Effect of sodium starch octenyl succinate-based Pickering emulsion on the physicochemical properties of hairtail myof ibrillar protein gel subjected to multiple freeze-thaw cycles
Huinan Wang,Jiaxin Zhang,Xinran Liu,Jinxiang Wang,Xuepeng Li,,Jianrong Lia,,
a Key Laboratory of Food Nutrition and Safety, Ministry of Education, College of Food Science and Engineering, Tianjin University of Science and Technology, Tianjin 300457, China
b College of Food Science and Technology, Bohai University, National R&D Branch Center of Surimi and Surimi Products Processing, Jinzhou 121013, China
Keywords: Pickering emulsion Myof ibrillar protein Gel properties Freeze-thaw stability Intermolecular interactions
ABSTRACT A Pickering emulsion based on sodium starch octenyl succinate (SSOS) was prepared and its effects on the physicochemical properties of hairtail myof ibrillar protein gels (MPGs) subjected to multiple freeze-thaw (F-T)cycles were investigated.The whiteness,water-holding capacity,storage modulus (G’) and texture properties of the MPGs were significantly improved by adding 1%-2% Pickering emulsion (P < 0.05).Me anwhile,Raman spectral analysis demonstrated that Pickering emulsion promoted the transformation of secondary structure,enhanced hydrogen bonds and hydrophobic interactions,and promoted the transition of disulf ide bond conformation from g-g-g to g-g-t and t-g-t.At an emulsion concentration of 2%,the α-helix content decreased by 10.37%,while the β-sheet content increased by 7.94%,compared to the control.After F-T cycles,the structure of the MPGs was destroyed,with an increase in hardness and a decrease in whiteness and waterholding capacity,however,the quality degradation of MPGs was reduced with 1%-2% Pickering emulsion.These f indings demonstrated that SSOS-Pickering emulsions,as potential fat substitutes,can enhance the gel properties and the F-T stability of MPGs.
1.Introduction
Suri mi-based products are a popular category of aquatic processed food that offers rich nutrition,resilience,convenience,and cost benef its[1].In general,surimi products are subjected to quick-freezing to inhibit microbial growth and extend shelf life.However,prolonged freezing can degrade the product’s quality due to ice crystallization and recrystallization from repeated freeze-thaw cycles caused by inevitable temperature fluctuations during production,distribution,and sale[2].The reformation of ice crystals candeteriorate the muscle tissue,reduce water-holding capacity (WHC),and denature proteins,impacting the texture and gelation of the surimi product[3].Therefore,improving the surimi product stability during freeze-thaw (F-T) cycles is of great significance to extend product shelf life,reduce losses during transportation and storage,and enhance consumer acceptance.
At present,anti-freezing agents,such as polysaccharides and complex phosphates,are commonly used to reduce damage to frozen food[4].In addition,some emerging techniques,such as ultrasoundassisted freezing,electromagnetic-assisted freezing,and highpressure-assisted freezing,can limit the rate and size of ice crystal formation by better controlling the freezing conditions[5-6].However,these methods are costly.Some studies reported that the addition of protein or starch to surimi gel products signif icantly improves their freezing stability[7-8].With the increasing demand for nutritious and healthy products,f inding new and suitable additives that can reduce freeze damage to surimi products has become the research focus of surimi processing.
Recently,Pickering emulsions stabilized by solid particles have attracted wide attention from various industries for their strong thermodynamic and kinetic stability.Studies showed that solid particles in Pickering emulsions provide electrostatic and spatial repulsion to form thicker interfacial layers or highly elastic interfacial films around oil droplets[9].This viscoelastic layer and gel network structure can effectively prevent damage to emulsion structure from ice crystals[10-11].Therefore,Pickering emulsions may have the potential to maintain the F-T stability of frozen food and we will explore this in this work.As a biodegradable and inexpensive natural polymer,starch is emerging as a promising emulsifier for Pickering emulsions after hydrophobic modification[12].In recent years,octenyl succinate starch (OS-starch) has garnered a great deal of interest in emulsion systems owing to its low cost and excellent emulsification properties,particularly as a granule stabilizer for Pickering emulsions[13].Sodium starch octenyl succinate (SSOS) is a semiesterified product of low-substituted starch with hydrophobic and lipophilic octenyl succinic anhydride[14].Pickering emulsions prepared from OS-starch have been reported to have excellent stability[13-15],and are used as a fat substitute in frozen foods.At present,Pickering emulsions are often used as active substance encapsulants,food packaging materials,and lipid substitutes in a wide range of food applications.However,little is known about their application in surimi product processing.Myofibrillar protein (MP) is the main component in surimi products.The gelation properties of MP significantly affect the quality of surimi products.However,the effect of a starch-based Pickering emulsion on the gelation properties and F-T stability of myofibrillar protein gels (MPGs) is unknown.This study investigated the effects of SSOS-based Pickering emulsions on the physicochemical properties of hairtail MPGs,especially considering the influence of F-T cycles on product stability.The results suggest that Pickering emulsions can be used to improve surimi gelation quality reducing damage from F-T cycles.
2.Materials and methods
2.1 Materials
SSOS (food grade) was provided by Beijing Qingyuan Food Additives Co.,Ltd.,rapeseed oil was purchased from a local supermarket;fresh chilled hairtail was purchased from the aquatic market;all other analytical-grade reagents were purchased from Sinopharm Chemical Reagent Co.,Ltd.(Shanghai,China).
