Multi-stimuli bilayer hydrogel actuator for remotely controllable transportation of droplets
Shuting Shen, N Pn, Chengfei Liu, Wenxin Fn, Kunyn Sui,*
a State Key Laboratory of Bio-Fibers and Eco-Textiles, College of Materials Science and Engineering,Shandong Collaborative Innovation Center of Marine Biobased Fibers and Ecological Textiles, Institute of Marine Bio-based Materials, Qingdao University, Qingdao, 266071, China
b School of Materials Science and Engineering, Liaocheng University, Liaocheng, 252000, China
Keywords:Bilayer hydrogels Actuator Multiple responsiveness Transportation of droplets
A B S T R A C T Under external stimuli, the bilayer hydrogels can change their shapes or move mechanically with fast, sensitive and adjustable motion characteristics.However, the main methods for preparing bilayer hydrogels have many limitations, such as they involve complicated synthesis processes, lead to fragile bilayer interface and structure,and result in single responsiveness and deformation,which hinders the practical applications of bilayer hydrogel in intelligent bionics.Here, we used the reaction-diffusion of sodium alginate (SA) and chitosan (CS) combined with the photopolymerization of N-isopropylacrylamide (NIPAM) to prepare (PNIPAM/SA)/(SA/CS) bilayer hydrogel film.The bilayer hydrogel film can well respond to the alteration of temperature,pH,salt concentration and thickness ratio with good interlayer bonding.This strategy can be applied to intelligent drive device to promote its practical application.
1.Introduction
Hydrogel is a kind of polymer material with unique threedimensional network structure.Being a typical soft and wet elastomer,hydrogel has been widely used in many fields such as tissue engineering[1-5], wearable devices [6,7]and consumer products [8,9].In recent years,stimulus-responsive hydrogels have attracted increasing attention,as they can undergo programmable three-dimensional (3D) shape transformation under external stimulation, leading to potential applications in areas such as soft robots[10,11], cell scaffolds[12], soft actuators [13,14] and drug delivery [15,16].Especially, inspired by plant tissues such as pine cones, wheat awns and orchid seeds, hydrogel actuators with double-layer structure have been widely designed with multiple responsiveness to humidity, pH and salt, etc [17-21].Under external stimuli, the bilayer hydrogels can change their shapes or move mechanically with fast, sensitive and adjustable motion characteristics.In the study of bilayer hydrogels, hydrogel actuators that can convert isotropic stimulus into anisotropic deformation have recently drawn a wide research attention [22,23].The bilayer hydrogel actuator can self-fold to form a three-dimensional structure with different shapes after responding to stimulation,and the bending degree and dynamics can be easily controlled by controlling the raw material concentration and gel thickness[18,19].
At present, the main methods for preparing bilayer hydrogel films include multi-step in-situ polymerization [24], electrophoresis-driven adhesion [25] and seamless supramolecular adhesion [26].However,these methods have many limitations, such as they involve complicated synthesis processes, lead to fragile bilayer interface and structure, and result in single responsiveness and deformation, which hinders the practical applications of bilayer hydrogel in intelligent bionics.
In the previous work, by utilizing the reaction-diffusion strategy [6,27,28], we have prepared a series of multilayered and gradiently-structured hydrogel materials by changing the diffusion rate difference between sodium alginate (SA) and chitosan (CS).Based on this, in this paper, we aim to further obtain a compact and complex SA/CS layer by changing the molecular structure parameters of SA and CS with the same fabrication process,and at the same time,by combining with the photopolymerization of N-isopropylacrylamide (NIPAM), to prepare (PNIPAM/SA)/(SA/CS) bilayer hydrogel.The interpenetrating network formed between SA/CS layer and PNIPAM/SA layer during the reaction can solve the problem of weak interface bonding.In addition,thanks to the temperature sensitivity of PNIPAM and the polyelectrolyte properties of SA and CS, the as-prepared bilayer hydrogel is endowed with multiple responsiveness to temperature, pH and salt.We systematically studied the effects of different environmental stimuli,as well as the thickness ratio of the two layers, on the bending deformation performance of hydrogel films.In addition,by adding Ag nanoparticles with photothermal conversion ability, we also demonstrate the near-infrared responsiveness of the bilayer hydrogel to achieve remotely controllable transportation of droplets.
