Novel umami peptides from two Termitomyces mushrooms and molecular docking to the taste receptor T1R1/T1R3
Lanyun Zhang,Li Zhang,Jsus Pérz-Morno,Lu Bin,Fngming Zhang,Fuqiang Yu
a The Germplasm Bank of Wild Species, Yunnan Key Laboratory for Fungal Diversity and Green Development, Kunming Institute of Botany,Chinese Academy of Sciences, Kunming 650201, China
b College of Chemical Science and Technology, Yunnan University, Kunming 650091, China
c College of Food Science and Technology, Yunnan Agricultural University, Kunming 650201, China
d Colegio Postgraduados, Campus Montecillo, Edafología, Texcoco 56230, México
e Key Laboratory for Forest Resources Conservation and Use in the Southwest Mountains of China, Southwest Forestry University, Kunming 650224, China
Keywords: Termitomyces Non-volatile f lavor compounds Umami peptides Taste characteristics Molecular docking
ABSTRACT Wild edible Termitomyces mushrooms are popular in Southwest China and umami is important f lavor qualities of edible mushrooms.This study aimed to understand the umami taste of Termitomyces intermedius and Termitomyces aff. bulborhizus.Ten umami peptides from aqueous extracts were separated using a Sephadex G-15 gel f iltration chromatography.The intense umami fraction was evaluated by both sensory evaluation and electronic tongue.They were identif ied as KLNDAQAPK,DSTDEKFLR,VGKGAHLSGEH,MLKKKKLA,SLGFGGPPGY,TVATFSSSTKPDD,AMDDDEADLLLLAM,VEDEDEKPKEK,SPEEKKEEET and PEGADKPNK.Seven peptides,except VEDEDEKPKEK,SPEEKKEEET and PEGADKPNK were selectively synthesized to verify their taste characteristics.All these 10 peptides had umami or salt taste.The 10 peptides were conducted by molecular docking to study their interaction with identif ied peptides and the umami taste receptor T1R1/T1R3.All these 10 peptides perfectly docked the active residues in the T1R3 subunit.Our results provide theoretical basis for the umami taste and address the umami mechanism of two wild edible Termitomyces mushrooms.
1.Introduction
In the past decades,mushrooms have been attracted a lot of attention around the world as a medicinal,nutritional and functional food[1].Termitomyces,or termed “termite mushroom” because of the symbiotic relationship with termites in infested soil,are popular in a number of countries including Australia,China,India,Nigeria,etc.[2].For instance,Termitomycesmainly distributed in Yunnan,Sichuan and Guizhou provinces in Southwest China.Termitomyceshave unique and delicious tastes,as being nutritious edible mushrooms for both medicine and food[3].
Termitomycesare a favorite of nutrition richening in vitamins,protein,minerals and other nutrients.Termitomycesare important food to acquire nutrients and keep nutrition balance in some malnutrition areas.Studies have revealed thatTermitomycesgenerate organic acids,cycloalkanes,phenolic compounds,polysaccharides,terpenes,alkenes[4]that can be commercially used for the synthesis of perfumes and cosmetics[5].Furthermore,Termitomyceshave biological activity[6]that are beneficial for our health,regulating blood pressure,blood lipid,immune response,inf lammation and apoptosis,etc.However,at present there are little studies on exploring the mechanism for its unique and delicious tastes.
The umami taste is caused by umami compounds bind to umami taste receptors T1R1/T1R3[7].The predominant f lavor of mushrooms is the umami taste which can enhance or induce overall food flavor.The umami taste of mushrooms is affected by many different factors such as growth stage,storage conditions,processing method and species type[8].The umami of edible mushrooms is notable and favorable,the typical taste is mainly triggered by water-soluble low molecular weight substances of edible mushrooms[9].Therefore,the purpose of this paper was to discover non-volatile flavor compounds,including free amino acid,5’-nucleotides,soluble sugar,organic acid,and umami peptides from the aqueous extract ofT.intermediusandT.aff.bulborhizus.In this study,the isolation and identification of peptides were by using a gel filtration chromatography (GFC),reversed-phase high-performance liquid chromatography (RP-HPLC)and liquid chromatography tandem-mass spectrometry (LC-MS/MS).Then the taste characteristics of selected umami peptides and synthesized peptides were determined by both the sensory evaluation and E-tongue technology.Furthermore,a homology modeling was taken to study interaction between the identified umami peptides and umami taste receptor T1R1/T1R3,and a molecular docking was operated to reveal the molecular mechanism of the flavor umami peptides.The main objective of this study is to address the delicious and umami mechanism of twoTermitomycesand the discovery of new flavor umami peptides from wild edible fungi resources.
2.Materials and methods
2.1 Materials and chemicals
Two species of freshTermitomyces,T.intermedius(TI) andT.aff.bulborhizus(TB),were separately collected from Wuding(25°32’57.33” N,102°24’39.09” E,alt.1 789 m) and Yiliang County(24°54’49.71” N,103°8’13.63” E,alt.1 535 m) in Yunnan,southwest China.Samples ofTermitomyces(HKAS 111931 and 111932) were confirmed to be ofT.intermedius(Accession No.MW391703)andT.aff.bulborhizus(Accession No.MW391704) by molecular identification in Kunming Institute of Botany,Chinese Academy of Sciences and stored at -80 °C for further experiments.Part of experimental samples were dried to a constant weight (drying time of 20 h) at 60 °C using an oven-drying and stored at a desiccator for analysis.The reagents used for RP-HPLC was HPLC grade.Other chemical reagents were analytical grade.All the lyophilized fractions of and synthetic peptides were stored at -80 °C for analysis.