2.2 Preparation of starch-based Pickering emulsion
The starch-based Pickering emulsion was prepared by using sodium starch octenyl succinate (SSOS) nanoparticles as emulsifier and rapeseed oil as oil phase following the method described by Li et al.[16]with some modifications.SSOS suspension (1.5%,m/V)was mixed with 30% rapeseed oil,and the mixture was sheared at 13 000 r/min for 4 min using an Ultra-Turrax T25 high-speed shear (Guangzhou Yike Lab Technology Co.,Ltd.,China) to obtain the primary emulsion.Then the final emulsion was homogenized at 50 MPa for 3 min using a high-pressure homogenizer (Atos Nanotechnology (Suzhou) Co.,Ltd.,China),and the obtained SSOS-based Pickering emulsion was stored at 4 °C for subsequent experiments.
2.3 Extraction of myofibrillar protein (MP)
The MP extraction from hairtail was carried out according to the method by Chin et al.[17]with minor modifications.The chilled hairtail dorsal muscle was stirred,mixed with 4 times the volume of Tris-HCl buffer solution (pH 7.2,10 mmol/L),and homogenized thrice at 13 000 r/min (for 1 min each time) using a homogenizer (Shanghai Fusion,China).The obtained mixture was double centrifuged at 10 000 ×gand 4 °C for 20 min each time using a high-speed centrifuge (Thermo,USA).The obtained precipitate was homogenized by adding 4 times the volume of 10 mmol/L Tris-HCl buffer (pH 7.2,containing 0.6 mol/L NaCl) and the mixture was centrifuged.The obtained supernatant was filtered through 4 layers of gauze,and the filtrate was centrifuged at 7 500 r/min for 15 min.The precipitate (MP)was stored at 4 °C.
2.4 Preparation of SSOS-based Pickering emulsion and MP composite gels (MPGs)
MP was dissolved in phosphate buffer (pH 7.5,20 mmol/L,0.1 mol/L NaCl) to adjust the concentration to 100 mg/mL.Then,SSOS-based Pickering emulsion was added to the MP solution,and the contents of the Pickering emulsion were 0%,1%,2%,3%,and 4% (m/m),respectively[18].The mixtures were placed in gel vials and heated by a two-stage heating method (40 °C/30 min,90 °C/20 min).Next,the gel was rapidly cooled and placed in a refrigerator at 4 °C overnight.
2.5 F-T treatment
The composite MPGs samples were frozen at -20 °C for 48 h and then were completely thawed at 4 °C.The F-T cycle was repeated three times.
2.6 Rheological determination
The rheological properties of SSOS-based Pickering emulsion and MP composite pastes were determined following the method of Wang et al.[19].Each sample (about 1.5 mL) was measured under heating using an AR2000ex rheometer (TA Instruments,Newcastle,USA).A 40 mm diameter parallel plate with a slit spacing of 1 000 µm and a fixed scanning frequency of 0.1 Hz at 2% strain was used to increase the temperature from 20 °C to 90 °C at a heat increasing rate of 2 °C/min.The changes in the storage modulus (G’) and loss modulus(G”) of the samples were recorded,and the temperature scan samples were sealed with paraffin oil to prevent water evaporation.
2.7 Determination of gel strength and textural properties
The gel strength and textural properties analysis (TPA) profiles of the composite gel samples were determined using a TA-XT Plus texture analyzer (Stable Micro Systems Ltd.,UK) and P/5S spherical and P/50 flat plunger probes,respectively,according to our previous method[19].The prepared gels were placed at room temperature(25 °C) for 2 h and cut into 2.5 cm cylinders before fracture evaluation.The test parameters of gel strength were as follows:pre-test and post-test speed,2 mm/s;test speed,1 mm/s;downward pressure height,15 mm,and trigger force,10 g.The gel strength values were calculated by equation (1).
Gel strength (g·mm)=Breaking force (g) × Breaking distance (mm) (1)
Textural parameters (hardness,springiness,gumminess and chewiness) were calculated to estimate the texture of composite gels.The measurement parameters were as follows: test speed,1 mm/s;strain value,40%,and trigger force,5 g.Five parallel experiments were performed for each sample,and the average value was calculated.
2.8 Determination of water-holding capacity (WHC)
WHC was estimated following the method of Li et al.[20].Briefly,the composite gel samples of different treatment groups were accurately weighed and recorded asW1(about 2 g),wrapped well with 3 layers of filter paper,placed in a 50 mL centrifuge tube,centrifuged at 5 000 r/min for 15 min,and then weighed again to record weight asW2.The water-holding capacity was calculated using equation (2).
2.9 Low-field nuclear magnetic resonance (LF-NMR)
The composite gels were sampled with a sampler (10 mm ×10 mm × 20 mm) and loaded into an NMR tube.The Carr-Purcell-Meiboom-Gill (CPMG) spin relaxation time (T2) of the gel samples was determined using a LF-NMR analyzer as in Mi et al.[21].The test parameters were as follows: τ-value,150 μs;the number of echoes(EchoCnt),3 500;proton resonance frequency,22 MHz;scans,8 repetitive scans with an interval time of 4 000 ms.Finally,theT2profiles were obtained by inversion using the instrument-provided software.
