Adjuvant therapy for hormone receptor-positive breast cancer:Perspective from a survey on breast cancer physicians’acceptance of practice-changing data
Asian Institute of Clinical Oncology (AICO) Expert Panel
Abstract A cross-sectional online survey was conducted.A high proportion of the Chinese breast cancer (BC) physician respondents (n=77) would prescribe extended adjuvant endocrine therapy (AET) with aromatase inhibitors (AI)beyond 5 years for postmenopausal females with BC,especially those with higher risk.Respondents with ≥15 years of clinical experience were more likely to prescribe a longer duration of AET for low-risk patients.Half of the respondents considered intermittent letrozole as an acceptable option.Most respondents would prescribe adjuvant chemotherapy to genomic high-intermediate risk [Oncotype DX recurrence score (RS) 21-25] females aged ≤50 years regardless of the clinical risk classification.
Keywords: Breast neoplasms;surveys and questionnaires;chemotherapy;adjuvant;practice patterns;physicians
Adjuvant endocrine therapy(AET)prescription pattern of Chinese breast cancer(BC) physicians
Results from the Gruppo Italiano Mammella 4 (GIM4),the Secondary Adjuvant Long-Term Study with Arimidex(SALSA),and the Study of Letrozole Extension (SOLE)trials on extended AET or prescribing intermittent AET are practice-changing for the management of postmenopausal females with BC (1-3),but it was unknown whether physicians would accept these trial results and adjust their AET prescriptions for this patient group.
Likewise,the decision to prescribe adjuvant chemotherapy for hormone receptor (HR)-positive patients with intermediate Oncotype DX recurrence score (RS) risk remains controversial and there has been a lack of data to support such decisions (4).The Trial Assigning Individualized Options for Treatment (TAILORx) trial found that some patients with intermediate RS risk,especially those aged 50 years or younger,had some benefits by adding chemotherapy to their adjuvant AET regimens (5),and the Microarray In Node negative Disease may Avoid ChemoTherapy (MINDACT) trial suggested that high genetic risk is a more important factor as compared to high-risk clinical features (6).However,we suspected that some BC physicians may consider the absence of high-risk clinical features as an indication to safely forgo adjuvant chemotherapy for those with intermediate RS risk.
In an attempt to answer these questions,we conducted a cross-sectional online survey involving BC physicians in China in January 2022.The link to the online survey was sent to Chinese BC physician groups through a social media platform.A total of 77 BC physicians responded,and they were asked to indicate their medical specialties,the number of years of clinical experience,the location of practice,and the number of BC patients managed annually(personally or as a team).Ethics approval was not required for this study.This work was conducted in strict accordance with the human subject protection program.
The survey questions on extended or intermittent AET were developed based on the GIM4,SALSA,and SOLE trial results,as well as referred to the AET decision tree(section BINV-K) based on the latest National Comprehensive Cancer Network (NCCN) guidelines(1-3,7).This AET decision tree forms the basis of the survey questions pertaining to the duration of prior treatments [2-3 years of tamoxifen (TAM),4.5-6.0 years of TAM,or 5 years of aromatase inhibitors (AI)].The survey questions on prescribing adjuvant chemotherapy for patients with intermediate RS risk were developed based on the results of the TAILORx and MINDACT trials,as well as the fact that there remains a lack of guidance for this group of patients (4-6).
Statistical analyses were performed using SPSS Statistics(Version 28.0;IBM Corp.,New York,USA).Nonparametric tests (Mann-Whitney U or Kruskal-Wallis,depending on the number of subgroups) were used to assess whether there was any difference in response between the subgroups.
Survey findings: What we learned
Most (62.3%) of the respondents were BC surgeons,24.7%were BC specialists,10.4% were medical oncologists,and 2.6% were general surgeons.The majority (71.4%) of respondents’ location of practice was in Tier 1 cities,followed by 10.4% in Tier 2 cities,9.1% in Tier 3 cities,2.6% in Tier 4 cities,and 6.5% in Tier 5 cities.Of note,this tier classification was based on the Chinese city tier system,with the most developed areas of the country being classified as Tier 1.Almost half (45.5%) of the respondents had a clinical experience of 15 years or more,whereas 54.5% had less than 15 years of clinical experience.The mean ± standard deviation (SD) number of BC patients managed annually was 762.10±1,254.50.
Prescribing extended AET for HR-positive,postmenopausal BC patients
Table 1shows the survey results when respondents were asked about their usual practice when prescribing AET for HR-positive,postmenopausal BC patients considering the tumor characteristics and prior AET regimens.For patients with pN0,tumor size of 0.5 cm or smaller who had received either 4.5-6.0 years of TAM or 5 years of AI,the individual responses of those who chose to “add other duration of AI” (31.2% and 51.9%,respectively) indicated that most of them would stop the AET.Amongst the 35.1% of respondents who chose to “add other duration of AI” for patients with pN2/pN3 who had received 2-3 years of TAM,the individual responses indicated that they would add AI for up to 8-10 years.