2.2 Extraction of Termitomyces
The extraction ofTermitomyceswas according to previous studies[10]with minor modifications.Approximate 200.0 g fresh samples were minced by a tissue grinder.The minced samples were transferred into a round-bottomed flask and responding in ultrapure water at a ratio of 1:3 (m/m),and again transferred to a heating mantle at 100 °C for 60 min,repeated three times.The samples were then filtered and centrifuged at 10 000 ×gat 4 °C for 15 min.The supernatant was concentrated to 0.4 L by using a rotary vacuum evaporator (B100,BUCHI Shanghai Ltd.,Shanghai,China) and lyophilized for 48 h by a vacuum freeze dryer (Scientz-12N,Ningbo Scientz Biotechnology Co.,Ltd.,Ningbo,China).The freeze-dried powder fromT.intermediusandT.aff.bulborhizuswere stored at-80 °C for further experiments.
2.3 Non-volatile flavor components
2.3.1 Freeaminoacidanalysis
The contents of free amino acids in twoTermitomyceswere quantified by a modified method[11].An aliquot (1.0 g) lyophilized powder of TI or TB was hydrolyzed with 50 mL of 0.01 mol/L hydrochloric acid and stirred.After 30 min,the extracts were filtered,and 2 mL supernatants were mixed with 2 mL 8% sulfosalicylic acid.Then supernatants were centrifuged at 3 000 ×gfor 20 min and filtered through a 0.22 µm micro-pore filter membrane.The supernatants were finally assayed by an automatic amino acid analyzer (L-8900,Hitachi Ltd.,Tokyo,Japan).
2.3.2 5’-Nucleotide analysis
These 5’-nucleotides analysis were according to previous studies[12].Briefly,the samples were analyzed by HPLC (254 nm,ZORBAX SB-C18,150 mm × 4.6 mm,5 µm,30 °C,1 mL/min,Agilent 1260 HPLC analysis system),with 10 mmol/L KH2PO4(pH 4.6)as the eluent.Five flavor nucleotides were identified by the calibration curve with 6 different concentrations (2,4,6,8,10,12 mg/L).
2.3.3 Solublesugarandpolyols
The soluble sugars were analyzed as described by Beluhan and Ranogajec[13]with minor modifications.The TI and TB sample (5 g)were ultrasonically extracted for 30 min by 5 mL of zinc acetate and potassium ferrocyanide that were added with ultrapure water to 100 mL.The supernatants were filtered by a 0.45 µm cellulose membrane.The samples were loaded onto an XBridge Amide column (Waters,4.6 mm × 250 mm,3.5 µm) at 50 °C.Soluble sugar and polyol were confirmed by the reference standard compound and quantified by calibration curves.
2.3.4 Organicacids
Organic acids were analyzed with a modified method from Gu[14].The TI and TB sample (5 g) were extracted with 10 mL 80% ethanol and centrifuged at 5 000 r/min for 15 min.Then supernatants were filtered through a 0.45 µm micro-pore filter membrane and then analyzed by 1100 HPLC (Agilent Technologies,USA) with a UV detector at wavelength 210 nm.The mobile phase was potassium dihydrogen phosphate (0.01 mol/L) at a flow rate of 1 mL/min.The injected volume was 20 µL and loaded onto a BEH-C18column (2.1 mm × 50 mm,1.7 µm,Waters) at 30 °C.Each organic acid was confirmed by a reference standard compound and quantified by a calibration curve.
2.4 Analyses of peptides
The TI or TB lyophilized powder from the section 2.2 dissolved in 2 mL ultrapure water (60 mg/mL) was filtered through a 0.45 µm cellulose membrane.The supernatants were fractionated by a gel filtration chromatography (GFC) column (1.6 cm × 100 cm;Huxi Instrument Co.,Ltd.,Shanghai,China) and then filled with Sephadex G-15 gel (Sigma,Sweden).The eluent was ultrapure water with a 1.0 mL/min flow rate.The samples were monitored by a HD-3 UV detector (Huxi Instrument Co.,Ltd.,Shanghai,China) at 220 nm.Four peaks were separately designated as I1-I4 and B1-B4 for either TI or TB.Effluent from these four peaks (I1-I4,B1-B4) were then combined and lyophilized,and then stored at -80 °C.Next,these fractions were tested by a sensory evaluation and an electronic tongue determination,and the most intense umami taste fractions were further purified by RP-HPLC.
The lyophilized powders of 8 fractions (I1-I4 and B1-B4) were perceived by electronic tongue.Lyophilized powders were dissolved in 2 mL ultrapure water (20 mg/mL) and filtered through a 0.45 µm cellulose membrane.Then,the strongest umami taste fractions I4 and B1 were further separated by RP-HPLC (220 nm,ZORBAX SB-C18,9.4 mm × 150 mm,5 µm) at 35 °C and the sample loading volume was 80 µL.The mobile phase was consisted of 5% solvent A (methanol)and 95% solvent B (ultrapure water containing 0.05% (V/V)trifluoroacetic acid) for 20 min (2 mL/min).The separated peaks were collected and freeze-dried for next experiment.