2.Experimental
2.1.Materials
Chitosan (CS) with a 90% degree of deacetylation and a weightaverage molecular weight (Mw) of 5000 Da was purchased from Xi'an Realin Biotechnology Co.,Ltd.(Xi'an,China.).Sodium alginate(SA)with a viscosity of 485 mPa s was purchased from Qingdao Hyzlin Biology Development Co., Ltd.(Shandong, China.).N-isopropylacrylamide(NIPAM) was purchased from Aladdin Reagents Co., Ltd.(Shanghai,China).2,2'-Azobis(2-methylpropionamide) dihydrochloride (V50) and N,N'-Methylenebisacrylamide (BIS) was purchased from Aladdin Reagents Co., Ltd.(Shanghai, China).All chemicals were used without further purification.
2.2.Preparation of CS solution
A certain mass of CS powder is weighed and added into a beaker containing a certain amount of distilled water, and then stirred by magnetic force for 1 h to obtain CS solution.
2.3.Preparation of NIPAM-SA solution
A certain mass of SA powder was added into a beaker filled with distilled water, and stirred magnetically for 4 h at room temperature,then stirred continuously for 30 min in a constant temperature water bath at 50°C,and the SA was fully swollen and dissolved,resulting in a uniform solution of 1 wt%SA.Then NIPAM,crosslinking agent BIS and photoinitiator V50 were added, dissolved by magnetic stirring, mixed evenly, and then stood for 1 h to defoam.
2.4.Preparation of (PNIPAM/SA)/(SA/CS) bilayer hydrogel
Firstly, we prepared the concentrated low-molecular-weight (LMW)CS(Mw=5000 Da)solution,and the NIPAM-SA solution.Thereafter,the CS solution was dried inside a culture dish at normal temperature and pressure, and the NIPAM-SA (SA, Mw= 388 kDa) solution was poured onto the dried CS membrane.The LWMCS would gradually diffuse into the NIPAM-SA solution to react with SA due to the strong electrostatic complexation between them.The LWMCS would gradually diffuse into the NIPAM-SA solution to react with SA due to the strong electrostatic complexation between them.And their thicknesses increased with increasing the reaction time.After 30 min of reaction,the obtained SA/CS hydrogel film was exposed to 365 nm ultraviolet lamp for 2 min,and finally SA/PNIPAM double network hydrogel was formed.The gel was taken out,the unreacted solution was washed with deionized water,and then soaked in deionized water to swelling equilibrium, and the deformation experiment was carried out.
2.5.Preparation of Ag-embedded (SA/CS)/(PNIPAM/SA) bilayer hydrogels
After the bilayer hydrogel was immersed in 0.5 M AgNO3solution for 12 h,the unreacted AgNO3solution was washed off with water.Then,a layer of aluminum foil was coated on the surface of the hydrogel at different positions, and then irradiated by ultraviolet light for 1 h (L =365 nm) to obtain bilayer hydrogels loaded with Ag nanoparticles at different positions.
2.6.Characterizations
The morphologies of hydrogels were characterized by scanning electron microscope (FEI Quanta 250 FEG) operating at 10 kV.The samples were prepared by freeze-drying and then cryogenically fractured in liquid nitrogen.Before the SEM characterization,the fractured surface was coated with a thin layer of gold by the sputtering method.
3.Result and discussion
3.1.Strong interfacial adhesion between the bilayers of the hydrogel
The(PNIPAM/SA)/(SA/CS)bilayer hydrogel was synthesized using a two-step method as schematically illustrated in Fig.1.During the first step, the instantaneous electrostatic interaction between two polyelectrolydes at the interface of two solutions resulted in the formation of SA/CS complexes layer.As CS molecules continued to diffuse into SA solution, driven by osmotic pressure, the thickness of the SA/CS layer increased gradually with reaction time.The obtained SA/CS layer was exposed to UV light,to form the PNIPAM/SA layer on the SA/CS layer in a very short time (~2 min) by UV-light induced photopolymerization.The bilayer hydrogel was therefore obtained.

