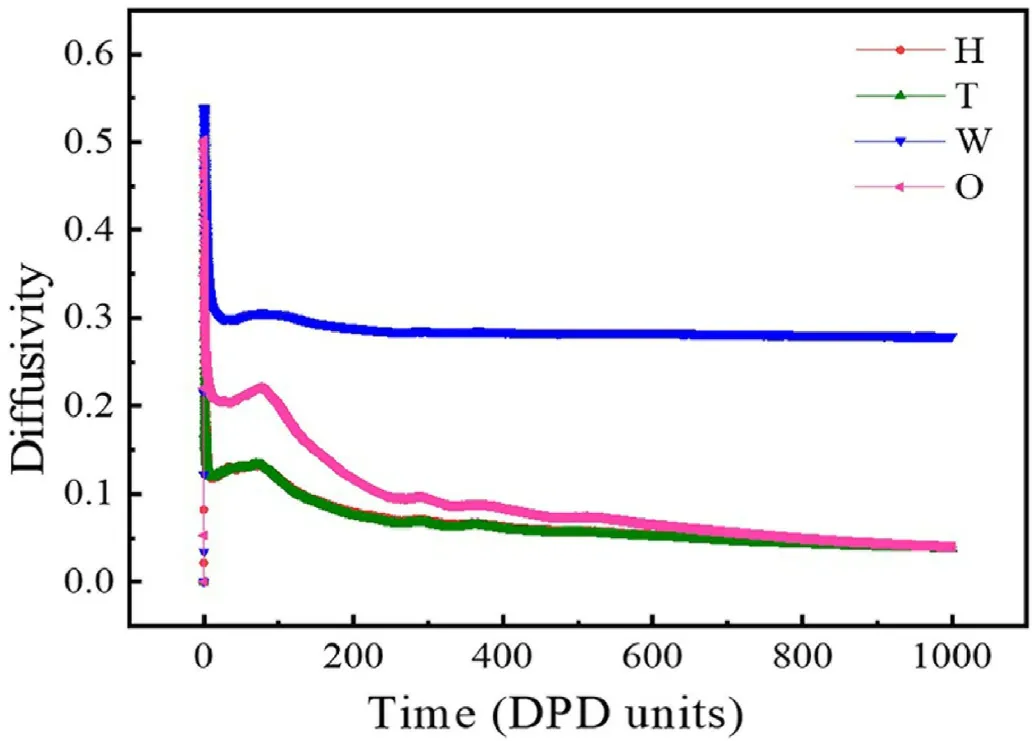
Fig.2.The diffusivity of all beads with increasing the DPD time.
This simulation adopts a set of interaction parameters calculated by Papavassiliou et al.[36]for similar oil-water-surfactant systems shown in Table 1.The simulation contains about 10125 particles, simulating 15000 steps to reach the equilibrium state.The simu lation system reaches equilibrium through the Gibbs canonical ensemble.
3.Results and discussion
3.1.Effect of water-oil ratio on the formation of W/O microemulsion
The water-oil ratio directly affects the formation of W/O microemulsion.The translucent dimensional structures formed by three surfactants with different oxygen vinyl numbers (a: H1T2、b: H5T2、c:H10T2) changing with water-oil ratio are shown in Fig.3.From the structural changes, the hybrid systems of the three surfactants offer similar changes.The aggregates changed from lamellar to columnar and then to microemulsion droplets with increasing the mass fraction of the oil phase.The water-oil ratio of forming the microemulsion could reflect the effect of the oxygen-vinyl group number on the formation of the microemulsion droplets.The water-oil ratios required to create W/O microemulsion are 1:5,1:6 and 1:8, respectively for H1T2with the leastoxygen vinyl groups (Fig.3a), H5T2(Fig.3b) and H10T2with the most oxygen vinyl groups (Fig.3c).That is to say, the difficulty order of forming W/O microemulsion is H10T2>H5T2>H1T2.Therefore, H1T2with the least oxygen vinyl base needs the smallest oil phase volume fraction to form the W/O microemulsion;H10T2has the largest number of oxygen vinyl, requires the largest oil phase volume fraction to form the W/O microemulsion.It can be seen that the larger hydrophilic group is disadvantageous to the formation of W/O microemulsion, which is consistent with the results observed previously on the effect of surfactant hydrophilic chain length on microemulsion formation observed by our group [35].This should be due to the fact that hydrophilic groups associate in the water-phase core when straight chain polyoxyethylene ether nonionic surfactants induce the formation of W/O microemulsion.The oil-phase molecules are more difficult to wrap the water-phase molecules with more oxyethylene groups reducing the greater repulsion between hydrophilic groups and the more apparent steric-hindrance effect.Therefore,more oil-phase molecules are needed to form the W/O microemulsion.