2.5 Identification of peptides by LC-MS/MS
The identification of umami peptides of I4 and B1 were performed with a Q Exactive Hybrid Quadrupole-Orbitrap(Thermo Fischer Scientifc,Waltham,USA) apparatus connected to a NanoLC Ultimate 3 000 system (Thermo Fischer Scientifc,Waltham,USA).Samples were dissolved in 15 µL of mobile phase A (0.1% formic acid solution).The separation gradient was 66 min from 5% to 30% mobile phase B (0.1% formic acid in acetonitrile).The chromatographic flow rate was 600 nL/min,the peptides were collected fromm/z300-1 800.
The results of taste test of TI and TB (I1-I4 and B1-B4) indicated that the I4 fraction form TI was the most intense umami taste fraction.These identified peptides form I4 were then synthesized (Leon Biotechnology Co.,Ltd.,Nanjing,China) and purity was over 99%.The taste characteristics of these synthetic peptides were further assessed by the experts and electronic tongue.
2.6 Taste test
2.6.1 Electronictongue
The GFC fraction samples and synthetic peptide samples were dissolved in 10 mmol/L KCL (0.2 mg/mL) and measured by the INSENT TS-5000Z (INSENT,Inc.,Kanagawa,Japan).This taste sensor system equipped with five sensor probes,umami and richness taste (aftertaste-umami) caught by AAE,saltiness caught by CT0,sourness caught by CA0,bitterness and aftertaste-bitterness caught by C00,astringency and aftertaste-astringency caught by AE1.
First,samples were separated by GFC from TI (I1-I4) and TB(B1-B4).Then the most intense umami taste fraction I4 and B1 were further purified by RP-HPLC.The synthesized peptides from I4 were tasted by an E-tongue with the method from the above paragraph,three of these synthesized peptides were chosen and added to the fresh chicken soup (2 mg/mL) to further test the taste characteristics of synthesized peptides in soup.
2.6.2 Sensoryevaluationofsyntheticpeptides
The sensory evaluation panelists consisted of 10 members(5 men and 5 women,aged 23-35 years,selected from 23 people)who had previously been trained to comprehend 5 basic tastes(sourness,sweetness,bitterness,saltiness,and umami).All sensory evaluation was performed in a sensory analysis laboratory at (23 ± 2) °C.
The umami taste thresholds of synthetic peptides were determined by a taste dilution analysis (TDA) according to Ottinger and Hofmann[15].These synthetic peptides were dissolved in ultrapure water at 1.0 mg/mL and the solutions were diluted stepwise in ultrapure water (volume ratio of 1:1) with.Until all panelists were not able to identify the peptide solution from one peptide solution and two ultrapure water solutions,the recognition thresholds of synthetic peptides were calculated with the average value of the last and penultimate concentration of the synthetic peptides recorded by panelists.Meanwhile,panelists were asked to describe their feeling of the 5 basic tastes.
2.7 Homology modeling and computational molecular docking of T1R1/T1R3 receptor with the identified peptides
The amino acid sequence of T1R1/T1R3 was retrieved from UniProtKB database (https://www.UniProt.org/).The homology modeling process usually includes three parts,an appropriate template,model construction,optimization and evaluation.In this study,the homology model of the umami receptor was constructed by using the metabotropic glutamate receptor as the template (PDB ID: 1EWK)[16-17].Then,the 3D structures of the umami peptides and optimization of model was by the Discovery Studio (2017 R2;Accelrys Inc.,San Diego,Canada).
Molecular docking was performed according to the method of Dang et al.[18]with slight modifcations.It was performed in CDOCKER protocol and optimized in minimization ligand protocol.
2.8 Statistical analysis
All experiments were performed three times.The results were expressed as means ± SD (n=3).An IBM SPSS Statistics 20.0 version(IBM,Corporation,Armonk,NY) was used to evaluate the experimental data with one-way analysis of variance (ANOVA).Level of statistical significance was set to values ofPless than 0.05 in all tests.
3.Results and discussion
3.1 Free amino acids,5’-nucleotides,and equivalent umami concentrations (EUC) from T.intermedius and T.aff.bulborhizus
Beluhan and Ranogajec[13]showed that non-volatile compounds including free amino acids,5’-nucleotides,soluble sugar and polyols were responsible for the taste of mushrooms,and such free amino acids were classified into 4 groups (umami,sweet,bitter and flavorless).
Total 17 free amino acids were detected from TI and TB (Table 1).The concentrations of total free amino acids,umami,sweet amino acids and bitter amino acids were respectively higher in TB (37.17,11.49,10.03 and 12.36 mg/g dry weight (DW)) than in TI (27.91,9.54,7.44 and 7.61 mg/g DW).The umami and sweet amino acids respond for pleasant taste of food.The bitter amino acids or umami amino acids may represent the main component that influences the taste of TB or TI[15].
Nutritionally,essential amino acids (EAAs) are those that must be acquired in the diet.EAAs are generally insufficiently synthesized to suit physiological function needs for maintenance,growth,development,and health[19].Both TI and TB had 7 types of EAAs,the total EAAs concentrations were significantly higher (P< 0.05) in TB ((14.31 ± 0.10) mg/g DW) than in TI ((9.32 ± 0.03) mg/g DW).Flavor 5’-nucleotides that can contribute unique flavor and umami to mushrooms were identified as 5’-GMP,5’-IMP,and 5’-XMP[18].Here,the concentrations of 5’-GMP and 5’-XMP were calculated into the total umami 5’-nucleotides.