2.10 Determination of whiteness
The composite gel samples were equilibrated at room temperature(25 °C) for 2 h,and then,the sample planes were measured using a CR-400 colorimeter (Konica Minolta,Japan) to obtain the values ofL*(brightness),a*(red-greenness) andb*(yellow-blueness).The measurement was repeated five times for each group of samples.The whiteness value was calculated using equation (3)[19].
2.11 Protein secondary structure determination
The secondary structure of MPGs was analyzed according to our previous method[19].The composite gel samples were cut into about 2 mm × 2 mm slices,placed on slides,and analyzed for 400-3 600 cm-1spectra through Raman spectroscopy (Horiba,Japan)at 532 nm,the acquisition time of 30 s,nof 2,and resolution of 1 cm-1.For each sample,three different areas were monitored.Raman spectroscopy data acquisition and processing were performed using Thermo Scientific OMNIC™xi software.The relative percentages of protein secondary structures (α-helix,β-turn,β-sheet,and random coil structural units) were calculated following the method of Alix et al.[22].
2.12 Scanning electron microscopy (SEM)
The microstructure of the composite gel samples was observed by the S4800 field emission scanning electron microscope (Hitachi,Japan).Gel samples were cut into 1-2 mm pieces,fixed with glutaraldehyde solution,washed with phosphate buffer,successively dehydrated with ethanol,freeze-dried,and sputter-coated with gold.The microstructure of the samples was observed at an acceleration voltage of 1 kV and 5 000 × magnification.The SEM images were binarized using Image J software (version 1.8.0,National Institutes of Health,USA) to analyze the 3D network structure of the composite gels and calculate the average porosity.
2.13 Statistical analysis
Statistical analyses were performed using SPSS (version 19.0),and the Duncan method was used for multiple comparisons;P< 0.05 indicates a significant difference.Data are expressed as mean ± standard deviation (SD).The graphs were plotted using Origin (version 9.0).
3.Results and discussion
3.1 Dynamic rheological properties
The dynamic thermo-mechanical technique can be used to monitor the conversion of complex MP paste into the gel matrix.Rheological properties indicate the viscoelasticity of proteins.TheG’ value indicates the elastic modulus of the MP-Pickering emulsion composite system during thermal gelatinization.A higherG’ normally represents better gelation ability with tighter internal structures and better elasticities.Fig.1A showed theG’ values as a function of temperature.In the control group (without F-T),G’ increased slowly between 30 and 45 °C indicating the cross-linking of myosin heads forming a weaker gel network structure[23].G’ decreased rapidly between 45 and 50 °C,this phenomenon can be attributed to endogenous protein hydrolase activity that dissociates myosin fibers disrupting the protein network structure[24].From 50 °C to 70 °C,G’ increased linearly suggesting cross-linking of complex molecular interactions in MP and formation of an irreversible gel network structure.The change inG’ showed a concentration-dependent characteristic of SSOS-stabilized Pickering emulsion,which plateaued at 2% SSOS.Yu et al.[25]demonstrated that the addition of SSOS stabilized the emulsion and improved elasticity (G’) of Pacific cod minced fish because the Pickering emulsion acted as a filler in the gel network[26],hindering protein interactions and cross-linking.As shown in Fig.1B,G” also showed a similar trend for all the samples,whileG’remained significantly higher thanG” suggesting the formation of an elastic-based gel system.
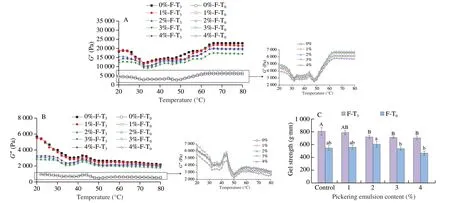
Fig.1 Effect of SSOS-based Pickering emulsion on the (A) G’ and (B) G” of myofibrillar protein before (inset) and after freeze-thaw cycles,and (C) the strength of myofibrillar protein gel before and after freeze-thaw cycles.Different capital and lowercase letters within the same column addition indicate a significant difference (P < 0.05).
BothG’ andG” increased substantially after the samples were subjected to F-T cycles,and the trends were the same among different treatment groups.This can be attributed to the freeze-denaturation of MP during freezing.Studies have shown that aggregation between protein molecules and unfolding of protein polypeptide chains are the two main routes of protein denaturation in fish meat during freezing[5].Before freezing,the bound water in the fresh fish tissue remains firmly and tightly bound to the protein molecules.As the tissue freezing gradually increases,a part of the bound water becomes detached making protein molecules dehydrated causing the aggregation of certain groups.Aggregation between proteins is triggered by the formation of chemical bonds such as disulfide and hydrophobic bonds.In addition,the formation and growth of ice crystals during freezing are not conducive to the orderly aggregation of proteins to form gel structures[27].Compared to the control group,SSOS-stabilized Pickering emulsion showed a lower reduction in the growth ofG’ andG” of the MPGs after F-T cycles treatment.This could be explained that the addition of Pickering emulsions resulting in a larger number of oil droplets immobilized in the gel matrix,which were used as active fillers to enhance the viscoelasticity of the MP matrix[28].