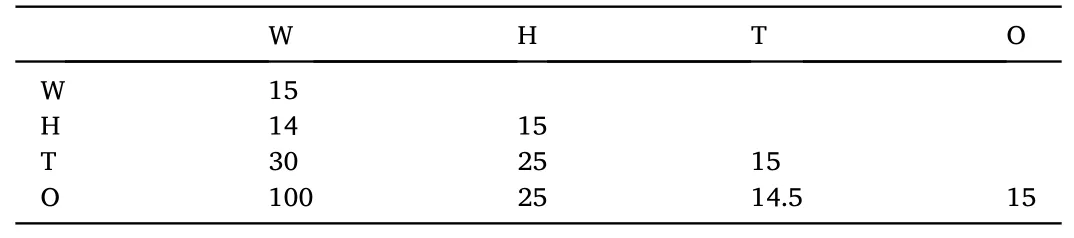
Table 1 Interaction parameters used in the simulation.
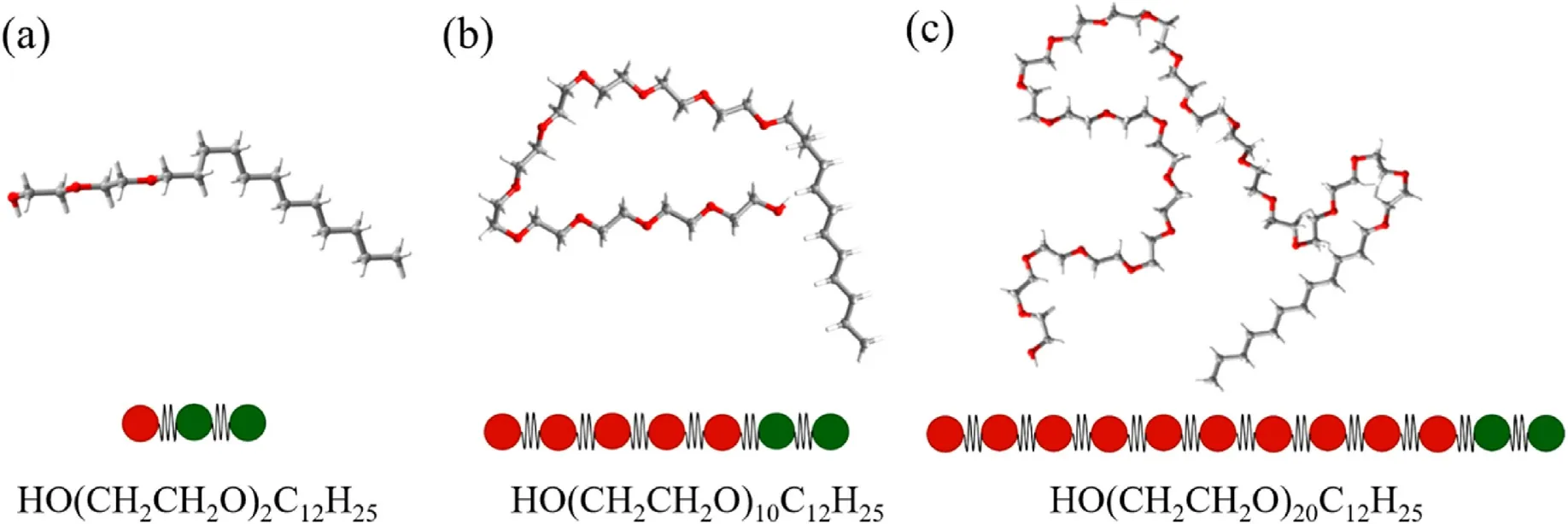
Fig.1.The molecular models of polyoxyethylene ether surfactant.(a) H1T2, (b) H5T2, (c) H10T2.(Red beads: hydrophilic heads; Green beads: hydrophobic tails).