Table 1 Respondents’ responses when asked about their usual practice when prescribing adjuvant AET for HR-positive,postmenopausal BC patients considering tumor characteristics and prior AET regimens
For patients with pN0 and tumor size of 0.5 cm or smaller,respondents with 15 years or more of clinical experience were more likely to prescribe a longer duration of AET as compared to those with less than 15 years of clinical experience (P=0.025 for patients who had received prior 4.5-6.0 years of TAM and P=0.038 for those who had received prior 5 years of AI).
Prescribing intermittent AET for HR-positive,postmenopausal BC patients
More than half (57.1%) did not agree with the hypothesis that AI resistance can be reversed by intermittent letrozole treatment (the SOLE trial hypothesis),but 50.6%considered intermittent letrozole as an acceptable option for patients who would prefer a treatment interruption,especially for those with 3 or less positive nodes (79.5%),tumor grade 1 (84.6%),tumor size of 2 cm or smaller(82.1%),and human epidermal growth factor receptor 2(HER2)-negative disease (74.4%).Two-thirds (66.7%) of the respondents did not consider intermittent AET as acceptable for patients who had only received selective estrogen receptor modulators (SERMs).
The respondents’ responses were almost divided when considering other factors such as whether the patient had received prior chemotherapy (59% of respondents voted that intermittent AET is acceptable),underwent mastectomy (59% voted yes),and received radiotherapy(43.6% voted yes).
Prescribing adjuvant chemotherapy for node-negative,HR-positive BC patients with intermediate RS risk
For patients aged 50 years or younger with RS of 16-25,almost all (97.4%) of the respondents would prescribe adjuvant chemotherapy.However,for patients aged older than 50 years with RS of 16-25,two-thirds (68.8%) of the respondents would not prescribe adjuvant chemotherapy.
For patients aged 50 years or younger with highintermediate RS (defined as RS of 21-25) and high-risk clinical features,almost all (98.7%) of the respondents would prescribe adjuvant chemotherapy.For patients aged 50 years or younger with high-intermediate RS,but without high-risk clinical features,the majority (87.0%) of the respondents would still prescribe adjuvant chemotherapy.
Key takeaways: Analyzing survey results and uncovering three critical findings
The results of this survey show several important findings(Table 2).Firstly,a high proportion of the BC physician respondents would prescribe extended AET with AI beyond 5 years for postmenopausal females with BC,especially for those with higher risk.Secondly,intermittent letrozole was considered as an acceptable option for lowrisk patients.Thirdly,forgoing adjuvant chemotherapy in younger females with genomic high-intermediate risk (RS of 21-25),but clinical low risk was not considered as acceptable by the BC physician respondents.