3.2 Changes in textural properties
Textural properties can be used to characterize the structure,histomorphology,and taste of the product.The effect of different concentrations of SSOS-based Pickering emulsion was examined on the hardness,springiness,cohesiveness,and chewiness of MPGs before and after F-T cycles (Table 1).The addition of SSOS-based Pickering emulsion significantly (P< 0.05) improved the hardness and springiness of MPGs within a certain range (1%-2%).This can be attributed to the uniform dispersion of fine emulsified droplets in the protein matrix,which provided the composite gel with better resistance to external forces improving gel hardness.Meanwhile,the cooling regeneration of starch granules in the mixed system after heating also increased gel hardness[29].Also,the increased amount of SSOS-based Pickering emulsion hindered MP cross-linking,weakened the gel network structure,and decreased the hardness.These results are consistent with gel strength.The springiness of the composite gels is closely related to the changes inG’.The cohesiveness of MPGs,which describes how food products resist damage and maintain structure during chewing,decreased with the increase in Pickering emulsion dosage (P< 0.05).The chewiness of food products is related to their hardness,springiness,and cohesiveness indicating the amount of energy required from chewing to swallowing.Similar results were observed by Cen et al.[18],who found that the addition of quinoa protein Pickering emulsion could improve the hardness,gumminess,and chewiness of golden threadfin bream MPGs,which was due to the increase of hydrophobic interaction,secondary structural changes and cross-linking of quinoa protein and MP.
The hardness of MPGs increased significantly (P< 0.05) after F-T cycles.The moisture distribution in the meat tissue shifted during freezing,disrupting hydrogen bonding and hydrophobic interactions mediated by water deteriorating the gel characteristics.Jia et al.[30]reported that the hardness of control surimi gels and starch-surimi gels increased obviously,under both quick and slow freezing(P< 0.05).It was suspected that surimi gels became harder because of the increased protein concentration caused by the pressure from ice crystals on the protein gel during frozen storage and drip loss after thawing.Furthermore,the formation of ice crystals during frozen storage changes the binding state between protein molecules and bound water,and the internal chemical bonds of protein molecules are broken,accompanied by the formation of new bonds,such as disulfide bonds,hydrophobic interactions,ionic bonds and hydrogen bonds.At the same time,the extrusion of ice crystals forces changes in the spatial structure of protein molecules,such as the original highly hydrated state of the protein is altered by the formation,and exposure of hydrophobic groups and changes in the protein polypeptide chains with reduced hydration[31-32].Connell et al.[33]reported that the product quality of fish meat decreased due to increased hardness and decreased WHC upon freezing.Therefore,an increase in gel hardness caused by repeated F-T deteriorates gel quality.Compared with the control sample,the addition of SSOS-based Pickering emulsion reduces the hardness increase of MPG after the F-T cycles,indicating that the addition of emulsion inhibits the deterioration of gel quality.No significant increase in gumminess and chewiness properties occurred in the different samples.
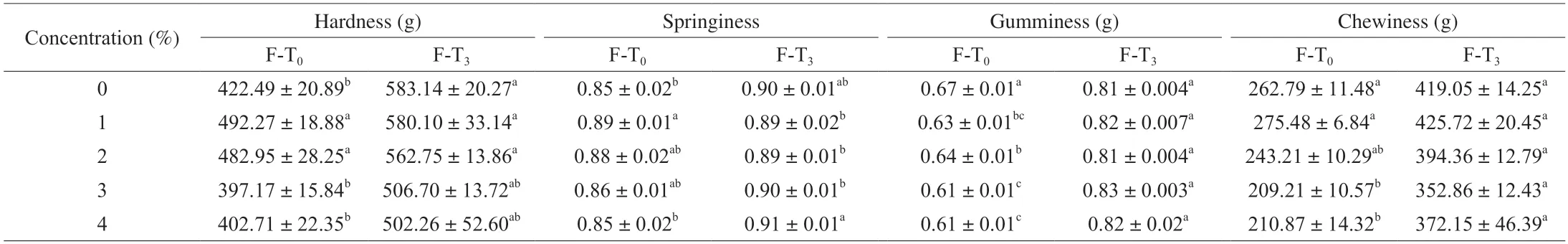
Table 1 Effect of SSOS-based Pickering emulsion on the texture properties of myofibrillar protein gel before and after freeze-thaw cycles.
3.3 Changes in the gel strength of MPGs
Fig.1C showed changes in MP gel strength before and after F-T cycles with additions of SSOS-based Pickering emulsions (0%,1%,2%,3%,or 4%);the gel strength reached the maximum at 2%(605.93 g·mm).The increase in gel strength is related to the crosslinking between MP and SSOS (dextrination and regeneration) in Pickering emulsion,intermolecular interactions between MPs and SSOS,and the excluded volume effect of Pickering emulsion particles in the mixed system.
