Epicatechin attenuates lead (Pb)-induced cognitive impairment in mice:regulation on Nrf2 signaling pathway,and interference on the interaction between Pb with albumin
Di Cheng,Qinqin YuKexin ZhuDingdong BuZijin Wu
a State Key Laboratory of Food Nutrition and Safety, Tianjin University of Science &Technology, Tianjin 300457, China
b School of Biotechnology and Food Science, Tianjin University of Commerce, Tianjin 300134, China
Keywords: Lead Epicatechin Bovine serum albumin Interaction Oxidative stress
ABSTRACT Epicatechin (EC) was used in this study to antagonize the cognitive dysfunction caused by lead (Pb) exposure in mice.Eight-week-old male Kunming mice were treated with PbCl2 (20 mg/kg) and/or EC (50 mg/kg)by gavage administration for 4 weeks.Morris water maze test showed that EC could improve memory dysfunction induced by Pb.EC antagonized Ca2+ overload,activated Nrf2 signaling pathway and reduced the accumulation of Pb in the brain and serum,which suggested that EC might alter Pb distribution in mice.In vitro,spectroscopic analysis,potentiometric titration and docking studies were applied to inquiry into the interaction between bovine serum albumin (BSA) and Pb2+ in presence or absence of EC.EC was proved to chelate Pb2+ and reduced the interaction between BSA and Pb2+.In summary,EC might protect Pb-induced cognitive impairment by activating Nrf2 signaling pathway,and suppressing Pb accumulation via interference on the binding of Pb to albumin.
1.Introduction
Heavy metal pollution is increasingly threatening the ecosystem and human body,and a growing number of organisms are exposed to environmental pollutants.Lead (Pb) is a persistent environmental pollutant,which has been identified as one of 10 chemicals of major public health concern due to its high toxicity,persistent bioaccumulation and non-biodegradability[1].The Institute for Health Metrics and Evaluation estimates that 63.8% of people with intellectual disabilities are caused by Pb exposure[2].Despite years of awareness and a range of intake standards,185 million children around the world are still reported to have high levels of Pb in their blood.Pb can enter the body through contaminated soil,water,canned food,etc.Dietary intake of Pb is the main source of human exposure to this heavy metal[3].As it reaches the bloodstream,Pb can be combined with erythrocytes or interact with thiol and sulfhydryl-containing proteins in the plasma,such as serum albumin[4].Pb enters tissues with the blood,distributes in the liver,kidneys,lungs,skin,nervous system and eventually accumulates in bone.The brain,as a major component of the nervous system,is the most sensitive organ to Pb exposure[5].Pb can cross the blood-brain barrier and deposit in the hippocampus and cerebral cortex,causing cognitive impairment.The cognitive dysfunction caused by low dose Pb exposure during development has attracted worldwide attention[6].
Pb induces excessive production of reactive oxygen species and consumes endogenous antioxidants,which is the main toxicological mechanism of Pb[7].Pb has a high affinity for the sulfhydryl group (-SH) and can form mercaptan salts with -SH,thus affecting antioxidant enzymes in the body[8].Chronic Pb exposure can cause damage by inactivating nitric oxide and increasing hydrogen peroxide formation.At present,the chelating agent is mainly used to intervene in Pb poisoning in clinic.For example,dimercaptosuccinic acid is registered in the United States as an oral chelator of Pb poisoning in children[9].However,chelating agents are mainly used for acute Pb poisoning,and there are non-negligible side effects[10-11].It is particularly important to find natural foods with small side effects to eliminate or avoid the toxicity of Pb to human body.The special molecular structure of phenol determines its good antioxidant activity and coordination to metal ions.More and more studies have shown that phenols play an important role in antagonizing Pb damage[12].
Epicatechin (EC) belongs to the class of catechins,which has diverse biological properties (Fig.1).EC supplies hydrogen to free radicals and binds to them,which prevents the oxidation of other small biological molecules.EC supplementation reduced short-term cognition impairment in high fat diet-induced mice via antioxidant and anti-inflammatory properties[13].Chang et al.[14]found that EC was a natural activator of nuclear factor erythroid-2-related factor 2 (Nrf2)and could increase the activity or expression of antioxidant enzymes.Ren et al.[12]showed that apple polyphenol extract improved cognitive impairment,depression and anxiety-like behavior caused by Pb,and that EC was the main phenolic compound in apple polyphenol extract.Our previous study found thatMalus micromalusMakino phenolic extract (MMPE) prevented oxidative stress in Pb-treated mice and reduced the level of Pb in the blood.It is worth noting that EC(approximately 58.6%) is the most abundant component in MMPE[15].Therefore,we further evaluated the potential protective effect of EC on Pb-induced biotoxicity.
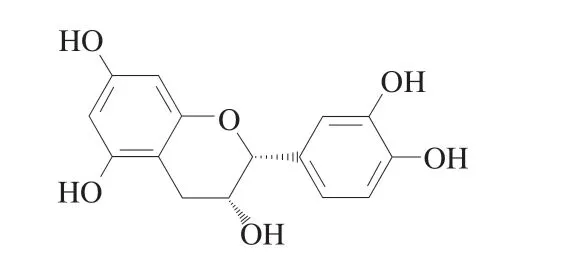
Fig.1 Structural formula of epicatechin (EC).
In the present study,we first evaluated the effect of EC on the learning and memory function of Pb-exposed mice through the water maze test,and then investigated the protective effect of EC on Pb-induced oxidative damage of erythrocytes,brain tissue,liver and kidney in mice.Based on the observation that epicatechin reduces the level of Pb in serum,the albumin in plasma was selected as the research object.Spectroscopic analysis,potentiometric titration and docking studies were applied to inquire into the interaction between BSA and Pb2+in the presence or absence of EC.The results are anticipated to provide essential insights into EC antagonism of Pb-induced toxicity.