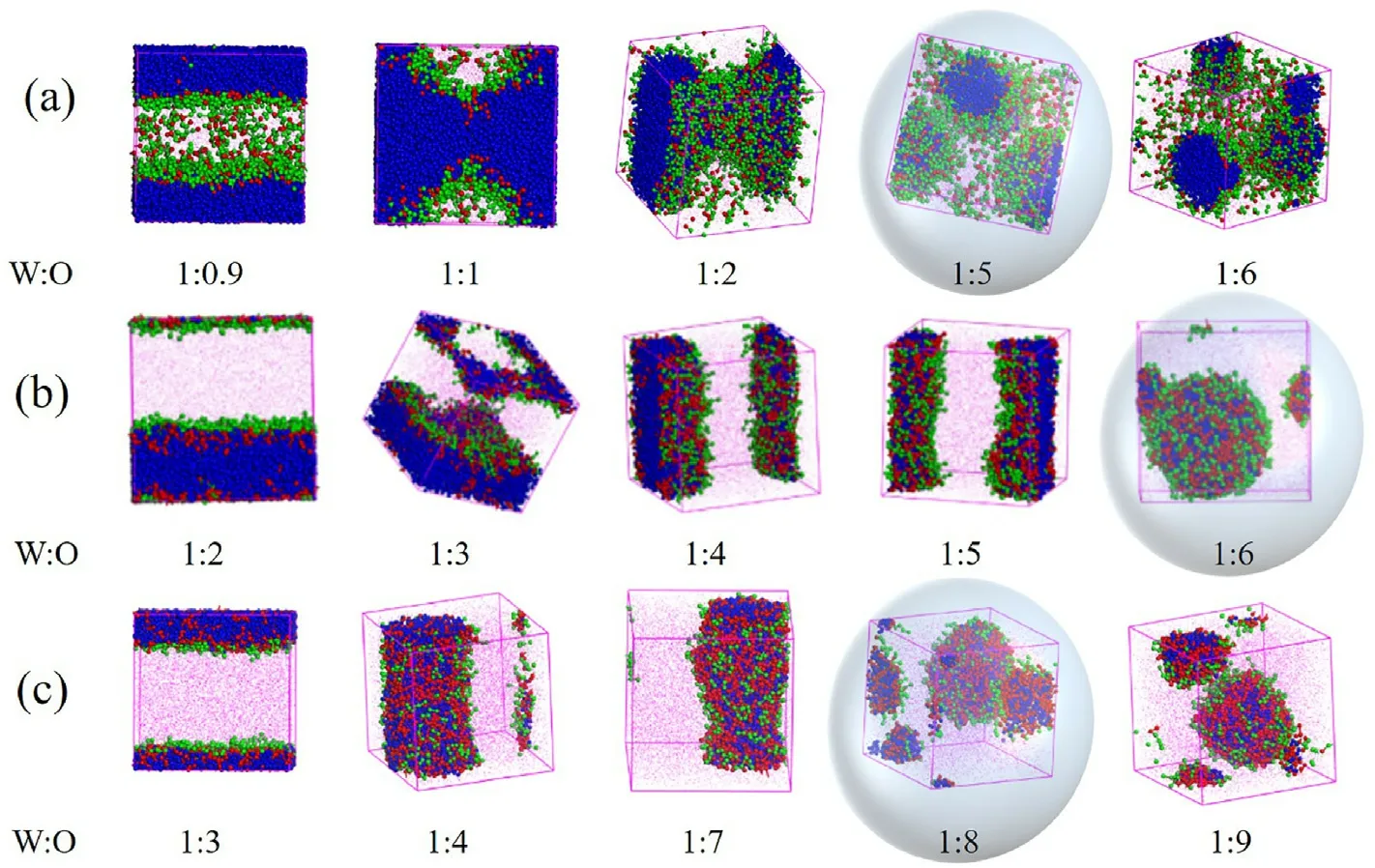
Fig.3.The translucent dimensional structures at different water-oil ratios.(a)H1T2; (b) H5T2; (c) H10T2.