Specifically,Pickering emulsion fat globules get filled in the MP matrix and participate in gelation during heating.Starch granules(SSOS) in the protein matrix are swelled and pasted during heating,which increases the viscoelasticity of the mixed matrix.Therefore,the presence of emulsified droplets increases the crosslinking between protein molecules in MPGs contributing to gel strength.In addition,Fan et al.[34]reported that the interaction between amylose and MP enhances the gel network structure.Yu et al.[25]showed that emulsion droplets stabilized by ovalbumin and SSOS act as active fillers improving gel properties of the emulsified surimi gels.This is because the interfacial layer between oil and water interacts with the protein continuous phase or directly participates in the formation of the MPGs network.However,the addition of 3% emulsion significantly decreased the gel strength (P< 0.05) possibly due to the limited protein wrapping of emulsified droplets[35].Pickering emulsion as a dispersing phase may destroy the continuous phase matrix of MP,affect cross-linking between MPs and reduce gel strength.After F-T cycles,the gel strength of all samples tended to increase,which is consistent with gel hardness test results confirming the deterioration of MPGs quality.However,the addition of Pickering emulsion slowed the increase in gel strength;the minimum protein gel strength increase was 300.6 g·mm at 3% emulsion addition.Buamard et al.[36]showed that lipid oxidation products (especially aldehydes or ketones) produced by the oxidation of oil during freezing and storage of emulsion gels were able to induce protein cross-linking,thus increasing the breaking force of surimi gels.Furthermore,when the oil is pre-emulsified to form an emulsion,the oil droplets in the emulsion are less susceptible to oxidation by the interfacial film,thus reducing the fracture that occurs in the gel structure.Despite some disruption in the initial gel structure,the addition of the SSOS-based Pickering emulsion improved the stability of test samples compared to the control group.
3.4 Changes in WHC of MPGs
Moisture content is closely related to tenderness,juiciness,and textural characteristics of meat products.During the heat treatment,the cross-linking of myosin creates a three-dimensional network structure,which improves the binding of water molecules[37].As shown in Fig.2A,with the increasing concentration of SSOS-based Pickering emulsion,the WHC of MP composite gel first increased and then decreased.2% SSOS-Pickering emulsion produced the maximum WHC of 63.06%,which is consistent with the gel strength results.Also,the SSOS-based Pickering emulsion has a viscosity and filling effect,which limits water migration reducing water precipitation in the protein gel during heating.However,the ability of MP in the gel network to wrap emulsion droplets may be limited.As a result,the excess emulsion may interfere with the formation of composite gel structures,and the WHC decreases[35].This is consistent with the findings of Xu et al.[38],showing that the increasing amount of diacylglycerol pre-emulsion from 1% to 5% first increased the WHC of surimi gel and then decreased slightly if the emulsion amount reached 7%.Wang et al.[39]used cellulose nanoparticles as a stabilizer and palm oil as the oil phase (instead of fat) to prepare a Pickering emulsion for sausages,which effectively increased the sausage moisture content making it juicier.
After three F-T cycles,the WHC of MPGs dropped significantly(P< 0.05) because water molecules immobilized in the protein gel network froze into ice crystals at low temperatures.This reduced the amount of immobilized water decreasing the WHC of protein gels.The WHC of MPGs without Pickering emulsion dropped by 11% after F-T cycles (Fig.2A).It has been reported that F-T cycles may severely affect the water loss of meat[40].In addition,the solid particles in the emulsion get dispersed into the MPG network structure producing a thickening effect that reduces damage to the gel network structure and water loss from ice crystals.3% SSOS-Pickering emulsion produced the least decrease in WHC of MPGs (3.54%).These results suggested that the starch-based Pickering emulsion may help stabilize the water in the MPGs system.
3.5 Changes in water distribution within MPGs
T2is the time required for the sample to return to equilibrium after being subjected to an external transient disturbance in relation to the binding force of the hydrogen proton and its degree of freedom.TheT2relaxation curves of MPGs with different amounts of Pickering emulsion are shown in Fig.2B.Four hydrogen proton peaks are present inT2.T21represents the monolayer of water bound to polar groups on the surface of macromolecules;T22represents the bound water inside the gel network;T23represents the moderate mobilized water (immobilized water) trapped in the gel network,accounting for more than 80% of the total water content of the gel;T24is the free water,which is free to move and lies outside the MP network structure[41].Compared with the control samples,theT23andT24relaxation time peaks of the composite gel underwent a discernible change,indicating moisture migration in the gel.The distribution and binding state of the hydrogen protons in the composite MPGs were analyzed by accumulating the peak areas in the integrated spectra(Fig.2B).P21+P22represents the peak area of bound water (Fig.2C).At emulsion amount > 1%,the amount of bound water and immobilized water increased significantly (P< 0.05),which indicated that the addition of SSOS-based Pickering emulsion improved the binding of MPGs structure to water molecules.P23represents the relaxation peak area of immobilized water (Fig.2D).The addition of SSOS-based Pickering emulsion significantly increasedP23(P< 0.05)due to an increase in the gel strength of MPGs,which is consistent with the change in WHC.P24represents the relaxation peak area of free water (Fig.2E).The addition of 1%-2% SSOS-based Pickering emulsion reduced free water levels,but the changes were not significant (P> 0.05).
After three F-T cycles,the amount of immobilized water (P23)in the protein gel dropped significantly,while the amount of free water (P24) increased.This was due to the recrystallization of water molecules in the protein gel during the F-T cycles,which destroys the protein gel network structure.Fan et al.[42]reported that the F-T cycle induced the transfer of immobile water to free water to a certain extent,reducing the water abundance in the surimi gel.This indicated that the protein binding becomes loose and the water loss phenomenon occurs.Interestingly,the addition of SSOS-based Pickering emulsion delayed water migration to a certain extent.In all,mechanical damage caused by the recrystallization and redistribution of ice crystals is the main driving force for water transfer and loss in MPGs.