Total 5’-nucleotide was markedly higher in TB ((132.85 ± 0.15) mg/g DW)than in TI ((119.86 ± 0.12) mg/g DW) (Table 2).5’-GMP was the major 5’-nucleotide in TI ((92.14 ± 0.10) mg/g DW) and TB((92.06 ± 0.15) mg/g DW),followed by 5’-CMP ((23.01 ± 0.03) mg/g DW in TI,and (15.42 ± 0.03) mg/g DW in TB),5’-AMP ((2.11 ± 0.01) mg/g DW in TI and (25.37 ± 0.11) mg/g DW in TB).Previous studies have shown that 5’-AMP also provides the sweetness and effectively inhibits the formation of bitterness.[10].The concentrations of 5’-XMP was (2.60 ± 0.02) mg/g DW in TI,but was not found in TB.
Yamaguchi et al.[20]reported that the interaction between MSG-like components and 5’-nucleotides components enhanced the umami taste and EUC values of flavor components.The EUC values of mushroom were divided into 4 levels: 1) > 1 000 g/100 g DW,2) 100-1 000 g/100 g DW,3) 10-100 g/100 g DW,and 4) < 10 g/100 g DW[21].The EUC values was higher in TI (245.68 g/100 g DW) than in TB (196.93 g/100 g DW) (Table 2),explaining that the overall flavor had more umami in TI than in TB.
ai(%) is the concentration of each umami amino acid (Asp or Glu);aj(%) is the concentration of each umami 5’-nucleotide (5’-IMP,5’-GMP,5’-XMP,or 5’-AMP);biis the relative umami concentration(RUC) for respective umami amino acids to MSG;andbjis the RUC for umami 5’-nucleotides to 5’-IMP.
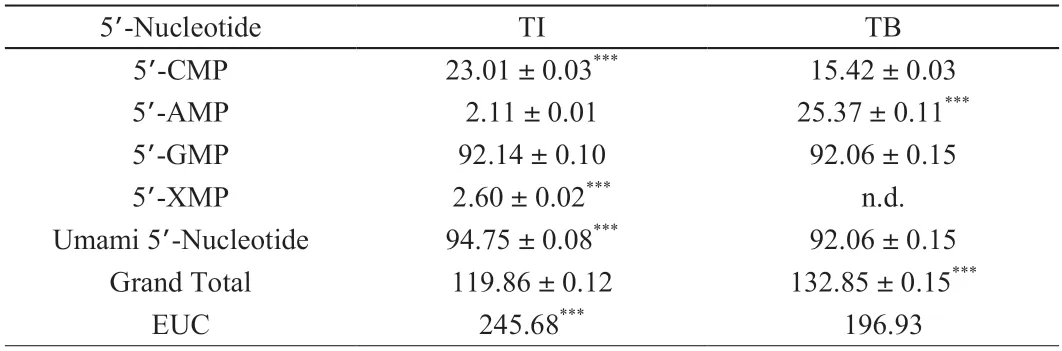
Table 2 Concentrations (mg/g DW) of 5’-nucleotides and EUC value (g/100 g) of TI and TB.
3.2 Determination of organic acids,soluble sugar and polyols
Studies had revealed that the organic acids and soluble sugar were important for the flavor of a little edible mushrooms.Mannitol and other soluble sugars are regarded as a taste-active ingredients that can contribute the sweet perception to mushroom.In addition,organic acids are closely related to the synthesis and metabolism of amino acids,aromatic compounds,esters and phenols[20].
Results from this present study showed that the sucrose (PubChem CID: 5988) was the major soluble sugar in TI ((13.90 ± 0.02) mg/g DW)and TB ((4.72 ± 0.12) mg/g DW) (Fig.1).The concentrations of mannitol,a contributor to the sweet flavor (PubChem CID: 6251)in TI were (2.73 ± 0.04) mg/g DW,but was not detected in TB.In addition,the concentrations of glucose (PubChem CID: 5793)were significantly higher in TB ((4.44 ± 0.17) mg/g DW) than in TI((0.75 ± 0.02) mg/g DW).The total concentrations of soluble sugar were significantly higher in TI ((17.38 ± 0.05) mg/g DW) than in TB sample ((9.17 ± 0.24) mg/g DW).
Results in Fig.1 showed that malic acid (PubChem CID: 525)was the major organic acid in TI ((2.96 ± 0.06) mg/g DW) and in TB((3.21 ± 0.13) mg/g DW),followed by citric acid (PubChem CID:5793,(1.76 ± 0.03) mg/g DW in TI and (1.94 ± 0.01) mg/g DW in TB),tartaric acid (PubChem CID: 444305,(0.05 ± 0.03) mg/g DW in TI and (1.54 ± 0.03) mg/g DW in TB),oxalic acid (PubChem CID:971,0.19 mg/g DW in TI and (0.34 ± 0.02) mg/g DW in TB),succinic acid (PubChem CID: 1110,0.13 mg/g DW in TI,0.26 mg/g DW in TB).The total concentrations of organic acids were significantly higher in TI((6.00 ± 0.06) mg/g DW) than in TB ((7.29 ± 0.17) mg/g DW).