2.Materials and methods
2.1 Materials
The lead chloride (PbCl2),epicatechin (EC,purity ≥ 99.0%),ibuprofen (Ibu,purity ≥ 98%) and indomethacin (Indo,purity≥ 98%) were purchased from Solarbio (Beijing,China).TheBovineserumalbumin (BSA,purity > 98%) was purchased from Biotopped (Beijing,China).Purchase blood urea nitrogen (BUN),creatinine (CR),aspartate transaminase (AST),alanine transaminase(ALT),superoxide dismutase (SOD),malondialdehyde (MDA),glutathione (GSH) commercial Assay kits from the Nanjing Jiancheng Bioengineering Institute (Nanjing,China).The primary antibodies include Nrf2 rabbit polyclonal antibody,KEAP1 rabbit polyclonal antibody,heme oxygenase 1 (HO-1) rabbit monoclonal antibody,NQO1 rabbit polyclonal antibody were obtained from Beyotime Biotechnology (Shanghai,China).β-Actin,anti-rabbit and anti-mouse antibodies were obtained from ABclonal Biotechnology (Wuhan,China).The other reagents used in this study were all analytical grades.
2.2 In vivo experiment
2.2.1 Experimental details of the animal
Animal experiments were carried out in accordance with the experimental standards of the Experimental Animal Center of Tianjin University of Science and Technology.Specific 32 pathogen-free(SPF) male Kunming mice (8 weeks old,(27 ± 2) g) were purchased from SPF (Beijing) Biotechnology Co.,Ltd.(Beijing,China) (SCXK(Beijing) 2019-0010).Animals were kept in a 12/12-h light/dark cycle with a temperature of (24 ± 2) °C and a relative humidity of (60 ± 4)%,and mice had unrestricted access to normal chow and water.After 7 days of adaptation,the mice were randomly divided into 4 groups:Control group: mice were given 0.2 mL saline (0.9% NaCl) by oral gavage.Pb group: mice were given 20 mg/kg PbCl2via oral gavage.Pb+EC group: mice were gavaged with 20 mg/kg PbCl2and 2 h after with 50 mg/kg EC with once per day.EC group: mice were given 50 mg/kg EC via oral gavage.Mice were treated once daily for 10 weeks.
2.2.2 Morris water maze(MWM)test
The MWM equipment was purchased from Zhenghua Biological Instrument Equipment Co.,Ltd.(Anhui,China).MWM tests include positioning cruise tests and space exploration tests[16].In the positioning cruise test,after 1 day of adaptive training,the mice were trained to find a hidden platform for 4 consecutive days.Each mouse was trained 3 times per day with a maximum duration of 60 s each training session.After 24 h,the platform was dismantled for a 1-day space exploration test.A camera attached to the tracking system was installed above the pool.A comprehensive analysis was performed for each test and the latency to find an escape platform was calculated[17].
2.2.3 Measurement of body weight and relative organ weight of mice
The body weight of the mice was recorded every four days until the end of the experimental period.The mice were sacrificed after 28 days of treatment,and the mice were anesthetized before dissection.The brain,liver,kidney,and spleen were taken out and weighed.
2.2.4 Determination of Pb content
About 0.1-0.2 g of samples were weighed into microwave digestion tubes and digested with nitric acid and perchloric acids.After digestion,the constant volume of the samples to 50 mL and then the Pb content was determined by ICP-MS (CX7000,Aglient Corporation,USA)[15].
2.2.5 Determination of osmotic fragility of erythrocytes
Erythrocyte suspension (0.2 mL) was added to a test tube containing a gradient concentration of saline solution (0.1%-0.9%NaCl,1.8 mL).After mixing gently and incubating at 37 °C for 30 min,erythrocyte suspension was centrifuged at 1 500 r/min for 10 min.The absorbance of the supernatant of the erythrocyte suspension at 540 nm was measured[18].
2.2.6 Biochemical analysis
Kidney function was evaluated by measuring serum BUN and CR.Similarly,liver function was evaluated by measuring serum ALT and AST[19].The experiment procedure was carried out according to the instructions listed in the kit (Nanjing Jiancheng Bio Inst,Nanjing,China).
2.2.7 Measurement of oxidative stress parameters
After homogenization in ice-cold normal saline (1:9,m/V),the brain,liver,and kidney tissue homogenate were centrifuged(10 000 r/min,4 °C,10 min).The supernatant and erythrocyte hemolysis were used to determine oxidative stress parameters.Advanced oxidized protein product (AOPP) was measured using an ultraviolet-visible (UV-vis) spectrophotometer[20].The levels of MDA,GSH,SOD and catalase (CAT) were measured by the kit (Nanjing Jiancheng Institute of Biology,Nanjing,China).
2.2.8 Histopathological analysis
The brain,liver,and kidney tissue samples were embedded in paraffin and cut into thick sections.After deparaffinized with xylene and rehydrated,the sections were stained with hematoxylin-eosin.The changes in tissue morphology were observed under an optical microscope and photographs were taken[19].
2.2.9 Western blot analysis.
The brain tissues of each group were homogenized,and the samples were loaded on each lane of a 10% SDS polyacrylamide gel(10% separation gel and 4% concentrated gel).After separation,the protein was transferred to nitrocellulose (NC) membrane.The NC membrane was incubated in PBST (PBS containing 0.1% Tween-20)with 5% skimmed milk to block non-specific sites,and then the primary and secondary antibodies were added to the membrane and incubated.Western blot analysis was performed using ImageJ software[21].
2.3 In vitro experiment.
2.3.1 Experimental details of the interaction systems
BSA was dissolved in phosphate buffer solution (PB solution,0.01 mol/L,pH 7.4) to prepare the BSA stock solution (2 × 10-5mol/L).The stock solution of PbCl2(2 × 10-2mol/L) was prepared by double distilled water.For EC,the working solution was freshly prepared with the PB solution.