3.6 Changes in the microstructure of MPGs
The uniformity and compactness of the gel network structure largely determine the WHC and gel properties.Fig.3A showed the microstructural changes in MPGs before and after F-T cycles with different concentrations of SSOS-based Pickering emulsion.MPGs without emulsion exhibited a loose structure with numerous pores in the middle of a continuous matrix.The addition of 2%SSOS-based Pickering emulsion made the gel structure more compact and homogeneous,and significantly reduced the number of gel pores.This indicated that SSOS molecules adsorbed on the surface of oil droplets increased cross-linking between myofibrillar protein molecules.Also,SSOS molecules not encapsulated on the oil droplet surface can be embedded in the MPGs network structure via hydrophobic interactions[25]occupying voids in the 3D gel network structure.However,at 3% emulsion,the surface microstructure of MPGs became rough and loose with larger pores.This indicated that higher amounts of emulsion hindered the formation of the MP network structure.Meanwhile,microstructure images can more intuitively verify the changes in gel strength and WHC of MPGs.
Microstructures of all samples were damaged to varying degrees after F-T treatments (Fig.3A).This was especially noticeable in the control group showing large cracks,the continuous gel matrix was divided,and the 3D structure appeared loose and rough.The repeated F-T cycles generated ice crystals,which destroyed the protein gel network structure[42],resulting in a faster rate of textural change.However,the addition of 1%-2% Pickering emulsion reduced such changes by increasing WHC,reducing the formation of larger ice crystals,and maintaining the original gel structure[43].It was reported that the addition of appropriate amounts of Pickering emulsion reduced the damage of ice crystals to muscle proteins,decreased the increase of solute concentration in surimi gels caused by ice crystal,inhibited the generation of free radicals and the oxidation of sulfhydryl groups and carbonylation of proteins,delayed the oxidation,denaturation,and aggregation of myofibrillar proteins,and thus attenuated the damage of gel structure by freeze-thaw cycles[44].

Fig.3 (A) Effect of SSOS-based Pickering emulsion on the microstructure of myofibrillar protein gels before and after freeze-thaw cycles.(B) Binarized gel microstructure images of gels (C) and the porosity of gel samples.Magnification: × 2 000.Different capital and lowercase letters within the same column addition indicate a significant difference (P < 0.05).
Image binarization can help visualize the topographic profile of a sample and has been applied to SEM images of hydrocolloid matrices.Further analysis of binary images of gel microstructure showed that with the increase in Pickering emulsion (Fig.3B),the mean area fraction of gel samples first decreased and then increased(Fig.3C).Compared to other treatments,the addition of 2%SSOS-based Pickering emulsion significantly lowered the gel porosity(40.41%,41.95%) of MPG before and after F-T cycles,which is consistent with the aforementioned results.
3.7 Changes in the color of MPGs
The whiteness value is an important sensory quality indicator of surimi products.L*,a*,andb*denote the sample brightness,red-greenness,and yellow-blueness,respectively.As shown in Table 2,the whitness vaule,L*,a*,andb*of MPFs increased significantly after emulsion addition (P< 0.05).Fang et al.[45]also demonstrated that the addition of emulsified lard improved theL*and whiteness values of the composite surimi gel.On one hand,the emulsion itself is creamy,which improves the whiteness of MPGs.On the other hand,oil droplets encapsulated within the emulsion get uniformly dispersed in the gel matrix enhancing light scattering and brightness[28].The whiteness of all gels decreased after F-T cycles(P< 0.05).TheL*andb*values of all samples tended to decrease anda*tended to increase.The protein network structure was disturbed by dehydration-condensation,which lowered the water retention capacity of protein gels reducing light reflection.In addition,after three F-T cycles,a*increased andb*decreased due to oil oxidation and pigmentation[46].The whiteness of different experimental groups tended to decrease but the effect was slowed upon Pickering emulsion addition.This may be due to the filling effect of the Pickering emulsion and the enhanced light scattering effect on the gel surfaceby the oil droplets in the emulsion[28,47].These results indicated that starch-based Pickering emulsion can inhibit the deterioration of protein gel chromaticity.
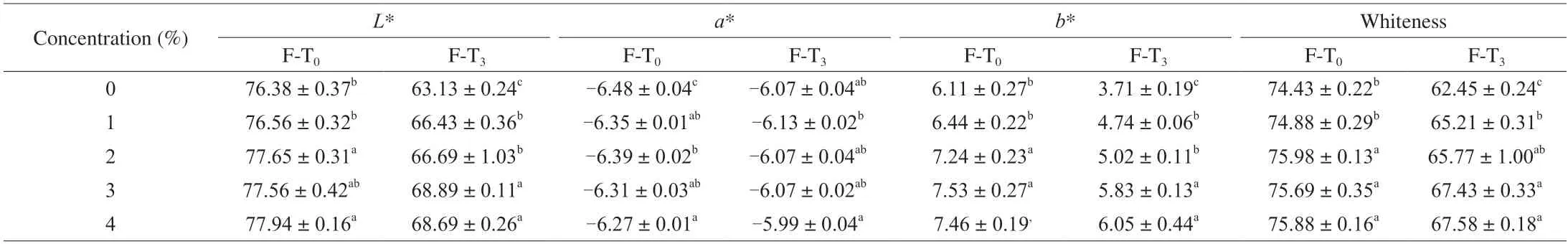
Table 2 Effect of SSOS-based Pickering emulsion on the whiteness of myofibrillar protein gel before and after freeze-thaw cycles.