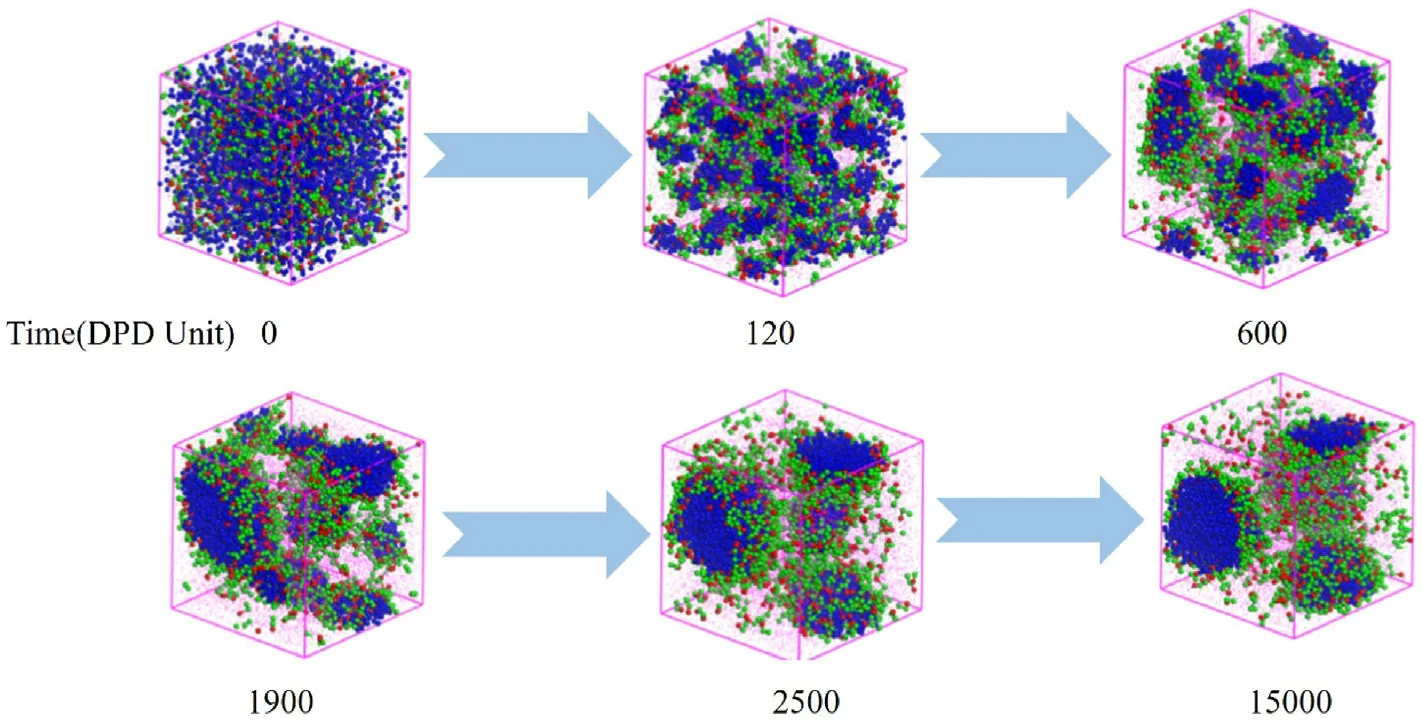
Fig.4.Formation dynamics of the W/O microemulsion of H1T2 with increasing simulation steps, where the W:O ratio was 1:5.
In the structures, water and oil are shown in blue and rose (the oil phase beads are not displayed to view the internal structure clearly).The symbols defined above are used in the following Figures.
To observe the formation process of the W/O microemulsion induced by polyoxyethylene ether nonionic surfactant more clearly,Fig.4 shows the change of the aggregate structure with increasing time,making H1T2as an example when the water-oil ratio is 1:5.At the beginning of the simulation(0 DPD time),all the beads were randomly distributed in the simulation grid.Increasing the DPD time to 120, the aqueous phase began to be wrapped into tiny droplets due to the emulsification capacity of H1T2.The tiny water droplets gradually merged into large droplets at 600 DPD time and then aggregated to two stable W/O microemulsion droplets with increasing DPD time to 2500, reaching an equilibrium state.This aggregate structure would not change until the DPD time is 15,000,and it is also further verified that the selection of simulation time is enough for the system to be stable.