Table 2 Key takeaway messages from the survey
A large meta-analysis involving 88 trials and 62,923 patients has previously found that the risk of BC recurrence persisted beyond the first 5 years of AET and continued to occur steadily for at least 20 years after the diagnosis (8).In an attempt to find the optimal duration of AET,so as to not overtreat and cause an increased incidence of side effects from the treatment,the findings from the GIM4 and SALSA trials can be collectively interpreted to suggest that a total of 7-8 years may be the optimal extended AET duration for BC patients with average risk (1,2).
BC physician respondents in the present study seemed to agree with the results of these trials and would prescribe extended AET with AI beyond 5 years for high-risk postmenopausal females with BC.Nonetheless,it isapparent from the survey results that some BC physicians remain conservative in terms of prescribing AET to lowrisk patients (defined as pN0,tumor size of ≤0.5 cm in this survey),with 22.1% choosing to add 5 years of AI to patients who had already received 4.5-6.0 years of TAM,as well as 15.6% choosing to add 5 years of AI to patients who had already received 5 years of AI.Since the more experienced BC physicians (i.e.,those with more than 15 years of clinical experience) were found to be more likely to prescribe a longer duration of AET for low-risk patients in subsequent analysis,and these findings may suggest that a more conservative approach in terms of AET prescriptions is preferred by BC physicians with more clinical experience.Considering that clinicopathologic features may not reliably predict who will benefit from extended AET,the use and validation of the Breast Cancer Index,which integrates theHOXB13:IL17BR(H/I) gene expression ratio and molecular grade index (MGI)components (9),are warranted for extended AET use in low-risk patients (10).
Multigene panel testing could also be used to guide extended AET decisions on patients with intermediate-to high-risk,as the BC physician respondents in this study were not comfortable with prescribing just 5 years of AET for this group of patients.Although 7-8 years of AET appears to be the “sweet spot” based on the results of GIM4 and SALSA,the optimal duration (i.e.,choosing between 5-10 years) remains unknown and therefore this decision should be personalized and discussed thoroughly with the patient.The addition of adjuvant S-1 for 1 year or abemaciclib for 2 years should also be considered based on the clinical benefits shown in the POTENT (adjuvant S-1 plus endocrine therapy for oestrogen receptor-positive,HER2-negative,primary breast cancer: a multicentre,open-label,randomised,controlled,phase 3 trial) and monarchE (Abemaciclib combined with endocrine therapy for the adjuvant treatment of HR+,HER2-,node-positive,high-risk,early BC) trials,respectively (11,12).
The SOLE study was initially designed to assess whether the recovery of circulating estrogen levels through intermittent AET with letrozole would result in an improved disease-free survival (DFS),but the results showed no improvement in DFS as compared to those who received continuous letrozole [7-year DFS of 81.4% in the intermittent groupvs.81.5% in the continuous group;hazard ratio: 1.03,95% confidence interval (95% CI):0.91-1.17] (3).Nonetheless,the less worsening in symptom-specific and global quality of life in those who received intermittent AET is an interesting finding which suggests that intermittent letrozole could be a viable option for patients who would require or prefer to do so (3).The respondents of the present study seemed to agree that intermittent letrozole is an acceptable option for low-risk patients,and this may represent an important strategy to combat patients’ potentially poor adherence to extended AET.
Lastly,seeing that the MINDACT trial found excellent survival in patients with genomic low risk and clinical high risk who forgo adjuvant chemotherapy,it appears that genomic risk plays a more important role in the decision to safely omit adjuvant chemotherapy in younger females (6).The respondents of the present survey seemed to agree with this consideration as well as the results of the TAILORx trial (5),and the vast majority of the BC physicians would prescribe adjuvant chemotherapy to genomic high-intermediate risk females aged 50 years or younger regardless of the clinical risk classification.This is understandable due to the fact that there is an enhanced chemotherapy effect in younger females with BC,which was three times larger in younger females than in older females,potentially due to chemotherapy-induced ovarian function suppression (6,13).However,it should be noted that it remains unknown whether an ovarian function suppression may replace chemotherapy and produce the same benefits (6,14).
To the best of our knowledge,this survey was the first to explore the BC physicians’ acceptance on the aforementioned novel,practice-changing data for the management of patients with HR-positive BC.Although the interpretations could be limited due to the nature of the survey,the findings provide insight into the real-world prescription pattern of BC physicians and their acceptance of new data.
Acknowledgements
We would like to thank Dr.Erich Ferdiansyah Lie for his medical writing assistance.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Working group members
AICO Expert Panel (listed alphabetically in order of surname;full list is available on https://www.aiconcology.org/):
Fan ChaiThe First Affiliated Hospital,Army Medical University
Yiding ChenTheSecond Affiliated Hospital,Zhejiang University
Louis Wing-Cheong ChowOrganization for Oncology and Translational Research
Xiaoxing DuanHuashan Hopital Affiliated to Fudan University
Peifen FuThe First Affiliated Hospital,Zhejiang University
Wenjing JianShenzhen Second People’s Hospital
Wen KangXiyuan Hospital of China Academy of Chinese Medical Sciences
Rui LingXijing Hospital,Air Force Medical University
Hongguang LiuShenzhen Second People’s Hospital
Simin LuoSun Yat-sen Memorial Hospital,Sun Yat-sen University
Hongmin MaGuangzhou Women and Children’s Medical Center
Ke QiNanshan District People’s Hospital
Die SangSanhuan Cancer Hospital
Li SuPeople’s Hospital of Ningxia Hui Autonomous Region
Zihan SunGuiqian International General Hospital
Cheng WangTheNinth People’s Hospital Affiliated to Shanghai Jiao Tong University School of Medicine
Nan WangPeking University Cancer Hospital and Institute
Ruliang WangHehai University
Wei WangShanghai General Hospital,Shanghai Jiao Tong University School of Medicine
Ying WangSun Yat-sen Memorial Hospital,Sun Yat-sen University
Wei WeiPeking University Shenzhen Hospital
Yu XiaoShenzhen Second People’s Hospital
Jiandong YeSuzhou Ninth Hospital Affiliated to Soochow University
Yinduo ZengSun Yat-sen Memorial Hospital,Sun Yat-sen University
Chao ZhangAffiliated Hospital of Jiujiang University
Yi ZhangTheFirst Affiliated Hospital,Army Medical University
Wenbin ZhouThe First Affiliated Hospital,Nanjing Medical University
Li ZhuShanghai General Hospital,Shanghai Jiao Tong University School of Medicine

Figure S1 Survival analysis in PSM cohort.(A) OS (P=0.827);(B) DMFS (P=0.454);(C) LRRFS (P=0.317) and (D) PFS (P=0.375).OS,overall survival;DMFS,distant metastasis-free survival;LRRFS,locoregional relapse-free survival;PFS,progression-free survival.

Figure S2 Survival analysis in IPTW cohort.(A) OS (P=0.863);(B) DMFS (P=0.808);(C) LRRFS (P=0.297) and (D) PFS (P=0.672).OS,overall survival;DMFS,distant metastasis-free survival;LRRFS,locoregional relapse-free survival;PFS,progression-free survival.