Fig.2.Characterization of the structure of the bilayer hydrogel.SEM image of the SA/CS layer cross section (A),the interfacial layer (B),and the PNIPAM/SA layer(C).SEM images of the surfaces of the SA/CS layer (D) and the PNIPAM/SA layer (E).
The bilayer structure of the as-prepared hydrogel was then observed by scanning electron microscope (SEM) characterization.As shown in Fig.2, the PNIPAM/SA layer and the SA/CS layer exhibited different porous microstructures.The SA/CS layer possesses a compact structure(Fig.2a,d), and the PNIPAM/SA layer shows porous network structures(Fig.2c,e).A dense interfacial layer was observed (Fig.2b), demonstrating the strong connection and adhesion between the two layers of the as-prepared bilayer hydrogel.More importantly, the PNIPAM/SA layer and the SA/CS layer are joined so tightly that they can't be separated during the bending-recovering process.This is probably because of that an inter-penetrating-network formed during the fabrication process,as the NIPAM monomers diffused into the first SA/CS layer and formed a robust interlocking interface layer after the photopolymerization step.
3.2.Thermo-responsive actuation behavior
PNIPAM is a well-known thermoresponsive polymer with a lower critical solution temperature (LCST) of ~32°C, which means that it swells at temperatures below LCST and shrinks at temperatures above LCST.In addition, the SA/CS layer has gradient structure generated during the reaction-diffusion process.Under high temperature conditions, the hydrogel will bend toward the direction of diffusion, because the larger pores impart the LC(low CS)side with the faster diffusion rate of counter ions, leading to the faster growing rate in the complexation density for the LC side.Thus,the obtained bilayer hydrogel is responsive to temperature change.
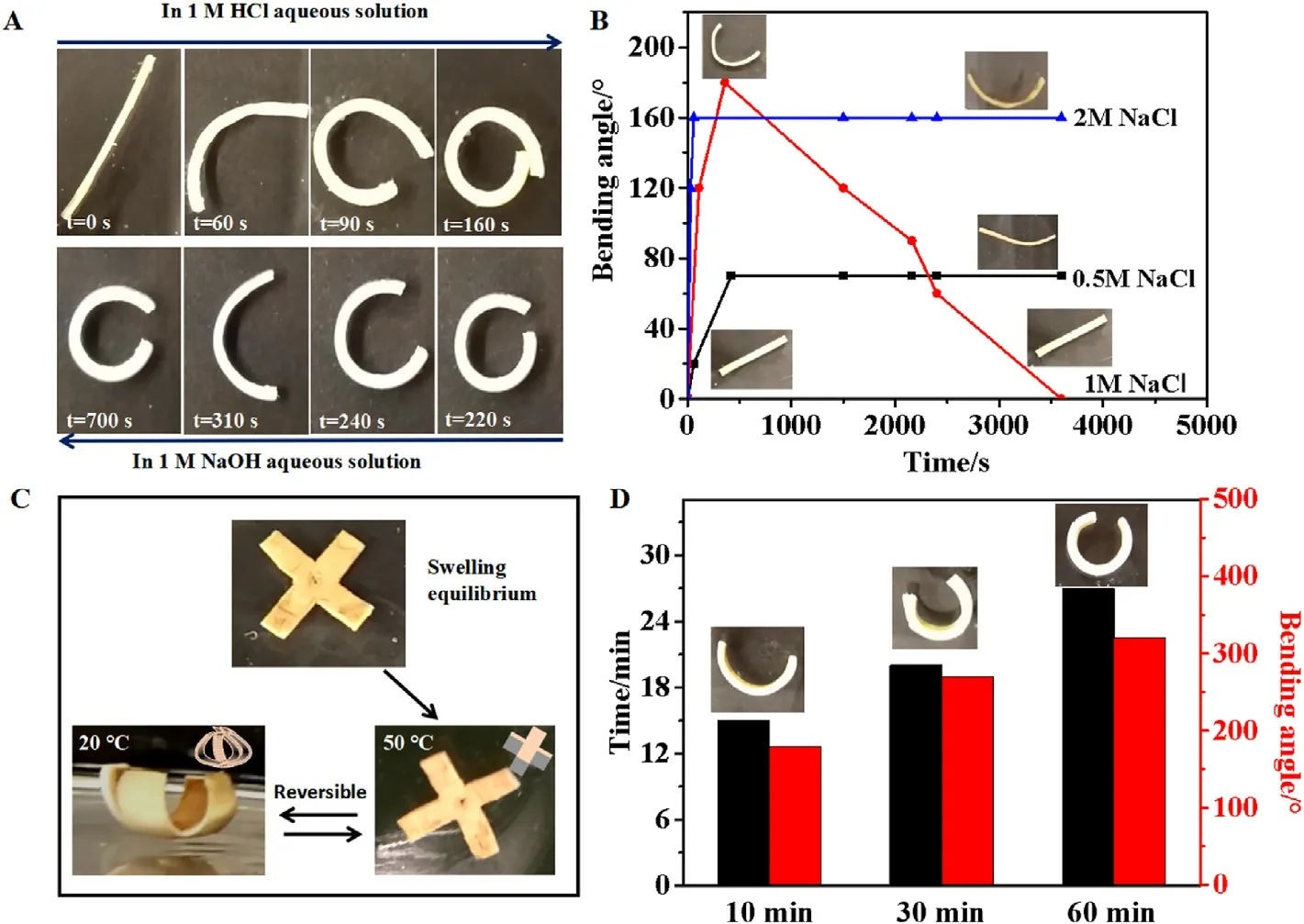