Interfacial tension is an essential parameter for measuring the emulsification capacity of surfactant molecules.In DPD simulation, the average interfacial tension is obtained by calculating the pressure tensor in each direction in the simulated box using Irving and Kirkwood methods,as shown in Equation(1).
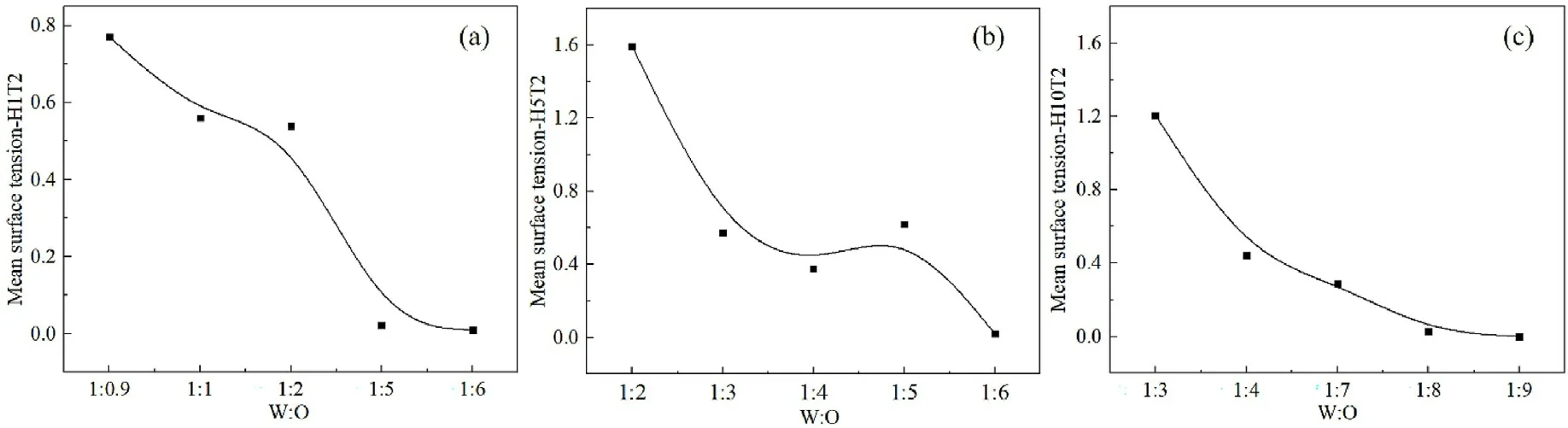
Fig.5.Mean interfacial tension at different W:O ratios.(a) H1T2; (b) H5T2; (c) H10T2.
Where Lx is the length of the box along the x-axis direction, perpendicular to the oil-water interface.Pijeg.Pxx,Pyy,Pzzare the pressure tensors on each plane, with reference to the findings of Milner et al.[37] and Chen et al.[38].Fig.5 shows the average interfacial tension value of the system at different water-oil ratios.It can be seen that the interfacial tension of the system decreases with increasing the oil content.H1T2reaches the lowest value when the water-oil ratio is 1:5,(Fig.5a); H5T2reaches the lowest level at a water-oil ratio of 1:6(Fig.5b);H10T2reaches the lowest level at a water-oil ratio of 1:8(Fig.5c).All the lowest value of the average interfacial tension virtually unchanged with increasing oil content.That is to say, the interfacial tension does not change, the surfactant has the strongest emulsifying ability and the system reaches the most stable state when W/O microemulsion is formed in the simulation system.