Table S1 Survival model parameters for model state transitions

Table S2 Initial cost and increment cost per cycle in Markov Iteration for each group

Figure S1 Secondary structure energies of ALKBH5 3’UTR with different sequence lengths.To avoid sequence length-dependent effects,a nucleotide sequence around miR-186-3p seed binding site and SNP site was extended to a length of 40-52 nt toward the 5’ end.The final target sequence was determined to be 52 nt.SNP,single-nucleotide polymorphism;nt,nucleotide.
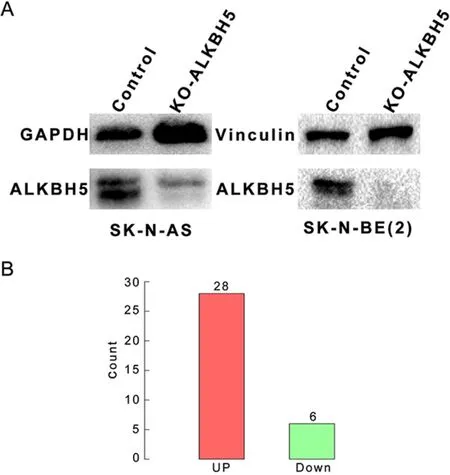
Figure S2 Stable knockout cell line construction and number of differentially expressed genes prior to transcriptome analysis.(A)We constructed neuroblastoma cell lines with stable ALKBH5 knockdown using CRISPR-Cas9 technology prior to transcriptome analysis;(B) Neuroblastoma cells with knockout of ALKBH5 expression showed 34 differentially expressed genes (28 up-regulated genes and 6 down-regulated genes).

Figure S3 Construction of ALKBH5 knockdown neuroblastoma cells.(A) Results of RT-qPCR analysis showed that ALKBH5 mRNA expression was significantly reduced in ALKBH5 knockdown neuroblastoma cell lines compared with the control group;(B) Western blotting data showed that ALKBH5 knockdown neuroblastoma cell lines had significantly reduced ALKBH5 protein expression;(C) EdU analysis also showed that proliferation of neuroblastoma cell lines with knockdown of ALKBH5 expression was inhibited.RT-qPCR,reverse transcription-quantitative real-time polymerase chain reaction.***,P<0.001;****,P<0.0001.

Figure S4 Relationship between rs8400 G>A and ALKBH5 expression was analyzed by cis-eQTL.(A) Compared with rs8400 G genotype,individuals with rs8400 A allele had significantly higher ALKBH5 mRNA levels in the whole blood and adrenal gland,with consistent results in other tissues (cerebral cortex,tibial nerve,cervical vertebra C1 of the spinal cord and cultured fibroblasts);(B) RT-qPCR results showed that mRNA expression of ALKBH5 in cell lines overexpressing rs8400 A allele was higher than that in cell lines overexpressing rs8400 G allele or control vector;(C) Western blotting results showed that in cell lines overexpressing rs8400 A allele,the protein expression of ALKBH5 was higher than that in the cell lines overexpressing rs8400 G or the control vector.eQTL,expression quantitative trait loci.****,P<0.0001.
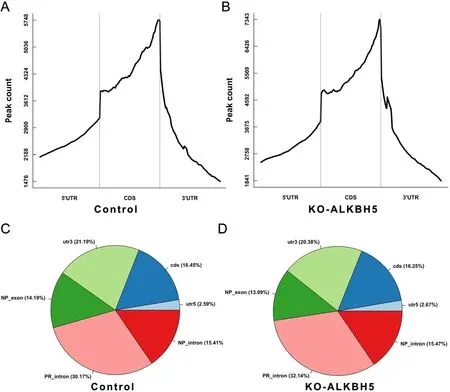
Figure S5 Distribution and counts of m6A peaks in control and knockout ALKBH groups.(A,B) m6A peak counts in control (A) and knockout ALKBH5 (B) groups;(C,D) Distribution of m6A peaks in control (C) and knockout ALKBH5 (D) groups.m6A,N6-methyladenosine.