Fig.4.pH and salt responsive actuation of the bilayer hydrogel.Bending behaviors of bilayer hydrogels in response to 1 M HCl and NaOH (A), and to different salt concentrations (B).(C) The hydrogel is reversibly self-deformed in water medium with temperature changing between 50 °C and 20 °C.(D) The time to reach the maximum curvature and the maximum curvature angle (in 20 °C) as a function of the immersion time in 50 °C water.
Fig.3a illustrates the controlled static bending motion of the hydrogel actuator in water of 20°C and 32°C.As expected,the bilayer hydrogel bends toward the PNIPAM/SA layer side at 32°C.After being cooled at 20°C in water,the bent hydrogel returned to its original state(Fig.3b),demonstrating the reversible deformation capability.Fig.3c shows the influence of different medium temperature on the actuating performance of the bilayer hydrogel.When the bilayer hydrogel was immersed in water at 35°C,it bent toward the PNIPAM/SA layer with an angle of ~440°in 210 s.After it was transferred into cold water at 20°C, it recovered to the straight state in 640 s.As the medium temperature increased to 50°C,the maximum bending angle increased to 540°within 40 s,which was 5 s faster than that at 40°C with the angle of ~450°.The reversible curling/uncurling process can be repeated by alternately soaking the hydrogel in 50°C and 20°C water for ten cycles (Fig.3d).Moreover, the curvature of the bilayer hydrogels in both equilibrium states exhibited good reproducibility (Fig.3d), without any decay or damage in the structures or properties.These results demonstrate that the bilayer hydrogel is robust against cyclic actuations, and further support the fact that the strong interfacial adhesion between the SA/CS layer and PNIPAM/SA layer leads to the excellent mechanical stability of the bilayer hydrogel during repeated deformation.Therefore, the bilayer hydrogel exhibited rapid,stable and repeated bi-directional self-bending behaviors.