BSA-Pb2+system (the binary system): The BSA and PbCl2solutions (from 0 to 500 × 10-6mol/L) were well-mixed.The final concentration of BSA is 2 × 10-6mol/L.The solutions were maintained for 15 min at 298 K.
BSA-Pb2+-EC system (the ternary system): EC solution (2 × 10-6mol/L)was added to the BSA-Pb2+system.
BSA-(Pb2+-EC) system (the ternary system): The EC and PbCl2solutions were well-mixed with the same conditions.BSA was titrated with Pb2+-EC mixture.The final concentration of EC and BSA were 2 × 10-6mol/L.
2.3.2 Fluorescence experiments
Fluorescence spectra were measured using a fluorescence spectrophotometer F-2500 (Shimadzu,Tokyo,Japan) equipped with 1.0 cm quartz cells.The excitation wavelength was 280 nm and the emission spectrum was from 300 nm to 500 nm.
The fluorescence quenching was expressed by the following Stern-Volmer equation[22]:
whereF0andFrepresented the fluorescence intensity of BSA in the nonexistence and existence of the ligand,respectively.[Q] was the concentration of the ligand,Kqdenoted the bimolecular quenching rate constant with units of L/(mol·s),τ0denoted the average fluorescence lifetime of the fluorophore without a ligand (10-8s).Ksvwas the slope of theF0/Fvs[Pb2+] curve,called the Stern-Volmer quenching constant.
For static quenching,the binding constant (Ka) and binding site number (n) were computed using the double-logarithm equation[23]:
The synchronous fluorescence spectra of BSA in the nonexistence and existence of ligands were displayed atλem=240-320 nm with Δλ(λem-λex) of 15 and 60 nm.Synchronous fluorescence quenching rate (RSFQ) was obtained by the following equation:
The three-dimensional (3D) fluorescence spectra were scanned atλex=200-300 nm andλem=300-450 nm with enhancements of 5 nm.
Site-selective experiments were conducted using two site markers,indomethacin (Indo) and ibuprofen (Ibu) for sites I (subdomain II A) and II (subdomain III A),respectively.After the BSA-Indo/Ibu complex was maintained for approximately 30 min,the experiment followed the binary and ternary system experimental methods as mentioned above.
2.3.3 UV-vis absorption spectroscopy
UV-3200 spectrophotometer (Mapada,Shanghai,China) was utilized for the BSA UV-vis absorption spectra from 200 nm to 450 nm at 298 K.
2.3.4 Energy transfer experiment
The absorption spectrum of Pb2+or Pb2+-EC and fluorescence spectrum of BSA were recorded in the wavelength range of 300-400 nm.The energy transfer efficiency (E) was reckoned as follows[24]:
Wherersignified the distance between ligand and BSA,andR0signified the critical distance whenEis 50%,which was evaluated by equation (5) below:
Jwas an overlap integral of the fluorescence spectrum of BSA and the absorption spectrum of the Pb2+/Pb2+-EC,which was acquired by the following equation:
whereF(λ) represented the fluorescence intensity of the BSA,ε(λ) represented the molar absorption coefficient of Pb2+/Pb2+-EC in L/(mol·cm).
2.3.5 Circular dichroism(CD)measurements
The CD spectrophotometer (New MOS-450,Biologic,France)was utilized for CD measurement.The spectra of solutions were measured from 200 nm to 250 nm.
2.3.6 Potentiometric titration experiments
Regarding the method described by Cheng[25],two titration schemes were implemented: (1) EC titration and (2) Pb2+-EC titration.An automatic titrator (T960,Hanon,Shandong) was used to measure the pH value.The values ofnA,nLandpLwere given by the equations(7)-(10) below:
The number of protons released by EC was expressed asn.The initial volume was recorded asV0.The molar concentration of NaOH was expressed byC1.The volume of alkalis instilled to reach the stable pH value in titration was expressed byV1.The initial concentration of EC was expressed byTL.The molar concentration of OH-was expressed by [OH].The molar concentration of H+was expressed by [H].The stability constant (lgK) was acquired from thenLvs.pLplot,and lgKwas represented by thepLvalues atnL=0.5.
2.3.7 Molecular docking
The AutoDock 4.2.6 was selected to accomplish molecular docking simulations of BSA and ligands.The crystal structure model of the BSA was provided by the Protein Data Bank (PDB ID: 4F5S)(https://www.rcsb.org).The 3D structure of EC was downloaded from PubChem (PubChem CID: 72276) (https://pubchem.ncbi.nlm.nih.gov).Water molecules were removed and hydrogen atoms were added for docking.The default genetic algorithm parameters were used for docking simulation to generate the 50 most reasonable models.The results were analyzed using PyMoL and Discovery Studio software.
2.4 Statistical analysis
The data were obtained through at least three independent experiments and were expressed as mean ± SD.Statistical significance was analyzed using SPSS 19.0 and determined by one-way analysis of variance (ANOVA) followed by LSD’s multiple test.ThePvalue < 0.05 was considered to be statistically significant.The data was produced using Origin 2019 software.