Figure S6 Workflow of current study.GTEx,Genotype-Tissue Expression;CCK-8,cell counting kit-8;ALKBH5,AlkB homolog 5;GO,Gene Ontology;KEGG,the Kyoto Encyclopedia of Genes and Genomes;SNP,single-nucleotide polymorphism;RT-qPCR,reverse transcription-quantitative real-time polymerase chain reaction;MeRIP,methylated RNA immunoprecipitation.
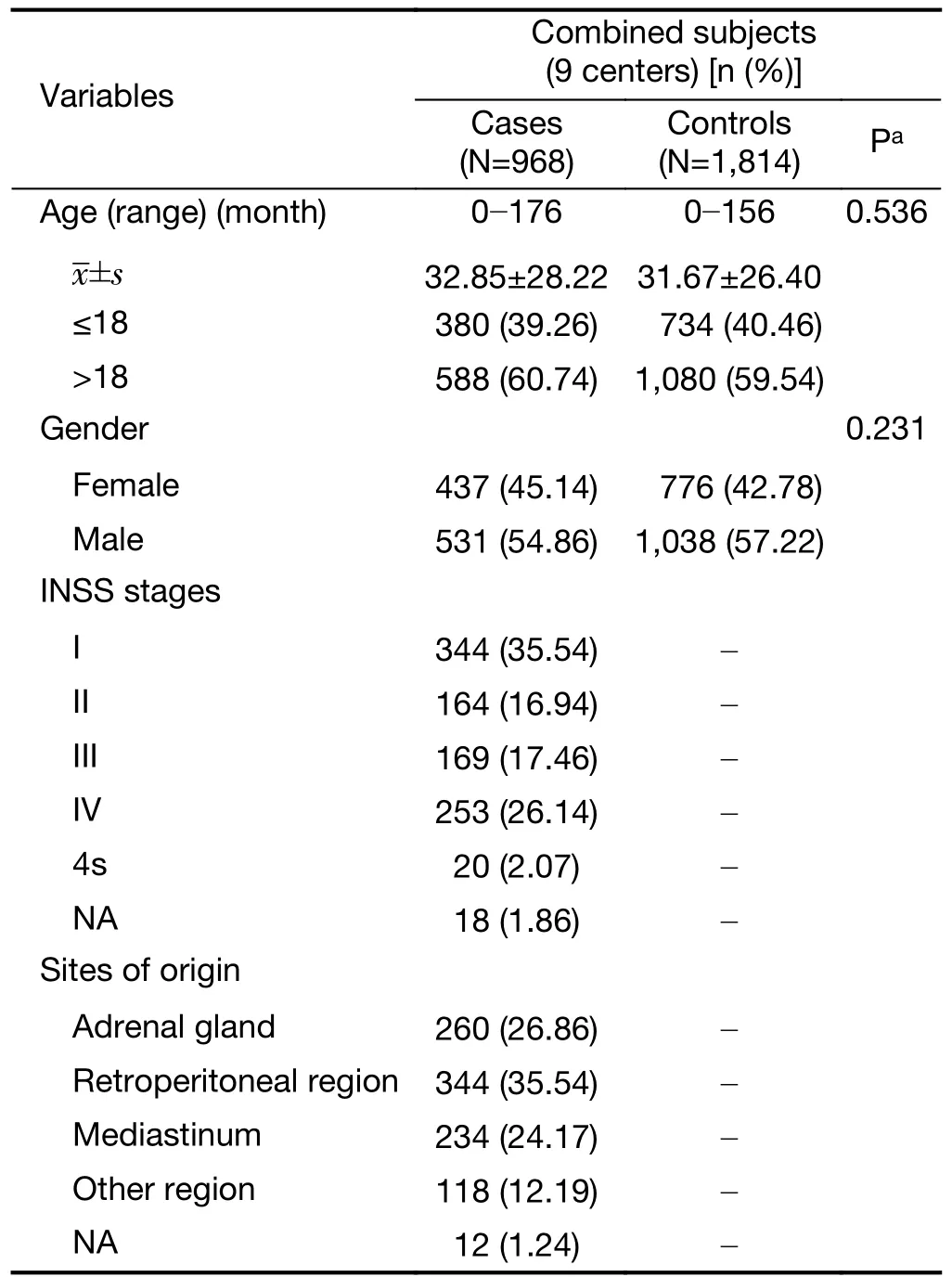
Table S1 Frequency distribution of selected characteristics in neuroblastoma cases and cancer-free controls

Table S2 Primers used in this study
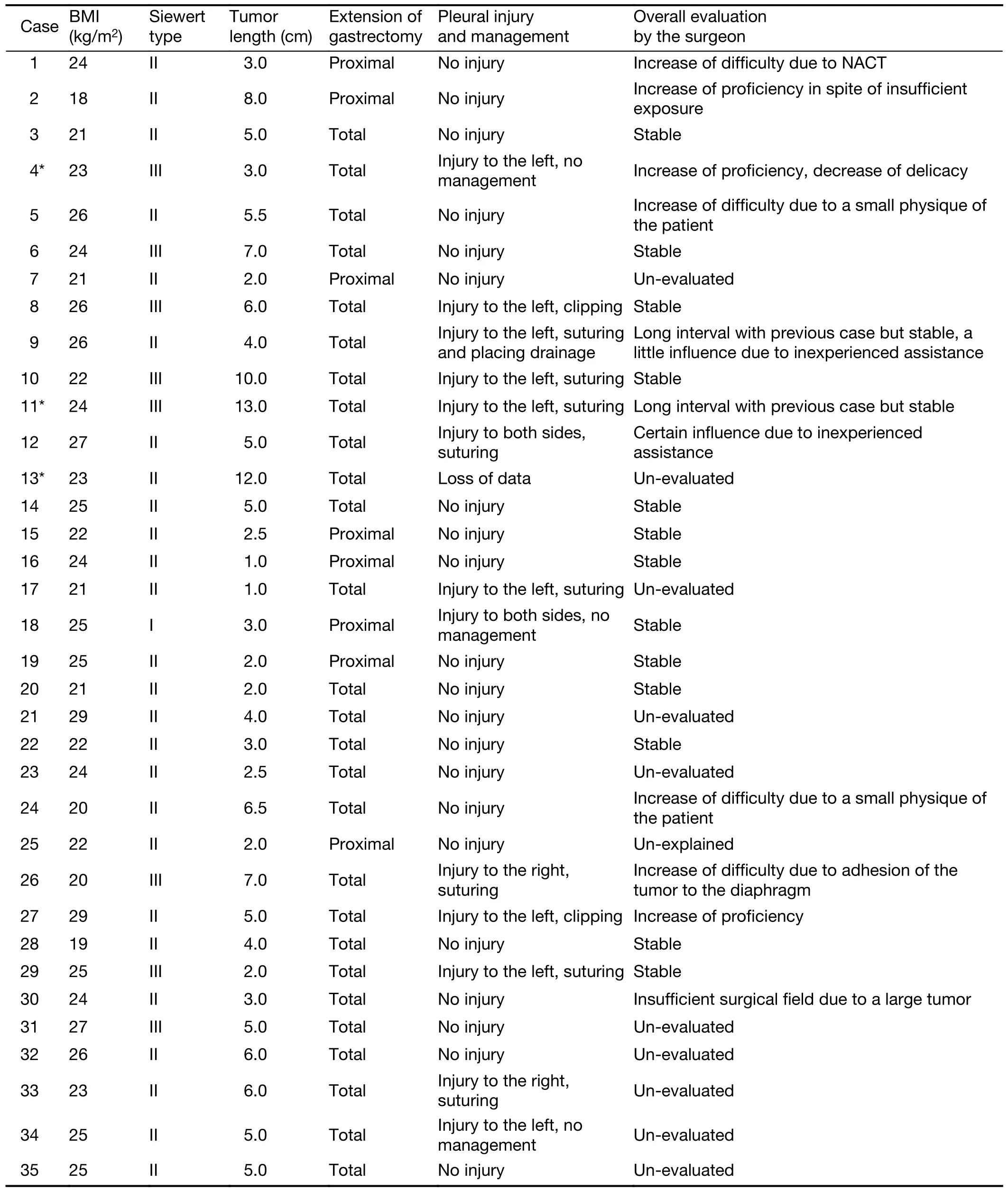
Table S1 Characteristics of each case