3.3.pH-responsive actuation behavior
Fig.4a shows the deformation of the bilayer hydrogel by changing the pH of the medium.In an acidic environment, the hydrogel bent toward the PNIPAM/SA layer and reached the equilibrium state in 160 s.When the solution was replaced with 1 M NaOH, which has a pH of 14, the hydrogel firstly tended to recover to its original shape, but then bent gradually toward the PNIPAM/SA layer and finally fixed the shape at the time over 700 s.Under the condition of strong acid,the COO-groups of SA combine with H+to form carboxylic acid.The -COOH groups of SA form hydrogen bonds with -CONH- in the PNIPAM network and the adjacent COOH,causing the PNIPAM/SA layer to shrink.For the SA/CS layer,the double folding deformation is triggered by the diffusion of H+ions into the layer, which leads to the electrostatic screen of both complex and noncomplex segments of the polyelectrolytes(denoted as SCand SN-C, respectively).Due to insufficient ions, the hydrogel only bends toward the LC side.Under alkaline conditions,the-COOH of SA dissociates to generate-COO-ions and the electrostatic repulsion between the-COO-.The action on adjacent groups will then be weakened, eventually causing the full extension of the gel chain and the increase of the swelling degree.After being in alkaline solution for 480 s,micro-crystallization of CS chains occurs, which acts as temporary crosslinking points to fix the deformed shape of the bilayer hydrogel[29,30].As a result,the hydrogel with a deformed shape was kept and would not unfold to its original state.
3.4.Ion-responsive actuation behavior
Fig.4b demonstrates the actuation behavior of bilayer hydrogels in response to salt solution(from 0.5 to 2.0 M NaCl).It can be clearly seen that (PNIPAM/SA)/(SA/CS) hydrogels bend toward the PNIPAM/SA layer at low salt concentration(0.5 M NaCl),but recover to their original straight shapes upon immersing in 1.0 M NaCl solution.As we know,ions will screen the charges of SA/CS layer and SA, and also influence the LCST of PNIPAM.LCST will decrease in the presence of ions,leading to the volume shrinkage of PNIPAM.Therefore, the variable deformation performance at different ion concentrations can be attributed to the synergistic effect of the above two factors.For the PNIPAM/SA layer, it only undergoes shrinkage upon ion stimulation owing to the volume variation of PNIPAM and the charge screening of SA.For the SA/CS layer,when the salt concentration is 0.5 M,due to the low SCdensity on the LC side (high SNCdensity), the molecular chain shrinks because of electrostatic shielding,and the gel bends toward the LC side(that is,toward