3.8 Changes in the secondary structure of MPGs
Raman spectroscopy can detect changes in protein secondary structures based on vibrational frequencies of amino acid side chains[48].The effects of different SSOS-based Pickering emulsion concentrations on change in secondary structures of MPGs are shown in Fig.4A.The proportions ofα-helices andβ-sheets in MPGs changed significantly between different treatment groups,whileβ-turns and random coils did not show significant changes.During gel formation,MP is thermally denatured,α-helices unwind exposing hydrophobic residues that may get converted intoβ-sheets to stabilize the protein structure[49].The lowest percentage ofα-helices and the highest percentage ofβ-sheets were observed with the addition of 2%SSOS-based Pickering emulsion,which is consistent with the results for gel strength.The dissociation ofα-helices facilitates the formation of protein gel structures.β-sheets have a large specific surface area and weak hydration strength,which facilitates cross-linking between proteins and the formation of gel network structure[50].
The results of Figs.4A and B showed that after three F-T cycles,the proportion ofβ-sheets andβ-turns in MPG decreases,while the proportion ofα-helices increases.This indicated that F-T cycles destroyed the ordered structure in MPs reducing their stability,which was consistent with the changes in gel texture properties.Zhou et al.[47]and Guo et al.[51]also demonstrated that the gel properties of surimi were positively correlated to the relative content ofβ-sheets and negatively correlated to the relative content ofα-helices.Compared with the other groups,3% emulsion addition allowed the lowestα-helical changes to increase by just 0.07%,while theβ-sheet proportion remained the highest at 25.50%.

Fig.4 Effect of SSOS-based Pickering emulsion on the secondary structure and disulfide bonds conformation of myofibrillar protein (A,C) before and (B,D)after freeze-thaw cycles.
3.9 Changes in disulfide bond (S-S) conformation of MPGs
Disulfide bonds are the main force for maintaining the tertiary structure of proteins.Protein Raman spectra of 500-550 cm-1are characteristic bands of disulfide bonds that can be used to study the conformational changes of disulfide bonds in mixed protein gels.Among them,the spectral peak around 500-515 cm-1is assigned to thegauche-gauche-gauche(g-g-g) configuration,the peak around 515-525 cm-1to thegauche-gauche-trans(g-g-t) conformation and the peak around 525-545 cm-1to thegauche-gauche-trans(t-g-t)conformation[52-53].As shown in Fig.4C,the addition of Pickering emulsion promoted the transformation of the protein disulfide bond conformation fromg-g-gtog-g-tandt-g-tconformations.It is generally believed thatt-g-tis a typical intermolecular disulfide bond configuration,g-g-gandg-g-tare intramolecular disulfide bond configurations[53],andg-g-ghas lower potential energy,making it the major disulfide bond conformation in proteins.The results showed that the addition of Pickering emulsion promoted the transition of MP from intramolecular to intermolecular conformation,whereas too much addition would reduce its intermolecular interaction.Li et al.[54]reported that 9 of the 16 cysteine residues in MHC are located in S1(a component structure of myosin),which has a very open structure and is susceptible to intramolecular S-S bonds formation under oxidation,allowing other cysteine residues in an unraveled rod to participate in intermolecular cross-linking through SH-SS interchange reactions.This might explain the pathway by which pickering emulsion affects the SH-SS reaction in the MP system.After the F-T cycles (Fig.4D),the relative content of theg-g-gconfiguration in the control group without the Pickering emulsion increased,while the relative content of thet-g-tconfiguration decreased,indicating that the F-T cycle destroyed part of the protein intermolecular disulfide bonds and reduce its intermolecular interactions[55].The addition of Pickering emulsion resulted in the change in the disulfide bond conformation,indicating that the Pickering emulsion filled the voids in the MP gel network and enhanced the stability of the MP gel structure.
3.10 Changes in hydrogen bonds conformation of MPGs
The intensity ratio of the two peaks (I850/I830) is the Fermi bimodal,which can be used to characterise the microenvironment of tyrosine residues and the nature of the hydrogen bonds formed by the phenolic hydroxyl group of Tyr[56].A highI850/I830ratio (0.9-2.5) indicates that the tyrosine residues are exposed to an aqueous solution or other polar microenvironments,or that they are acting as both hydrogen bond acceptors and moderate hydrogen bond donors[48].In contrast,a low relative ratio indicates that the tyrosine residues are buried in hydrophobic environments,or that they are only acting as hydrogen bond donors to enhance hydrogen bonding.As shown in Table 3,with the addition of Pickering emulsion,the ratio ofI850/I830in MPGs increased,suggesting that the polarity of the system environment was enhanced.The intensity of the control group was 0.988,indicating that the tyrosine residues in MPGs were buried in a hydrophobic environment[57].This may be due to the difficulty of MP gels with a highα-helix content to unfold.When the amounts of Pickering emulsion added were 1% and 2%,the hydrogen bond strength increased to 1.001 and 1.006,respectively.Changes in protein secondary structure may have exposed tyrosine residues to bind to H2O as hydrogen bond acceptors or donors,resulting in an increase in hydrogen bonds.In particular,bothα-helix andβ-sheet structures are stabilised by hydrogen bonds,so the changes in hydrogen bonds also verify the changes inα-helix andβ-sheet structures in Fig.4A.After F-T cycles,the corresponding decrease in theI850/I830ratio indicates that the degree of encapsulation of tyrosine residues increases,and the dehydration condensation induced by freeze-thaw cycles disrupts the gel network structure and also causes freeze-concentration of the gel system proteins and changes in the system microenvironment;however,there is no significant difference between the different groups (P> 0.05).