Table S2 No.of points under each sub-theme






Table S4 Postoperative recovery
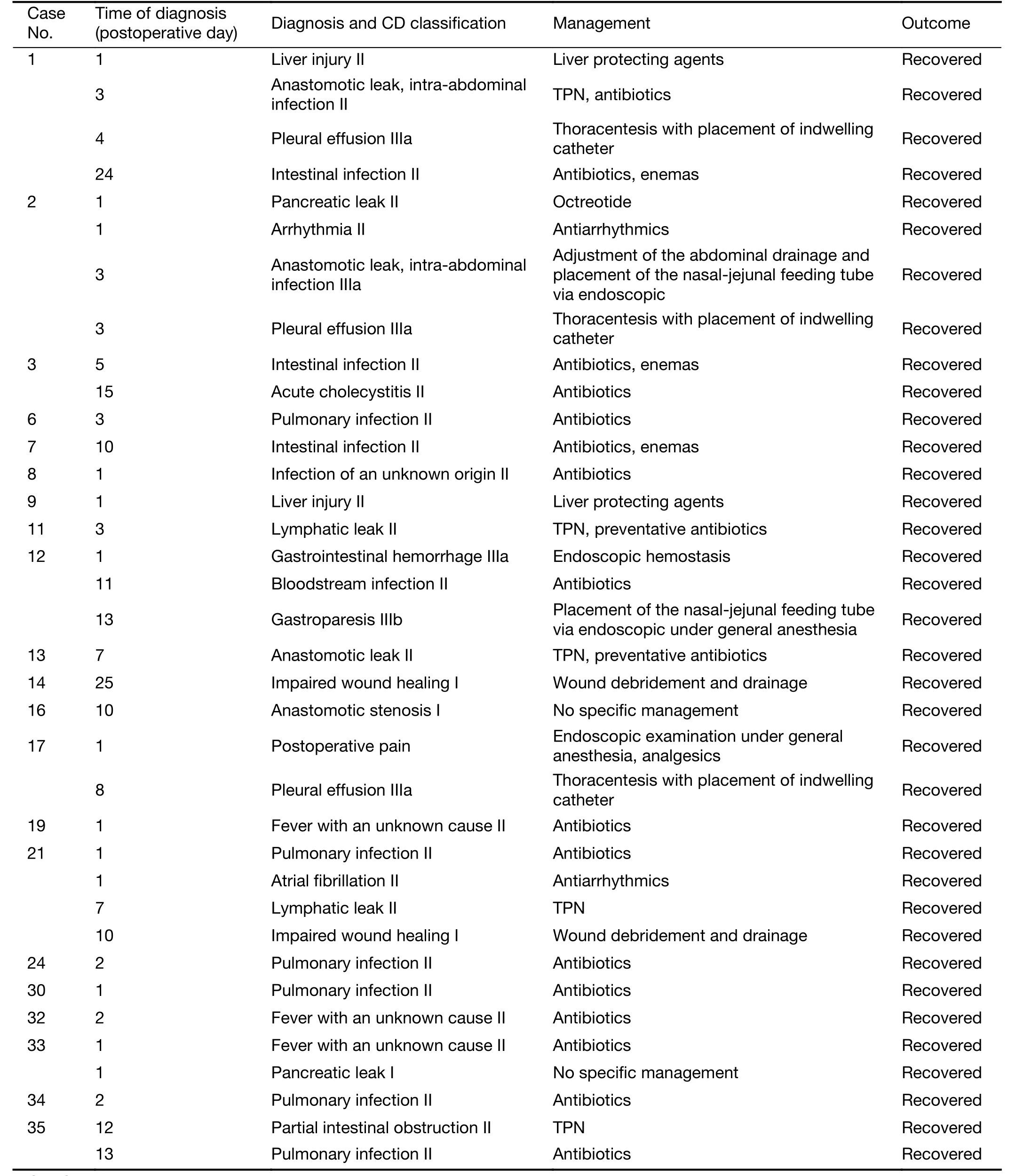
Table S5 Postoperative complications by case