the PNIPAM/SA layer).The low salt concentration does not destroy the gel complex structure.However,when submerging the bilayer into 1 M salt solution, the rate of ion entering the gel increases, and the enhanced electrostatic shielding destroys the gel complex structure [31].As a result, the bending degree toward the LC side firstly increases but then reduces, and the original shape of the hydrogel finally recovers.Moreover, the low complexation density endows the LC side with large swelling capacity.Therefore, the bilayer bent to the opposite direction,the HC (high chitosan) side.When the salt concentration increases to 2 M,the LC side of the hydrogel and the HC side compete for the electrostatic damage of the SCsegment.The bending angle to the LC side is reduced,and finally the bending balance is reached.
3.5.Influence of the thickness ratio on the actuation behavior
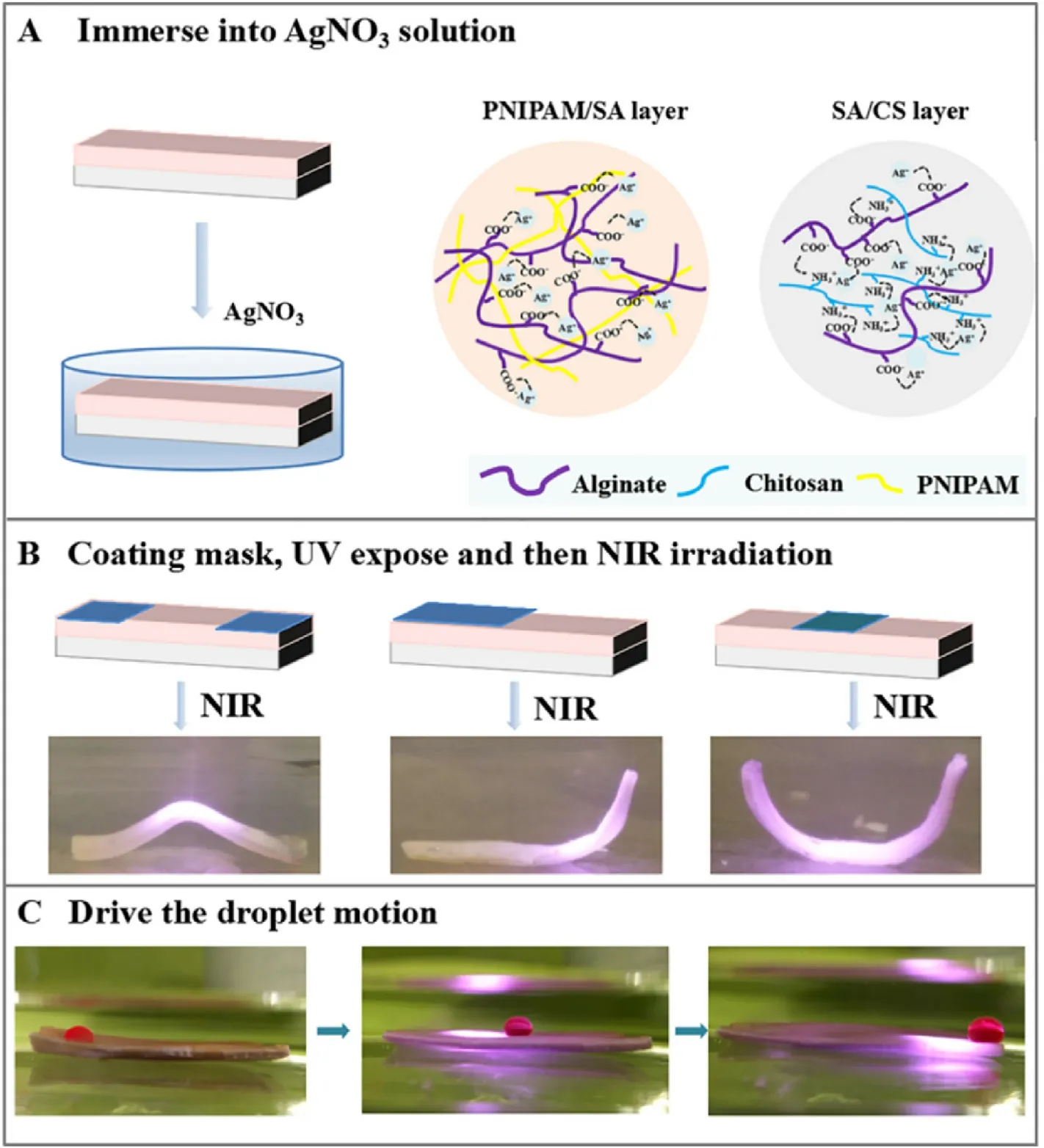
Fig.5.Photothermal-responsive actuation of the Ag nanoparticle embedded bilayer hydrogel.(A)Schematic illustration of the fabrication process of Ag nanoparticle embedded bilayer hydrogel.(B)Photothermal-responsive deformation of the patterned hydrogels loaded with Ag nanoparticles.(C)The oil droplet movement driven by the patterned bilayer hydrogel with Ag loaded under NIR irradiation.
The bending behavior can be adjusted by varying the thickness ratio between the PNIPAM/SA layer and the SA/CS layer.As shown in Fig.4c,when the thickness ratio of (PNIPAM/SA)/(SA/CS) is 4:1 (or 3:1), the bilayer hydrogel did not deform in 50°C water,while it bent toward the SA/CS layer in 20°C water.This is because of that the PNIPAM/SA layer is too thick to be easily driven by shrinkage force, as its bending resistance also increases with its thickness.Therefore, the thickness ratio between the two layers needs to be optimized to achieve a better performance,such as that when the thickness ratio is 2:1 and 1:1.Under the optimized configuration, the bilayer hydrogel restores enough elastic energy in 50°C water and fast releases the energy in 20°C medium to achieve the reversible deformation.Different amounts of elastic energy and release rate can be obtained when immersing the hydrogel in 50°C water for different time.As shown in Fig.4d,a maximum bending angle of ~180°was obtained when the immersion time at 50°C is 10 min,and the bending process finished within 15 min.When the immersion time extended to 30 min, the maximum bending angle increased to 280°which was achieved within a shorter time,i.e.,20 min.By immersing the bilayer hydrogel in 50°C water for 60 min,a maximum bending angle of~320°can be reached within 28 min.