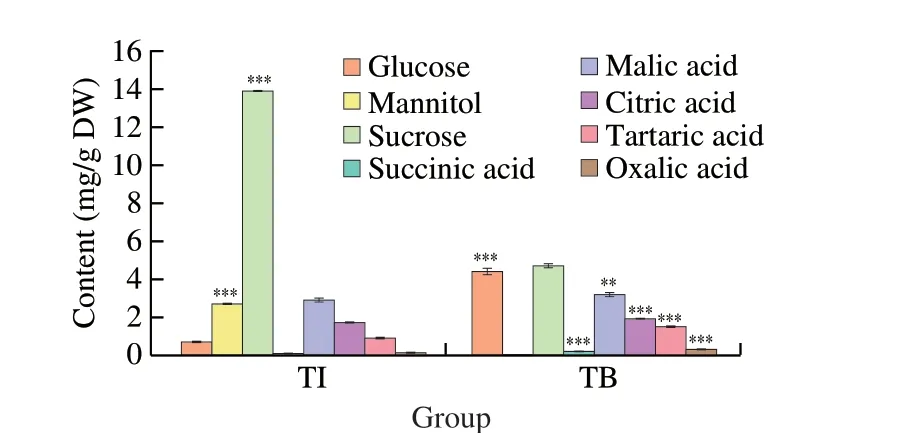
Fig.1 Content of organic acids,soluble sugar and polyols in TI and TB.All values are represented as mean ± standard deviation (SD) of triplicate analysis.**P < 0.05,***P < 0.01.
3.3 Separation and taste characterization of GFC fractions
In this study,GFC were used to separate the extracts of TI and TB.Sephadex G-15 had a good separation effect,and the different fractions were separately collected (Figs.2A and B).According to the absorbance (λ=220 nm),the extracts of TI was divided into 4 fractions (I1-I4,Fig.2A) and the extracts of TB was divided into 4 fractions (B1-B4,Fig.2B).The effluent was separately divided into 8 fractions and separately collected for next analysis.Results of GFC fractions from the electronic tongue,showed significant differences in the umami taste between TI and TB (Figs.2C and D).
All the GFC fractions presented umami taste,followed by the saltiness,and among the 6 basic tastes and 2 aftertastes,sourness was the weakest (Figs.2C and D).Among the 8 fractions,the I4 and B1 had the highest scores of umami intensity (9.18 and 5.81),followed by I3 (6.45),B4 (4.74),I1 (3.0),B2 (4.2),while I2 (1.24) and B3 (1.94)had very low scores.Besides the umami taste,the second strongest electric response was saltiness (Figs.2C and D).I4 had the strongest saltiness (5.32),then were I3 and B1,which scores respectively were 3.49 and 1.57.I4 and B1,with the highest umami intensity but the lowest unpleasant taste,were then further purified by RP-HPLC.
Except pleasant taste,there also existed bitter taste and astringency taste that were got nothing on flavor of mushroom.From Fig.2D,B4 had the intensest bitterness (5.14),followed by B3 (2.7),B2 (1.99)and B1 (1.7),all of them were more bitter than fractions of TI (from-0.29 to 0.74).It was inferred a correlation between the species of edible mushrooms and their taste characteristics.Meanwhile,the electric response results of aftertaste-astringency (Aftertaste-A)and aftertaste-bitterness (Aftertaste-B) were related to astringency taste and bitter taste.Toelstede et al.[20]had showed that long-lasting and thickness characteristic were much more representative than aftertaste-A and B for the kokumi properties of samples.
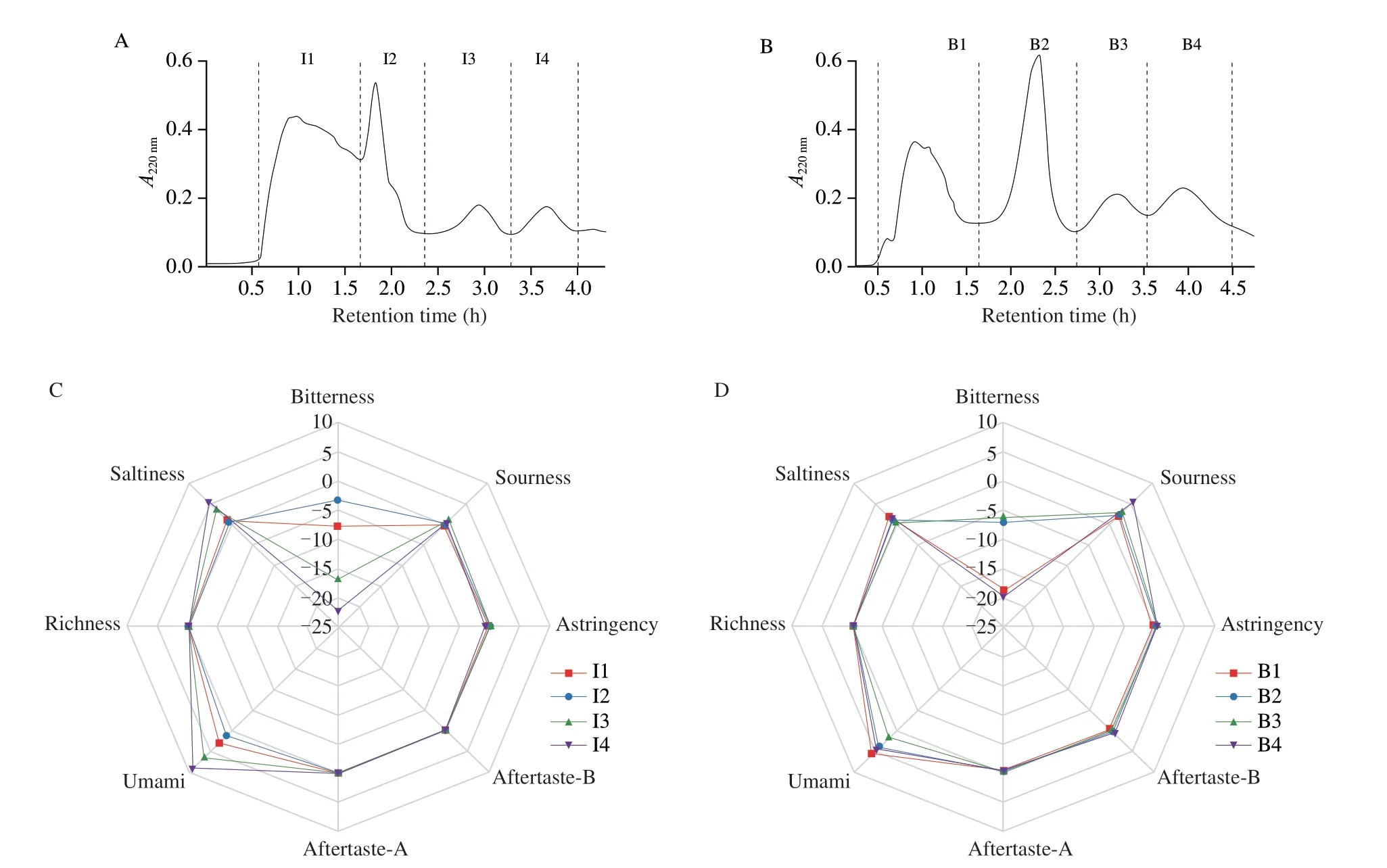
Fig.2 (A) Sephadex G-15 gel filtrations chromatogram of extraction from TI;(B) Sephadex G-15 gel filtration chromatogram of extraction from TB;(C) Radar fingerprint chart of 4 fractions (I1-I4) form TI that were tasted by E-togue;(D) Radar fingerprint chart of 4 fractions (B1-B4) form TB that were tasted by E-togue.P < 0.05.