The adsorption and arrangement of surfactant molecules at the oilwater interface can be reflected by the average end-to-end distance of the surfactant molecules.Fig.6 shows the average end-to-end distance of all three surfactant molecules at different water-oil ratios.The average end-to-end distance significantly decreased from about 3.90 to 1.40 with reducing the oxygen vinyl groups from H10T2(Fig.6a)to H1T2(Fig.6c).For anyone surfactant,it is found that the average end-to-end distance of all surfactants show some degree of reduction with increasing the oil content,and then reaches the minimum value when W/O microemulsion is formed.The interface area of the spherical droplet of formed W/O microemulsion is larger than the layered and cylindrical interface.Therefore,the surfactant molecules can be more flexibly associated with the more extensive oil-water interface of W/O microemulsion under the fixed surfactant molecules content,so the average end-to-end distance of surfactant molecules reaches the minimum value when W/O microemulsion is formed.
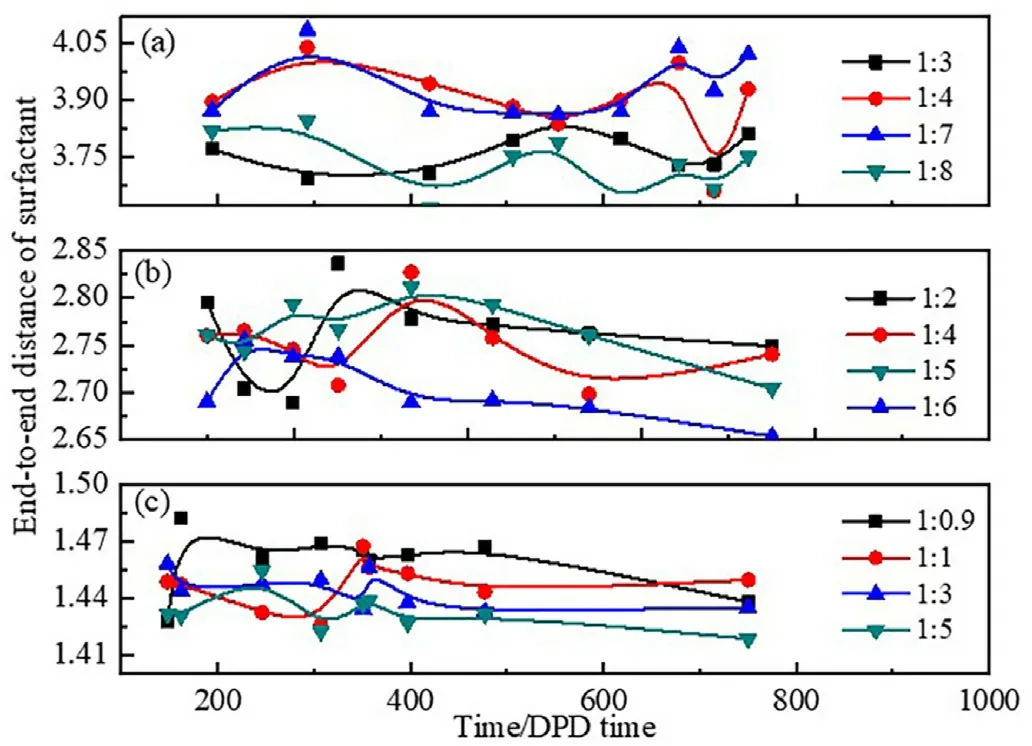
Fig.6.End-to-end distance of the surfactants at different water-oil ratios.(a)H10T2; (b) H5T2;(c) H1T2.
3.2.The temperature resistance stability of the W/O microemulsion
Temperature is one of the crucial factors that affect the stability of microemulsion.To investigate the influence of temperature on the stability of W/O microemulsion, the translucent dimensional structures of aggregates emulsified by surfactants with three types of polyoxyethylene chain lengths at different temperatures are shown in Fig.7.For H1T2,H5T2and H10T2, we can observe the tiny droplets gradually merge to form one large droplet with increasing the temperature.This is because the tiny droplets have more extensive interface area than one large droplet at the same water-oil ratio.Therefore, the number of surfactant molecules adsorbed at the unit interface area of tiny droplets is less than that of one large droplet at the same surfactant concentration.The emulsifying ability is weaker with little surfactants, causing the interfacial membrane of tiny droplets to be unstable, which gradually gather and form a relatively stable large droplet.What is noteworthy is that W/O microemulsion emulsified by H5T2deforms into columnar aggregates when the temperature rises to 1.8 kBT; The W/O microemulsion emulsified by H10T2deforms into worm-like aggregates when the temperature dropped to 0.1 kBT.We can observe that the W/O microemulsion with H5T2has poor high-temperature resistance, but the H10T2system has weaker low-temperature resistance.Analyzing the results of temperature resistance stability shows that the W/O microemulsion emulsified by H1T2has the best stability.