3.Results
3.1 EC improved learning and memory impairment induced by Pb in mice
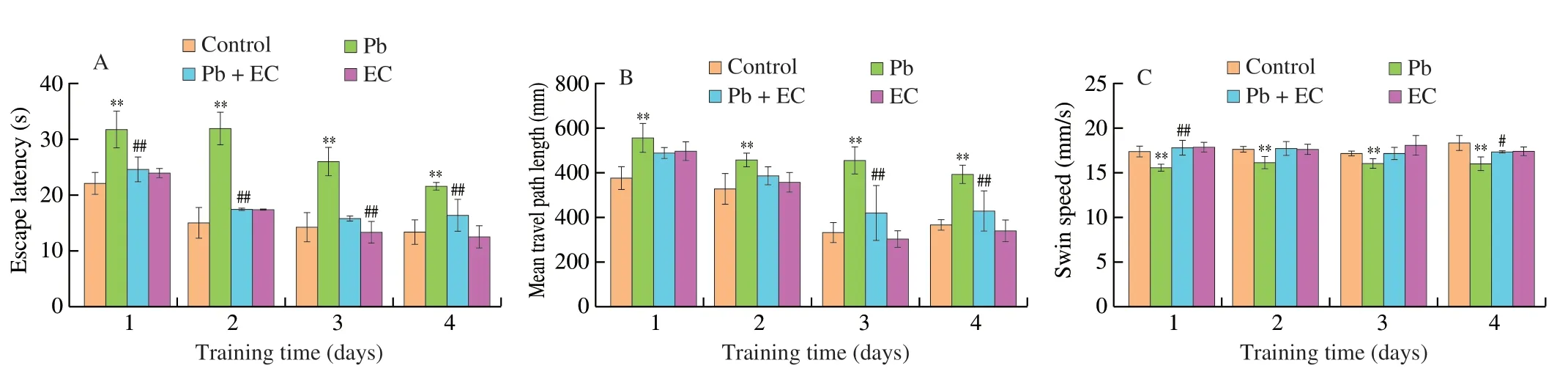
Fig.2 Effects of EC on escape latency (A),mean travel path length (B),mean swimming speed (C),and swimming trajectory (D) in Pb-exposure mice in the place navigation trial.Effects of EC on the percentage of path length and time spent in the target quadrant (E),number of platform crossing (F) and swimming trajectory (G) in Pb-exposure mice in the spatial probe trial.Mean ± SD,n=8;*P < 0.05,**P < 0.01 compared with the control group;#P < 0.05,##P < 0.01 compared with the Pb group.

Fig.2 (Continued)
On the 4thday of training,the escape latency ((21.6 ± 0.6) s)of mice in the Pb group was significantly longer than those in the control group ((13.5 ± 2.2) s) (Fig.2A).Compared with the Pb group,the escape latency and total path of the Pb+EC group were significantly shortened,and the swimming speed was increased.The effects of EC on the spatial exploration ability of Pb-induced mice were shown in Figs.2E-G.Compared with the Pb group,Pb+EC treatment significantly increased the time and distance spent in the target quadrant,and slightly increased the number of platform crossings.
3.2 EC had a protective effect on Pb-induced erythrocyte injury
As shown in Figs.3A and B,after treatment with EC,the osmotic fragility of erythrocyte and the erythrocyte hemolysis were decreased compared with Pb group,indicating that EC has a certain protective effect on Pb-induced erythrocyte damage.
Compared with the control group,the advanced oxidation protein products (AOPP) level in the Pb group was increased by 64.04%,and the malondialdehyde (MDA) level was increased by 55.11%.In the Pb+EC group,the AOPP and MDA levels were decreased by 22.84%and 28.19%,respectively,compared with the Pb group (Figs.3C and B).When EC was administered after Pb exposure,erythrocyte SOD and CAT activities increased by 37.04% and 10.95%,respectively(Figs.3E and F).
3.3 EC protected Pb-induced brain injury in mice
The morphology of neurons in the control group and the EC group was generally normal (Fig.4A).The cells shape was round and the cells were evenly distributed and closely arranged.The number of degenerated nerve cells increased,the nucleoli disappeared and the cell layer became thinner,which showed severe histopathological changes in Pb group.EC treatment after Pb exposure reduced the degree of tissue damage,the cell arrangement was more orderly than in the Pb group.
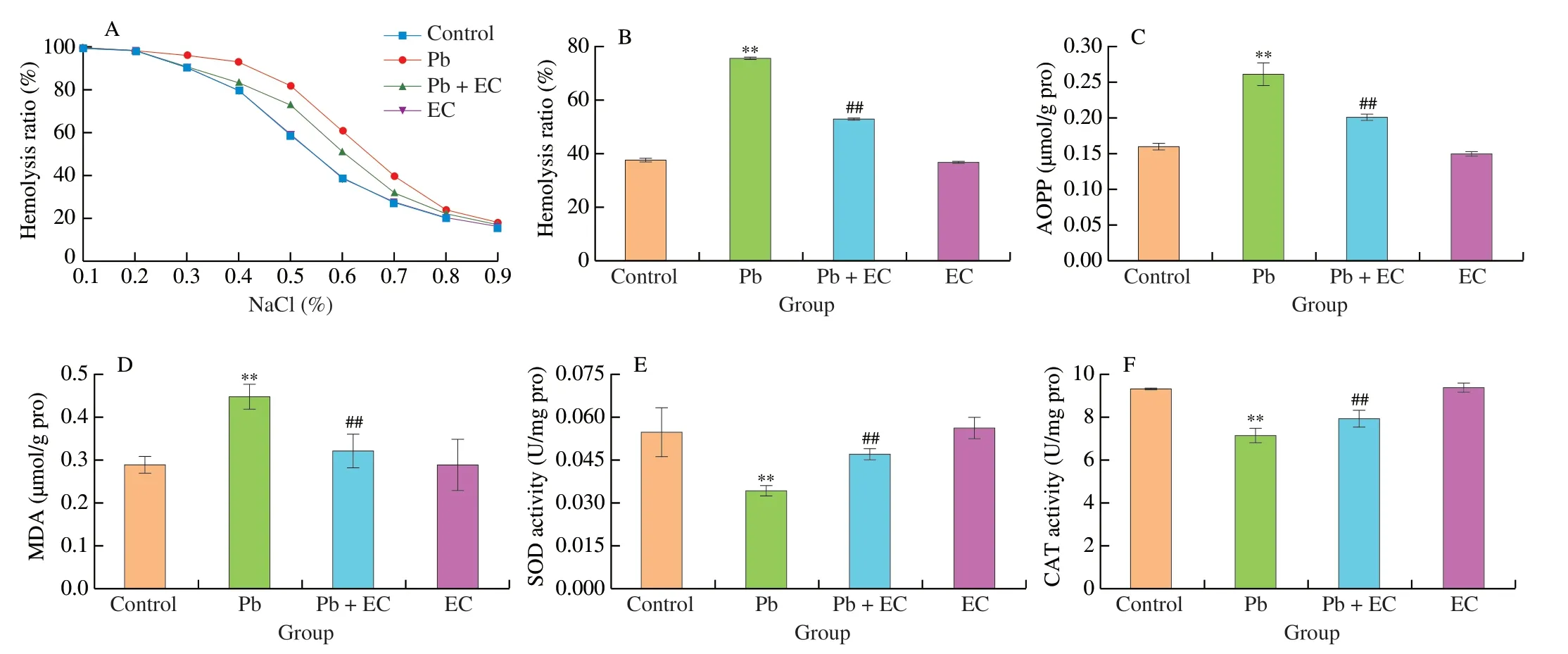
Fig.3 Effect of EC on osmotic fragility (A),hemolysis rate (B),AOPP content (C),MDA content (D),SOD activity (E) and CAT activity (F) of erythrocytes in Pb-exposure mice.Mean ± SD,n=8;**P < 0.01 compared with the control group;##P < 0.01 compared with the Pb group.