3.4 Identification of the taste peptides in I4 and B1 by nano-LC-MS/MS
RP-HPLC was used to purified I4 and B1,then nano-LC-MS/MS was operated to determine the amino acid sequence and molecular weight of these peptides.Ten peptides were identified including 7 peptides from I4 (No.1-7) and 3 peptides from B1 (No.8-10) (Table 3).The molecular mass of the 10 peptides were 983.54,1 109.535 2,958.636 1,950.449 8,1 354.625 2,1 534.689 5,1 090.552,1 344.640 9,1 204.545 9,954.477 Da (Fig.3).The peptide sequences were identified in the PEAK studio protein database and the 10 peptides were KLNDAQAPK,DSTDEKFLR,VGKGAHLSGEH,MLKKKKLA,SLGFGGPPGY,TVATFSSSTKPDD,AMDDDEADLLLLAM,VEDEDEKPKEK,SPEEKKEEET,and PEGADKPNK (Table 3).
Umami taste thresholds of these 10 peptides were respectively 0.76,1.24,0.64,0.55,0.70,0.35,1.24,0.70,1.18 and 1.20 mmol/L (Table 3).The related research showed that sequence of umami peptides generally including aspartic acid (D) and glutamic acid (E)[22].In addition,phenylalanine (F),alanine (A),serine (S),glycine (G),threonine (T),valine (V) and lysine (K) also contribute umami taste to peptides[23].
The concentration of umami-taste amino acid (D and E) in VEDEDEKPKEK and AMDDDEADLLLLAM were 54.5%and 35.7%,respectively.The umami-taste amino acid content of VGKGAHLSGEH and TVATFSSSTKPDD were 90.9% and 92.3%,respectively.However,the content of umami amino acids was not proportional to umami taste thresholds of TVATFSSSTKPDD.

Table 3 Sequences,E-togue evaluation of the synthetic peptides and the threshold value.
3.5 Flavor characteristics of seven synthetic peptides
To explore the taste characteristics of synthetic peptides,7 synthetic peptides were tested by electronic tongue.Then the 3 of most intense pleasant taste synthetic peptides were added to the fresh chicken soup for further test (Fig.4A).
The umami,saltiness and richness values of these 7 peptides were built to bubble chart.There were obvious differences in umami or salt taste among these peptides in this study (Fig.4A).AMDDDEADLLLLAM and DSTDEKFLR had the strongest umami intensity (scores were 5.24 and 4.23),then were MLKKKKLA(3.85),VGKGAHLSGEH (3.77) and KLNDAQAPK (2.93).TVATFSSSTKPDD had a lower umami score,but a higher saltiness.

Fig.3 MS/MS spectrum of the identified peptides in I4 (A) and B1 (B).