Fig.8 shows the end-to-end distance of the surfactants at different temperatures.As expected, an increase in the end-to-end distance of H1T2, H5T2and H10T2was obviously perceived with increasing the temperature from 0.1kBT to 2.0kBT, indicating that all surfactant molecular chains were more extended with higher temperature due to the drastic molecular motions.This change in the end-to-end distance can also be further observed from the surfactant molecular chain absorbed at the surface of the W/O microemulsion droplets in the translucent dimensional structures of aggregates(Fig.7).
4.Conclusions
In this paper, the influence of polyoxyethylene chain length of polyoxyethylene ether nonionic surfactant on the formation and temperature stability of W/O microemulsion was studied by dissipative particle dynamics simulation method at the mesoscopic level.The translucent dimensional structures, the average interfacial tension and the average end-to-end distance of polyoxyethylene ether nonionic surfactants under different water-oil ratios were analyzed and discussed.The results show that all three polyoxyethylene ether nonionic surfactant systems can form W/O microemulsion by decreasing the water-oil ratio.The required water-oil ratio is 1:5,1:6 and 1:8 respectively for H1T2,H5T2and H10T2.That is to say, H1T2with the lowest number of oxyethylene groups is easier to form the W/O microemulsion.In contrast, more oxyethylene groups are disadvantageous for the formation of the W/O microemulsion.Moreover, the results of the temperature stability of the W/O microemulsion show that H1T2with the lowest oxyethylene group as an emulsifier has good high-temperature and low-temperature stability.When the W/O microemulsion is formed, the interfacial tension of the system is the lowest, the end-to-end distance of surfactant molecules is the smallest,and the molecular chain is the most curved at the water-oil interface.The theoretical results can sufficiently support the practical application of the W/O microemulsion.
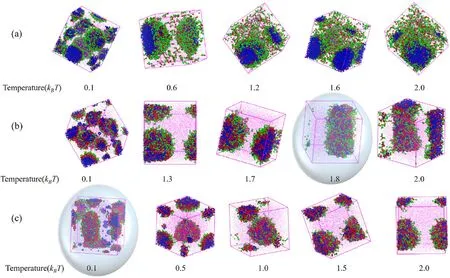
Fig.7.The translucent dimensional structures at different temperatures.(a) H1T2; (b) H5T2; (c) H10T2.

Fig.8.End-to-end distance of the surfactants at different temperatures.(a) H1T2; (b) H5T2; (c) H10T2.
Data availability
The datas regarding to support this finding are available upon reasonable request to the corresponding author.
Author contributions
Haixia Zhang: Data curation, Methodology, Investigation, Writing -original draft.Zongxu Wu:Conceptualization,Writing-review&editing.Chenguang Ding: Methodology, Investigation.Shoulong Wang: Supervision, review & editing.Jiahui xia: review & editing.Weimin Zhao:Supervision,review&editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The work was supported by the Research Foundation Project under Grant[BZXYLG2121];Shandong Provincial Natural Science Foundation under Grant [ZR202011050003]; the Shandong Province Natural Science Foundation of China under Grant[ZR2020KE034]; Shandong Natural Science Foundation of China[ZR2020MB032].
 Progress in Natural Science:Materials International2023年3期
Progress in Natural Science:Materials International2023年3期
- Progress in Natural Science:Materials International的其它文章
- Research progress of composite cathode materials for Solid oxide fuel cells
- A review on solidification of alloys under hypergravity
- Improving mechanical properties of Mg-Sn alloys by co-addition of Li and Al
- Multi-stimuli bilayer hydrogel actuator for remotely controllable transportation of droplets
- Towards high-efficiency of hydrogen purification in metal hydride
- Research advances on electrode materials for solid oxide electrolysis cells