Compared with the control group,the level of calcium (Ca2+)in the brain tissue of the Pb group was significantly increased,and the level of brain Ca2+in the Pb+EC group was 25.60% lower than that of the Pb group (Fig.4B).When EC treatment was given after Pb exposure,the Glu content in the brain tissue of the mice was decreased by 15.28% (Fig.4C).EC treatment mitigated the decrease in acetylcholinesterase (AChE) activity in brain tissue induced by Pb exposure (Fig.4D).
As shown in Figs.4F-I,the MDA content in the brain of the Pb group was significantly higher than that of the control group,while the MDA content in the Pb+EC group was significantly lower than that in the Pb group.In addition,compared with the control group,the SOD,CAT activities and GSH contents in the brain of the Pb group were significantly decreased.EC treatment after Pb exposure increased SOD,CAT activities and GSH content in tissues.
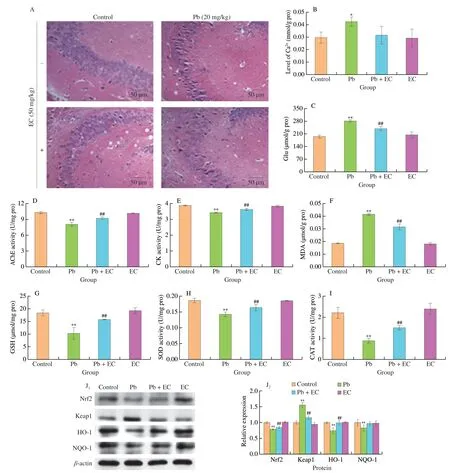
Fig.4 Effects of EC on brain tissue morphology (200×) in Pb-exposure mice (A).Effects of EC on Ca2+ level (B),Glu content (C),AChE activity (D) and creatine kinase (CK) activity (E) of brain tissue in Pb-exposure mice.Effect of EC on MDA content (F),GSH content (G),SOD activity (H),CAT activity (I) of brain tissue.Effect of EC on the protein expression levels of Nrf2,Keap1,HO-1 and NQO-1 in the brain tissue of Pb-exposure mice (J).Mean ± SD,n=8;*P < 0.05,**P < 0.01 compared with the control group;#P < 0.05,##P < 0.01 compared with the Pb group.
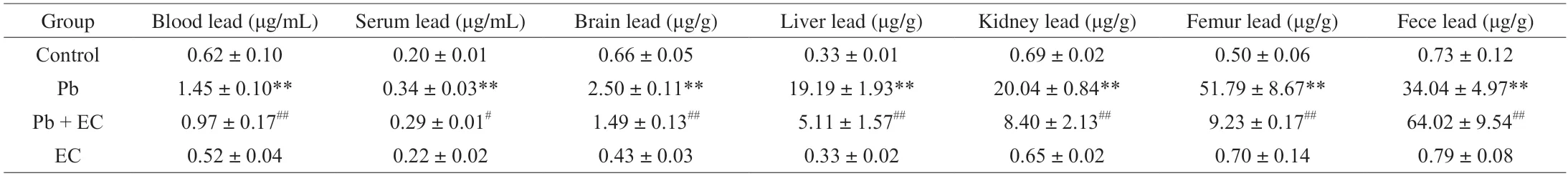
Table 1 Effects of EC on the Pb level in Pb-exposure mice.
The expression of Kelch-like ECH-associated protein 1 (Keap1),nuclear factor erythroid-2-related factor 2 (Nrf2),heme oxygenase 1(HO-1) and NAD(P)H: quinone oxidoreductase (NQO1) proteins were detected by western blotting.Compared with the control group,the protein expression of Keap1 in the Pb group was increased by 55.73%.Compared with Pb group,the expression level of Keap1 decreased by 25.83% in Pb+EC group.Compared with the control group,the expression levels of Nrf2,HO-1 and NQO1 were decreased by 21.46%,25.55% and 17.43%,respectively.EC treatment mitigated the reduction of Nrf2,HO-1 and NQO-1 expression levels in brain tissue induced by Pb exposure.