3.6.Photothermal-responsive actuation behavior
Photothermal-responsive bilayer hydrogel can be achieved by a simple Ag loading process.The hydrogel was immersed in AgNO3solution, allowing diffusion of Ag+cations in the hydrogel and fast complexation with the CS and SA chains, which also leads to the additional formation of interwoven networks (Fig.5a).As shown in Fig.5b, the surface of the hydrogel was then covered with a layer of aluminum foil mask at different positions.Ag nanoparticles were finally obtained by UV light (L = 365 nm) irradiation for 1 h.The bilayer hydrogel combined with patterned photothermal Ag nanoparticles enables the programmable volume change of the hydrogel triggered by NIR irradiation.As illustrated in Fig.5b,the masked regions of the hydrogel strips don't have the photothermal capability,and the uncovered region containing photothermal-responsive Ag nanoparticles is therefore endowed with bending ability, leading to the deformation of the hydrogel strips into different shapes upon NIR irradiating(Fig.5b).
Based on the photothermal bending capability of the Ag-loaded(PNIPAM/SA)/(SA/CS) hydrogel, we further designed a hydrogel actuator to control its bending direction and angle to drive the moving of the trichloromethane chloroform droplets, as shown in Fig.5c.When the hydrogel reached the equilibrium state of swelling, it showed superoleophobicity under water.Once illuminated by NIR light,the hydrogel bends toward the PNIPAM/SA layer side,therefore driving the droplet to move forward.Once the NIR illumination stops, the deformation of hydrogel relaxes back to its original state,and the droplet is successfully transported to the opposite end of the hydrogel strips.
3.7.Application of the bilayer hydrogel
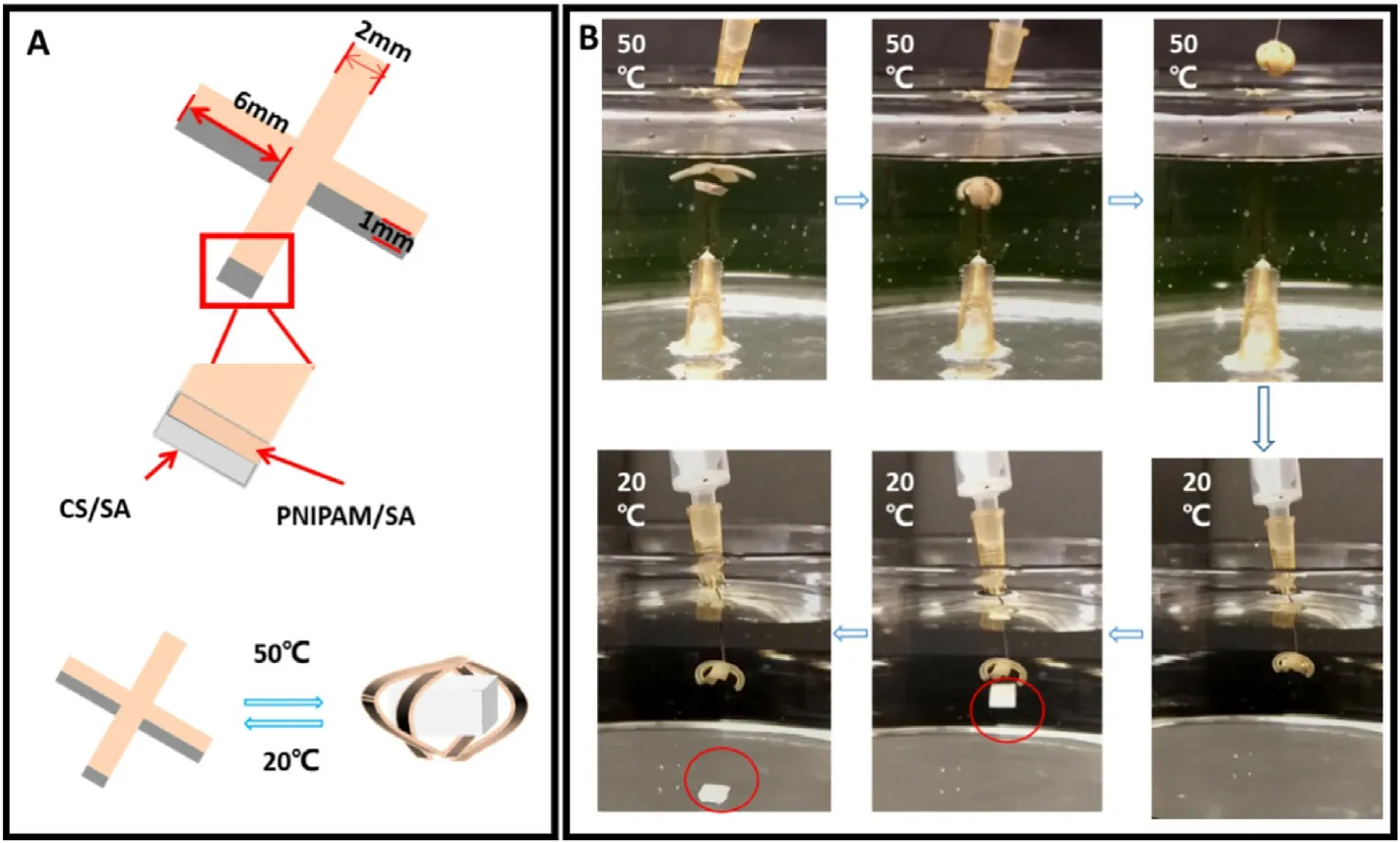
Fig.6.Application of the bilayer hydrogel actuator.(A)Schematic illustration of the gripping process of a four-arm hydrogel gripper.(B)Images demonstrating the grasping and releasing of gel block by the four-arm hydrogel gripper in water at different temperatures.