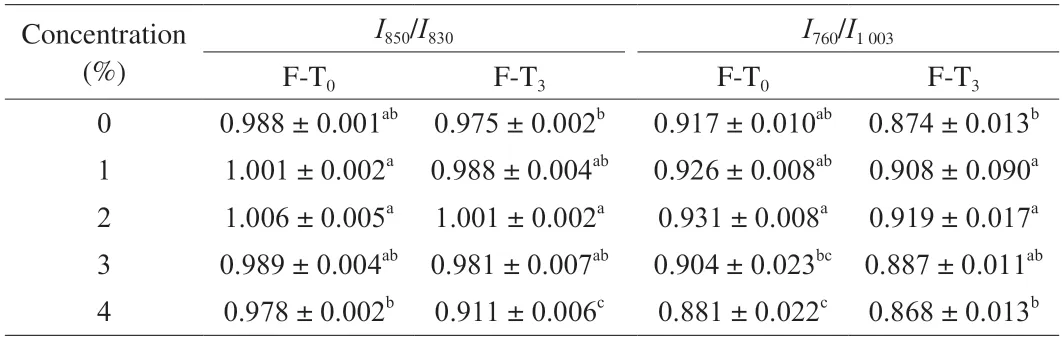
Table 3 Effect of SSOS-based Pickering emulsion on the hydrogen bond (I850/I830) and hydrophobic interaction (I760/I1003) of myofibrillar protein gel before and after freeze-thaw cycles.
3.11 Changes in the hydrophobic interaction conformation of MPGs
The normalised intensity values of Raman spectraI760/I1003can be used to reflect the hydrophobic interactions of MPGs[18].As shown in Table 3,with the addition of Pickering emulsion,the hydrophobic interaction of MPGs first increased and then decreased compared with the control group,and our previous study showed that hydrophobic interaction is the most important intermolecular interaction for the formation of protein gel network force[19],which is consistent with the previous results of gel strength (Fig.1C).The secondary structure change results (Fig.4A) showed that the addition of the emulsion promoted the transition of the MP secondary structure,exposing the MP hydrophobic groups and enhancing the hydrophobic interaction in the MPGs.It has been reported that theg-g-gconfiguration in disulfide bonds may hinder the exposure of hydrophobic groups,and the increase in the hydrophobicity of the protein surface may be related to the increase in the relative content of thet-g-tconfiguration[18].After adding Pickering emulsion,theg-g-gconfiguration in the disulfide bond was converted to theg-g-tandt-g-tconfigurations,verifying our previous findings.After the F-T cycles,the hydrophobic interaction of MPGs decreased to a certain extent,which may be related to the loss of immobile water in the network structure caused by the destruction of the gel structure by recrystallisation.The smallest change in hydrophobic interaction (0.12) was observed for the addition of 2%Pickering emulsion compared with the control group,which also indicated that the addition of Pickering emulsion could to some extent slow down the degradation of MPGs during the F-T cycles.
4.Conclusion
Pickering emulsion could improve the stability and water retention of the gel network before the F-T cycles.The addition of 1%-2% SSOS-stabilised Pickering emulsions significantly improved the whiteness,viscoelasticity,gel strength and WHC of MPGs.At an emulsion concentration of 2%,the gel strength maximized at 605.93 g·mm,an increase of 51.7 g·mm as compared to the control group.Meanwhile,the addition of SSOS-stabilised Pickering emulsionspromoted the transformation ofα-helices intoβ-sheets,increased the hydrogen bonding and hydrophobic interactions,and contributed to the transition of disulfide bond conformation from low potential energy (g-g-g) to high potential energy conformation(g-g-t,t-g-t).After the F-T cycles,recrystallisation and freezing shrinkage of the proteins disrupted the network structure of the gels,manifested as increased gel hardness and chewiness,decreased WHC,viscoelasticity,whiteness (P< 0.05),hydrogen bonding and hydrophobic interactions.However,the addition of 2% Pickering emulsion reduced the quality deterioration of MPGs and improved the freeze-thaw stability.These findings suggest that Pickering emulsions may have a role in protecting the gel structure and reducing damage to MPGs after freezing and thawing.This study highlights the possible application of Pickering emulsions in the processing of high-quality surimi products or other minced meat products.Specific interactions between starch-based Pickering emulsions and MP,as well as the flavor and nutrition characteristics in the composite system,should be further explored in future work.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (U20A2067,32272360).The authors would like to express gratitude to all the reviewers who participated in the review,as well as MJEditor (www.mjeditor.com) for providing English editing services during the preparation of this manuscript.
- 食品科学与人类健康(英文)的其它文章
- Protective effects of oleic acid and polyphenols in extra virgin olive oil on cardiovascular diseases
- Diet and physical activity inf luence the composition of gut microbiota,benef it on Alzheimer’s disease
- Inf luence of nitrogen status on fermentation performances ofnon-Saccharomyces yeasts: a review
- Ganoderma lucidum: a comprehensive review of phytochemistry,eff icacy,safety and clinical study
- Resveratrol combats chronic diseases through enhancing mitochondrial quality
- Demonstration of safety characteristics and effects on gut microbiota of Lactobacillus gasseri HMV18