3.4 EC suppressed Pb accumulation in lead-induced mice
The Pb levels in the brain,liver,kidney,bone and feces of the Pb group were significantly higher than those of the control group(Table 1).After EC supplementation,compared with the Pb group,the Pb content in the brain,liver,kidney and bone of the Pb+EC group was significantly decreased,and the Pb content in the feces was significantly increased.It was worth noting that compared with the control group,the Pb level in the serum of the Pb-induced mice was significantly increased,and the serum Pb level in the Pb+EC group was significantly lower than that in the Pb group.It suggested that EC might interfere with the binding of Pb to carrier proteins in serum,thereby affecting the distribution of Pb in the body.
3.5 EC reduced the binding force of Pb2+ to BSA
3.5.1 Fluorescence quenching
The effects of EC on the fluorescence emission spectra of the interaction between BSA and Pb2+were shown in Fig.5.The fluorescence peak of BSA was decreased gradually with the concentration of Pb2+increased (Fig.5A),indicating the occurrence of fluorescence quenching.The fluorescence quenching degree of the two ternary systems was both lower than that of the BSA-Pb2+system,indicating that EC interfered with the interaction of the BSA and Pb2+(Figs.5B and C).The quenching degree of BSA in the BSA-Pb2+-EC system was more potent than that in the BSA-(Pb2+-EC)system.The linearity property of the Stern-Volmer plot was found in the interactions of BSA-Pb2+,BSA-Pb2+-EC and BSA-(Pb2+-EC)(Fig.5D).The values ofKsvandKqwere estimated and listed in Table 2.Although theKsvvalue of the BSA-(Pb2+-EC) system was reduced by about 46.3% compared with the BSA-Pb2+system,theKqvalues of the three systems were far more than the diffusion-controlled rate constant (2 × 1010L/(mol·s)).It indicated that the fluorescence quenching mechanism of BSA induced by Pb2+was more likely to be a static quenching,and EC might not change the mechanism of fluorescence quenching.

Table 2 Effects of EC on the Stern-Volmer quenching constant (Ksv),quenching rate constant (Kq),binding constant (Ka),and number of binding site (n) for the interaction of BSA and Pb2+.
3.5.2 Binding constant and numbers of binding site
For static quenching,the values of the binding constant (Ka) and the numbers of the binding site (n) could be reckoned by the double-logarithm equation (5) (Fig.5E,Table 2).TheKavalue of the BSA-Pb2+system was estimated to be 6.83 × 106L/mol,and the value ofnwas approximately 2.12.Compared with the BSA-Pb2+system,theKavalue of the BSA-(Pb2+-EC) system was decreased to 0.43 × 106L/mol,and the degree of decrease was stronger than that of the BSA-Pb2+-EC system (Ka=4.21 × 106L/mol).
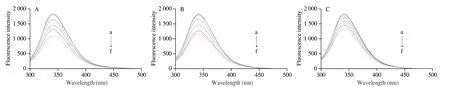
Fig.5 Effects of EC on the fluorescence emission spectra of the interaction between BSA and Pb2+: BSA-Pb2+ (A),BSA-Pb2+-EC (B),BSA-(Pb2+-EC) system (C).Stern-Volmer plots (D) and double-logarithm plots (E) for the BSA-Pb2+,BSA-Pb2+-EC,BSA-(Pb2+-EC) system.CBSA=CEC=2 × 10-6 mol/L,CPb2+(a-f)=0,100 × 10-6,200 × 10-6,300 × 10-6,400 × 10-6 and 500 × 10-6 mol/L.Effects of EC on the UV-vis absorption spectroscopy of the interaction between BSA and Pb2+:BSA-Pb2+ (F),BSA-Pb2+-EC (G),BSA-(Pb2+-EC) system (H).CBSA=CEC=2 × 10-7 mol/L,CPb2+(a-f)=0,100 × 10-7,200 × 10-7,300 × 10-7,400 × 10-7 and 500 × 10-7 mol/L.The overlapping part of fluorescence spectra of BSA (black line) and absorption spectra of Pb2+ (blue line) (I).The overlapping part of fluorescence spectra of BSA (black line) and absorption spectra of Pb2+-EC (blue line) (J).CBSA=CEC=2 × 10-6 mol/L,CPb2+=500 × 10-6 mol/L.
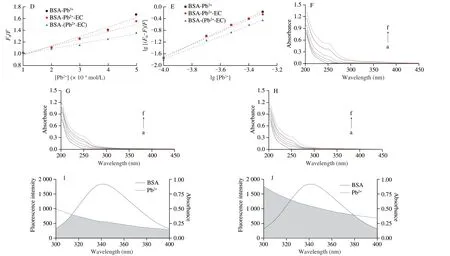
Fig.5 (Continued)
3.5.3 Intermolecular binding affinity
A significant overlap between the fluorescence emission spectrum of BSA and the ultraviolet absorption spectrum of Pb2+-EC complex was presented in Fig.5J.Based on equations (4)-(6),the overlap integral (J),the binding distance (r),the critical distance (R0),and the energy transfer efficiency (E) were calculated (Table 3).The binding distance between BSA and Pb2+-EC complex was increased to 2.23 nm,and the energy transfer efficiency of the interaction decreased to 26.49%,as compared with the interaction between BSA and Pb2+.Ther-value for BSA-(Pb2+-EC) binding was larger thanR0.
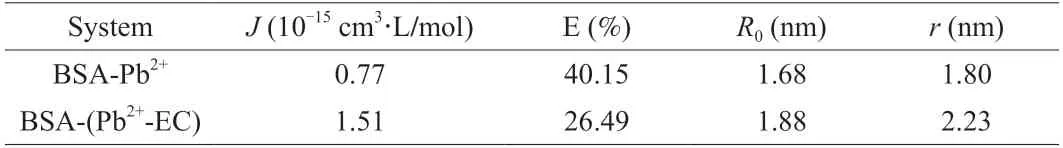
Table 3 Effects of EC on the overlap integral (J),energy transfer efficiency (E),critical distance (R0) and binding distance (r) of the interaction between BSA and Pb2+.