Soft actuators with variable shapes and wide adaptability are mostly preferred in intelligent sensors,circuit switches and soft fixtures.As our bilayer hydrogel can bend directionally in response to temperature and pH,it can be used as an actuator for environmental control.We therefore designed an intelligent clamp with four arms for an illustration, which can change its shape according to the change of temperature.The length,width and thickness of each arm are controlled at 6 mm×2 mm×1 mm,and the working principle of the clamping hydrogel is schematically illustrated in Fig.6a.Fig.6b demonstrates the process of the bilayer hydrogels to grasp heavy objects underwater.By fixing the block hydrogel on the underwater needle and then immersing the four-arm gripper in the 50°C water bath, the four arms bend to capture the underwater gel block.Then,by taking out the gripper and immersing it in the 20°C water bath,the gripper unfolds to release the gel block.
4.Conclusion
In conclusion,we have prepared a bilayer hydrogel film by a simple interface diffusion and photo-polymerization process, which can well respond to the alteration of temperature, pH, salt concentration and thickness ratio with good interlayer bonding.Moreover, by taking advantage of the photothermal conversion ability of Ag nanoparticles,the hydrogel can deform into different shapes and drive the moving of oil droplets under the control of NIR illumination.This study proposes a novel strategy for the synthesis of bilayer hydrogels with deformation capability under multiple stimulations, which could be applied to intelligent actuating devices.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This research was supported by National Natural Science Foundation of China (52003133 and 52273036), “Youth Innovation Team Plan” of Colleges and Universities of Shandong Province(2022KJ299),and State Key Laboratory of Bio-Fibers and Eco-Textiles, Qingdao University(G2RC202024 and ZDKT202006), and the Program for Taishan Scholar of Shandong Province.
 Progress in Natural Science:Materials International2023年3期
Progress in Natural Science:Materials International2023年3期
- Progress in Natural Science:Materials International的其它文章
- Research progress of composite cathode materials for Solid oxide fuel cells
- A review on solidification of alloys under hypergravity
- Improving mechanical properties of Mg-Sn alloys by co-addition of Li and Al
- The formation and temperature stability of microemulsion emulsified by polyoxyethylene ether surfactant
- Towards high-efficiency of hydrogen purification in metal hydride
- Research advances on electrode materials for solid oxide electrolysis cells