Fig.4B showed the taste value of the fresh chicken soup,the soup with DSTDEKFLR (Cs+D),the soup with AMDDDEADLLLLAM(Cs+A) and the soup with MLKKKKLA (Cs+M).Fresh chicken soup had an intense umami taste as AMDDDEADLLLLAM and DSTDEKFLR respectively enhanced the umami value of chicken soup to 3.79 (Cs+A) and 2.86 (Cs+D) (Fig.4B).The saltiness of soup was significantly increased after the addition of MLKKKKLA to the soup (Cs+M) that had a certain inhibitory effect on the bitterness of the soup.There were complex interactions between taste and taste receptors,the function of taste peptides on flavor was not just additive or subtractive.

Fig.4 Flavor characteristics of seven synthetic peptides.(A) The bubble chart of seven synthetic peptides,A (AMDDDEADLLLLAM),D (DSTDEKFLR),M (MLKKKKLA),V (VGKGAHLSGEH),K(KLNDAQAPK),T (TVATFSSSTKPDD),S (SLGFGGPPGY).(B) Chicken soup (Cs),Chicken soup+AMDDDEADLLLLAM (Cs+A),Chicken soup +DSTDEKFLR (Cs+D),Chicken soup+MLKKKKLA (Cs+M).
3.6 Computational molecular docking of umami peptides into T1R1/T1R3
To further investigate the molecular mechanism of the interaction between umami peptides and umami receptor T1R1/T1R3,molecular docking simulations were conducted utilizing the CDOCKER tool of Discovery Studio software.The homology model of the umami taste receptor T1R1/T1R3 is shown in Fig.5A and the Raman diagram of the optimized umami receptor model was calculated.It was found that 5.71% were in disallowed region and the coverage was 94.29%,which showed that the established homology model was reasonable(Fig.5B).In this study,all the 10 identified umami peptides could interact with T1R1/T1R3.While all 10 identified peptides had inserted into the active site of T1R3,which might be because T1R1 was closed and T1R3 was open.Fig.5C showed the best docking pose and the 2D interaction diagram of the umami peptides with the active residues of T1R3.These results were consistent with the report which provided main binding residue of umami peptides was T1R3[10].
The energy of molecular docking and the binding score of the binding sites of umami peptides with T1R1/T1R3 were displayed in Table 4.The conformation docking energy of the peptides increased in the following order: DSTDEKFLR,PEGADKPNK,VGKGAHLSGEH,VEDEDEKPKEK,AMDDDEADLLLLAM,SLGFGGPPGY,SPEEKKEEET,TVATFSSSTKPDD,KLNDAQAPK,MLKKKKLA.To insert to the binding region,DSTDEKFLR and MLKKKKLA required the lowest (-182.1 kcal/mol)and highest energy (-99.4 kcal/mol).In this study,docking energy and the peptide length did not exist remarkable relativity,which was consistent with the result from Li et al.[20].
Moreover,the binding sites of active residue of T1R3 mainly included Asp63,His47,Lys53,Tyr313 and Lys354 (Table 4).Asp63 appeared most frequently and followed by His47.Asp63 existed in KLNDAQAPK,DSTDEKFLR,VGKGAHLSGEH,MLKKKKLA,SLGFGGPPGY,VEDEDEKPKEK,SPEEKKEEET and PEGADKPNK.His47 frequently appeared in KLNDAQAPK,DSTDEKFLR,VGKGAHLSGEH,MLKKKKLA and SPEEKKEEET.Lys53 existed in 5 identified peptides,Tyr313 and Lys354 appeared in 4 identified peptides.These results indicated that Asp63,His47,Lys53,Tyr313 and Lys354 were important binding sites.The analysis of the binding sites of the above-mentioned ten identified peptides and umami receptor showed that the binding of identified umami peptides to T1R3 subunit was mainly through hydrogen bond and electrostatic interactions.Hydrogen bond and electrostatic interaction are usually the basic factors of umami receptor and ligand[17].

Fig.5 Homology model and molecular docking poses of ten umami peptides with T1R1/T1R3 receptor.(A) Structure of umami receptor T1R1/T1R3;(B) Ramachandran plot for the T1R1/T1R3 homology model;(C) Molecular docking results of 10 identified peptides with T1R1/T1R3 receptor active site.
4.Conclusion
Based on results of this present study,bothTermitomycesmushrooms ofT.intermediusandT.aff.bulborhizuscontained non-volatile flavor components of free amino acids,5’-nucleotide and organic acid.Ten new umami peptides from aqueous extracts were identified as KLNDAQAPK,DSTDEKFLR,VGKGAHLSGEH,MLKKKKLA,SLGFGGPPGY,TVATFSSSTKPDD,AMDDDEADLLLLAM,VEDEDEKPKEK,SPEEKKEEET and PEGADKPNK.Among them,7 strong flavor peptides,except VEDEDEKPKEK,SPEEKKEEET and PEGADKPNK,were synthesized to reveal their umami and enhancement of umami characteristics.The molecular docking visualization revealed that all these ten identified umami peptides could combined with the active residue of T1R3 by entering the binding pocket,and Asp63,His47,Lys53 and Tyr313 in T1R3 might make mainly contributions in the formation of umami taste.Moreover,the flavor mechanism of umami peptides and umami receptor T1R1/T1R3,hydrogen bonding and electrostatic interactions were considered as the important interaction factors.This study contributes to address the delicious and umami mechanism ofT.intermediusandT.aff.bulborhizus,and the discovery of new flavor umami peptides from wild edible fungiresources.Furthermore,there is still a need for more comprehensive study of umami taste and natural flavor from wild edible mushrooms.