3.6 EC reduced the conformational change of BSA induced by Pb
3.6.1 Ultraviolet-visible(UV-vis)absorption spectroscopy
For the BSA-Pb2+system (Fig.5F),the UV-vis spectrum of BSA was increased regularly as the concentration of Pb2+increased.When the Pb2+concentration was 500 × 10-7mol/L,Pb2+increased the absorbance of BSA at 202 and 287 nm by 75.89% and 17.28%,respectively.The BSA-(Pb2+-EC) system (Fig.5H) had a more apparent inhibitory effect on the elevation of BSA UV-vis spectrum induced by Pb2+,as compared with the BSA-Pb2+-EC system (Fig.5G).
3.6.2 Synchronous fluorescence spectroscopy
The addition of EC reduced the quenching degree of Trp and Tyr spectra by Pb2+.In the BSA-Pb2+-EC system,the spectra of Trp and Tyr residues were redshifted 1 nm with the increase of Pb2+concentration.In BSA-(Pb2+-EC) system,the Trp spectrum redshift was 1 nm and Tyr spectrum redshift was 0.5 nm (Figs.6A-F).
3.6.3 Three-dimensional(3D)fluorescence spectroscopy
In Figs.6H1and H2,peak I appeared at 280/340 nm (λex/λem),and peak II was detected at 230/340 nm.The fluorescence intensity of peak II was abated significantly with the accretion of Pb2+(Figs.6I1and I2).The quenching degree of the fluorescence peak I and peak II of the BSA-(Pb2+-EC) system were both significantly weakened(Figs.6K1and K2) compared with the BSA-Pb2+system.
3.6.4 Circular dichroism(CD)spectroscopy
The free BSA spectrum has two negative bands at 208 and 222 nm which are the representative structure of theα-helix of the BSA.Upon interaction with Pb2+,the BSA band intensity was decreased,and the content ofα-helix was decreased from 64.94% to 51.56% (Table S1).The BSA band intensity of the BSA-(Pb2+-EC) system was significantly higher than that of the BSA-Pb2+system,and the content ofα-helix was restored to 61.84% (Fig.6G).
3.7 EC inhibited the binding of BSA to Pb by chelating Pb2+
3.7.1 Site-selective experiment
To ascertain the binding regions of the ligands on BSA,we have carried out competitive binding studies.Comparing Ibu-BSA and Ibu-BSA-Pb2+solutions,the presence of Pb2+could significantly reduce the fluorescence intensity.It meant that Pb2+competed with Ibu to a certain extent.Nevertheless,compared with the Ibu-BSA solution,the fluorescence intensity of Ibu-BSA-EC did not change significantly,demonstrating that site II may not be the binding site of EC.Compared with the Ibu-BSA-Pb2+solution,the fluorescence intensity induced by the Ibu-BSA-Pb2+-EC system was increased.Indo was used to label site I of BSA in a similar way to Ibu (Fig.7B).
3.7.2 Protonation and stability constant
3.7.3 Molecular docking
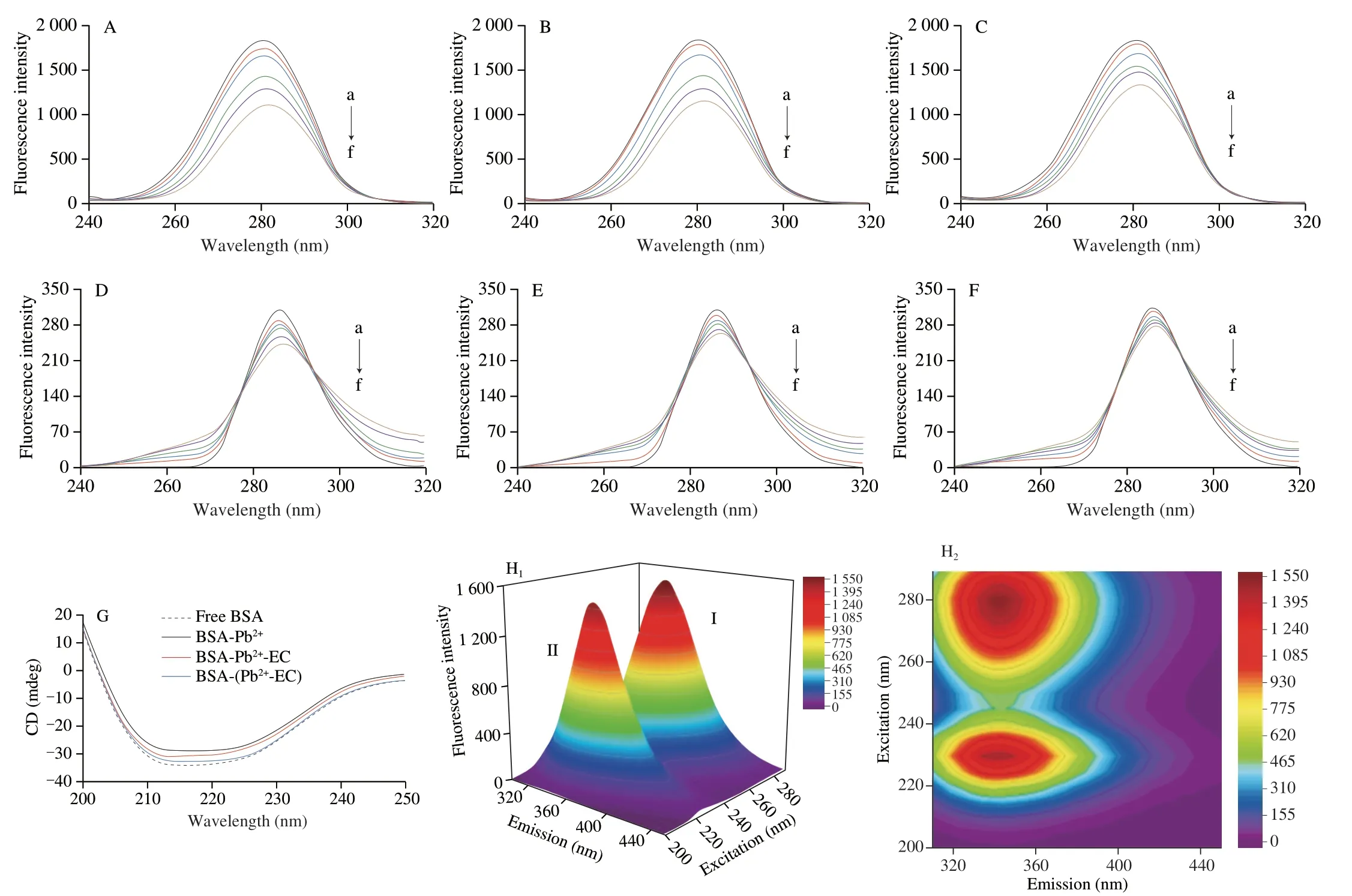
Fig.6 Synchronous fluorescence spectra of Trp (Δλ=60 nm),BSA-Pb2+ (A),BSA-Pb2+-EC (B),BSA-(Pb2+-EC) system (C),synchronous fluorescence spectra of Tyr (Δλ=15 nm),BSA-Pb2+ (D),BSA-Pb2+-EC (E),BSA-(Pb2+-EC) system (F),CBSA=CEC=2 × 10-6 mol/L,CPb2+(a-f)=0,100 × 10-6,200 × 10-6,300 × 10-6,400 × 10-6 and 500 × 10-6 mol/L.Circular dichroic (CD) spectra of free BSA,BSA-Pb2+,BSA-Pb2+-EC,BSA-(Pb2+-EC) system (G).SA=CEC=2 × 10-6 mol/L,CPb2+=500 × 10-6 mol/L.Three-dimensional (3D) fluorescence spectra of free BSA (H1,H2),BSA-Pb2+ (I1,I2),BSA-Pb2+-EC (J1,J2),BSA-(Pb2+-EC) system (K1,K2).CBSA=CEC=2 × 10-6 mol/L,CPb2+=500 × 10-6 mol/L.