Figure S1 Transcriptome analysis of A-GSP treated cells.(A) Differential gene volcano plot.There are 1,000 μg/mL drug-treated SCC15 cells for 72 h compared with control group;(B) Cluster heat map of differential gene expression between groups in SCC15 cell lines;(C)KEGG pathway enrichment scatter plot.A-GSP,aqueous-soluble sporoderm-removed G.lucidum spore powder;KEGG,Kyoto Encyclopedia of Genes and Genomes.

Figure S2 Serum biochemical indicators of each group of mice.Detection of serum ALB (A),ALP (B),ALT (C),AST (D),CK (E),CRE(F),D-BIL (G),GGT (H),LDH (I),T-BIL (J),TP (K),UA (L) and UREA (M).ALB,serum albumin;ALP,alkaline phosphatase;ALT,alanine transaminase;AST,glutamic oxaloaceti;CK,creatineknase;CRE,creatinine;D-BIL,direct bilirubin;GGT,γ-glutamyl transpeptadase;LDH,lactate dehydrogenase;T-BIL,total bilirubin;TP,total protein;UA,uricacid;UREA,urea.ns,P>0.05.
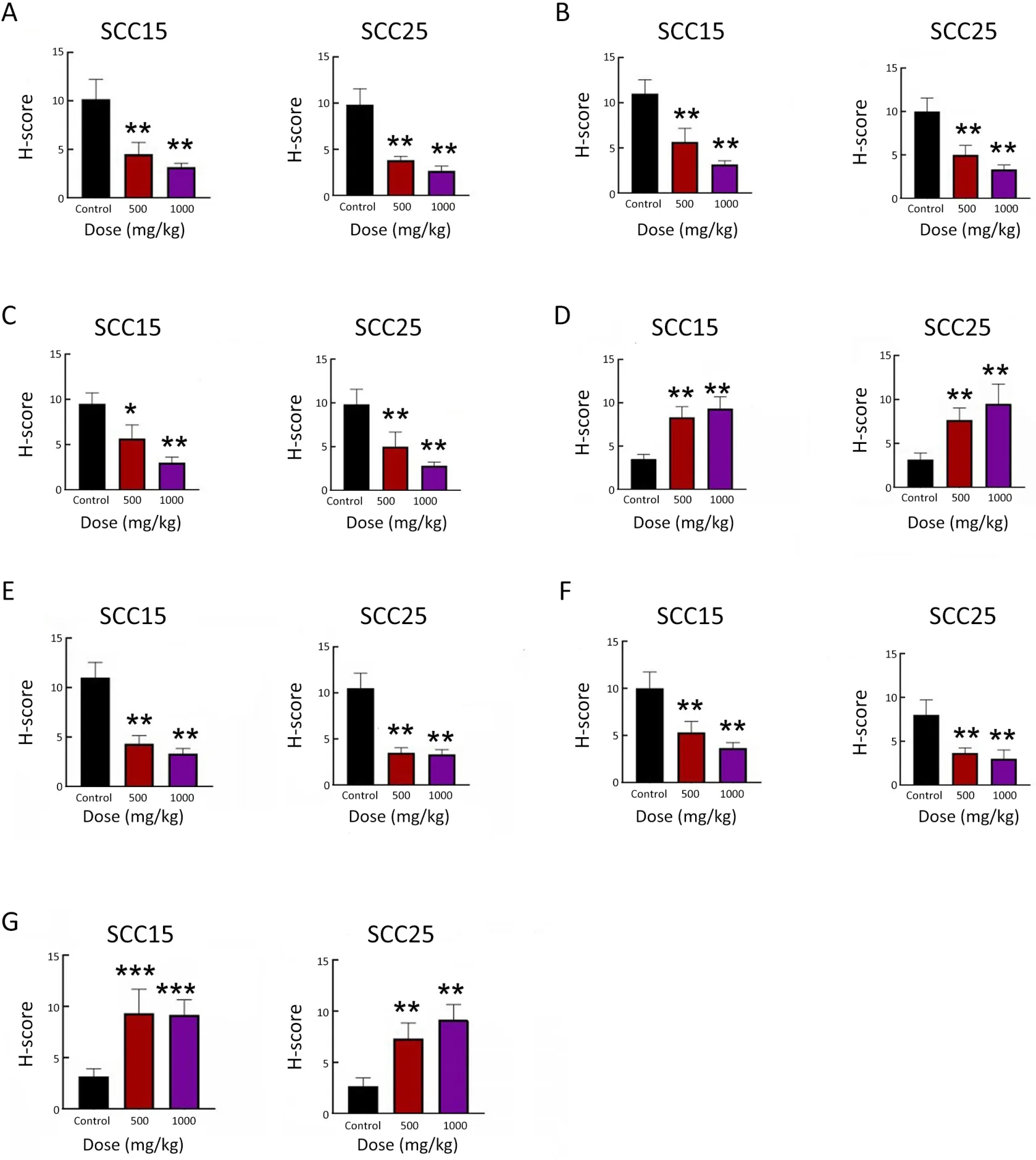
Figure S3 Immunohistochemical scoring of tumor growth markers and ferroptosis molecules.H-score of Ki67 (A),GPX4 (B),SLC7A11(C),ACSL4 (D),FTH1 (E),NRF2 (F) and NCOA4 (G).GPX4,glutathione peroxidase 4;SLC7A11,light chain subunit solute carrier