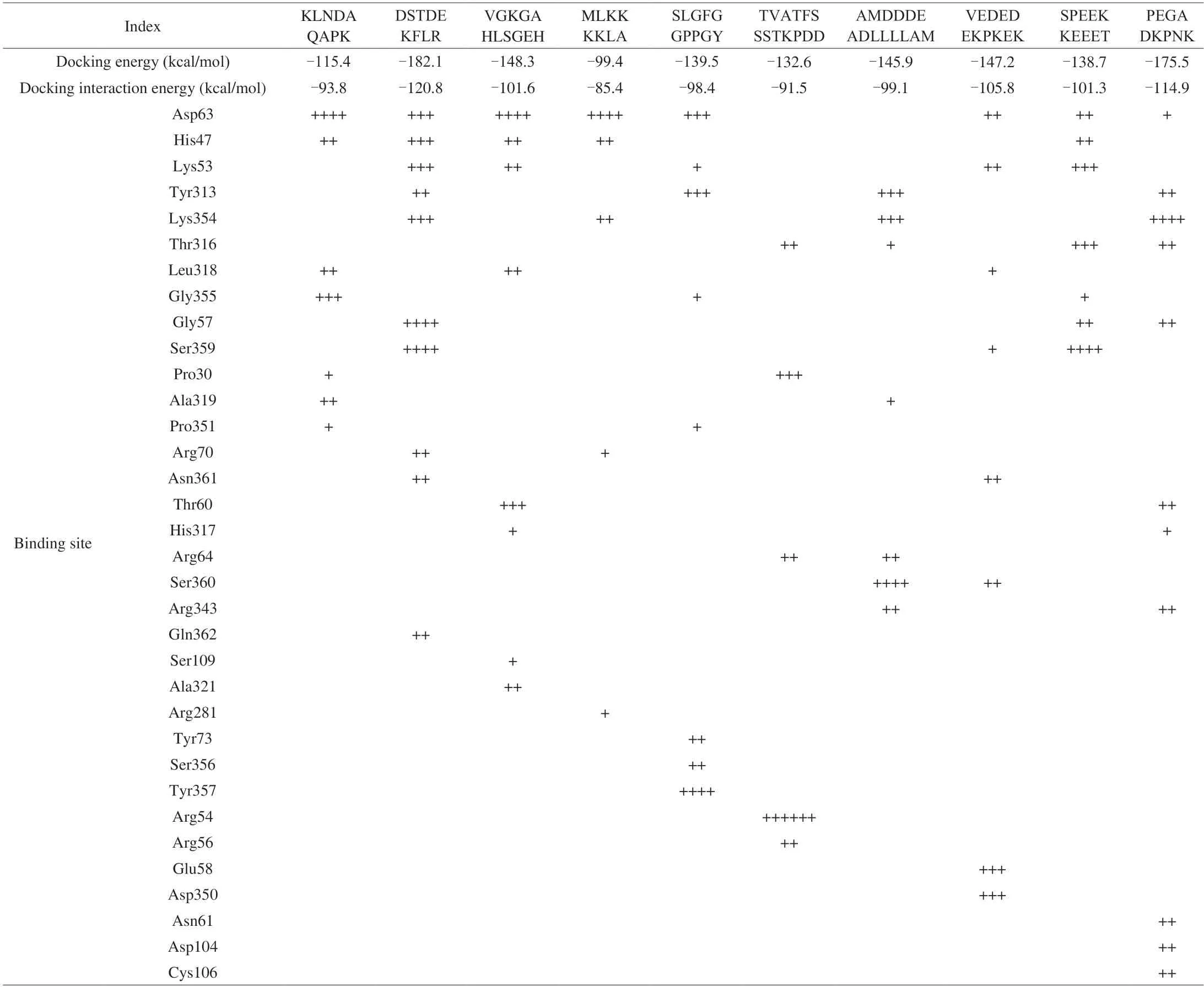
Table 4 Docking energies of the umami peptides and key amino acid residues around the active site in T1R1/T1R3.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
We are grateful to Ran Wang and Jianwei Liu for mushroom identification,Professor Zhili Zuo for molecular docking,and Professor Xinhua He for reviewing the manuscript and providing useful suggestions.This work was supported by the Yunnan Key Project of Science and Technology (202202AE090001),Postdoctoral Directional Training Foundation of Yunnan Province (E23174K2)and Postdoctoral Research Funding Projects of Yunnan Province,China (E2313442).
- 食品科学与人类健康(英文)的其它文章
- Protective effects of oleic acid and polyphenols in extra virgin olive oil on cardiovascular diseases
- Diet and physical activity inf luence the composition of gut microbiota,benef it on Alzheimer’s disease
- Inf luence of nitrogen status on fermentation performances ofnon-Saccharomyces yeasts: a review
- Ganoderma lucidum: a comprehensive review of phytochemistry,eff icacy,safety and clinical study
- Resveratrol combats chronic diseases through enhancing mitochondrial quality
- Demonstration of safety characteristics and effects on gut microbiota of Lactobacillus gasseri HMV18