family 7 member 11;ACSL4,acyl-coA synthetase long chain family member 4;FTH1,ferritin heavy chain 1;NRF2,nuclear factor erythroid 2-related factor;NCOA4,nuclear receptor coactivator 4.*,P<0.05;**,P<0.01;***,P<0.001.
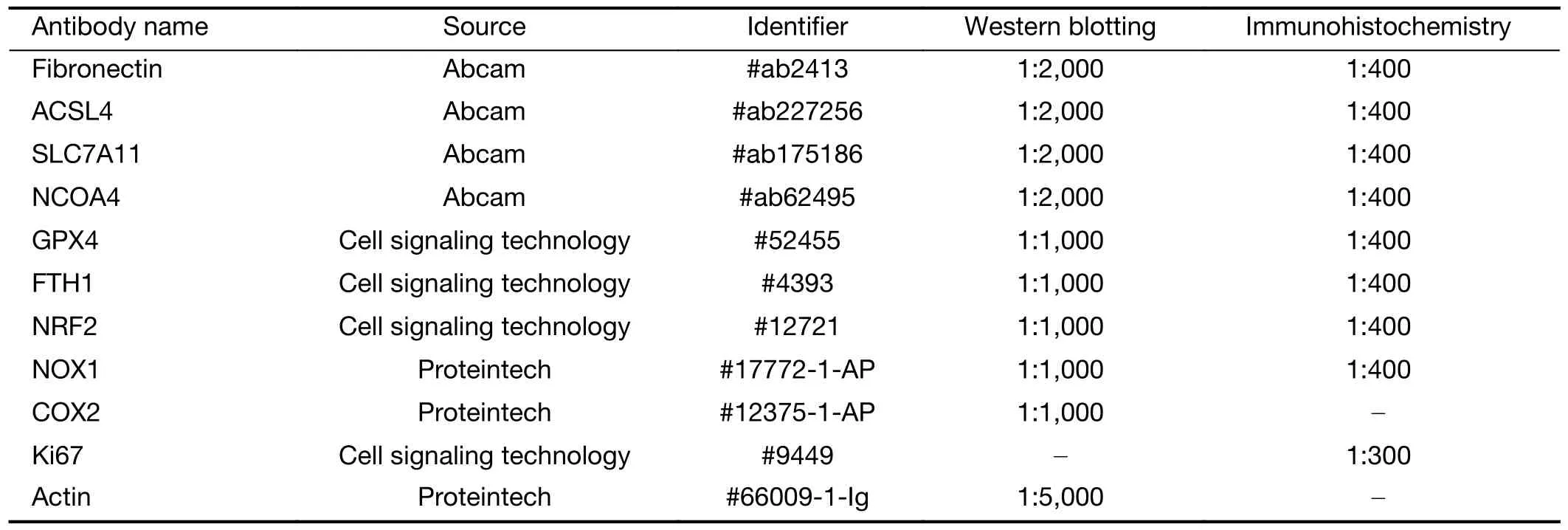
Table S1 Antibody information
 Chinese Journal of Cancer Research2023年2期
Chinese Journal of Cancer Research2023年2期
- Chinese Journal of Cancer Research的其它文章
- Stomach cancer burden in China: Epidemiology and prevention
- When immunotherapy meets liver transplantation for hepatocellular carcinoma: A bumpy but promising road
- Transforming cancer cells for long-term living with cancer:An inspiring new approach
- Integrated strategies for chemotherapy cycles in nasopharyngeal carcinoma patients: Real-world data from two epidemic centers guiding decision-making
- Variant rs8400 enhances ALKBH5 expression through disrupting miR-186 binding and promotes neuroblastoma progression
- Exploration and optimization of surgical techniques for laparoscopic transhiatal lower mediastinal lymph node dissection for adenocarcinoma of esophagogastric junction: A prospective IDEAL 2a study with qualitative design