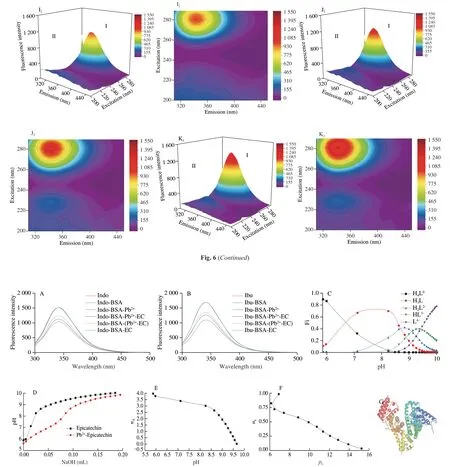
Fig.7 Fluorescence quenching spectra of each system in the presence of indomethacin (Indo) (A) and ibuprofen (Ibu) (B).CIbu=CIndo=CBSA=CEC=2 × 10-6 mol/L,CPb2+=500 × 10-6 mol/L.Effects of pH on the dissociation distribution of EC in water (C),the potentiometric titration curves of EC and Pb2+-EC (D),the formation curve of EC’s proton constant (E),the complexation stability constant of EC and Pb2+ (F).EC equivalent to quaternary weak acid,denoted by H4L.CEC=CPb2+=5 × 10-5 mol/L.The 3D view of the binding mode between PbCl2 and sites of BSA (G).The 2D schematic generated using Pymol shows the interaction between PbCl2 and the neighboring residues of BSA (H).The 3D view of the binding mode between EC and site of BSA (I).The 2D schematic generated using Pymol shows the interaction between EC and the neighboring residues of BSA (J).The 3D view of the binding mode between Pb2+-EC and sites of BSA (K).The 2D schematic generated using Pymol shows the interaction between Pb2+-EC and the neighboring residues of BSA (L).The 2D schematic generated using Discovery Studio shows the interaction between Pb2+-EC and the neighboring residues of BSA (M).

Fig.7 (Continued)
The binding sites of PbCl2on BSA were displayed in Fig.7G.It could be seen that PbCl2was placed on sites I and II.The interaction between BSA and PbCl2exhibited a particular polarity because of several ionic and polar residues near the bound ligand (Fig.7H),similar to the experimental results of synchronous fluorescence.It was predicted that the EC binding site was situated at site III of BSA(Figs.7I and J),identical to the consequences of the site competition experiment.For the ternary system,we first predicted the possible structure of Pb2+-EC based on the result of the potentiometric titration study.The combination of the Pb2+-EC and BSA was shown in Figs.7K-M.Compared with the docking result of BSA-Pb2+,one of the binding sites of Pb2+-EC was far away from site II.Pb2+-EC formed hydrogen bonds (green dotted lines) with Gln32,Asp107,Gln220,Glu339,Val342,Lys439,Cys447,and formed hydrophobic interactions (pink dotted lines) with Lys465,Pro446,Ala341 at the optimal and sub-predicted positions.
4.Discussion
Pb is the main source of heavy metal pollution in food.This heavy metal is negatively correlated with growth,intellectual development and delayed puberty.It is urgent to search for natural products to control the hazard of Pb in the human body.More and more studies have shown that plant polyphenols have antioxidant and chelation properties.Plant phenolic compounds take phenol as the basic skeleton,combined with multiple hydroxyl chemical structures[26].Phenol hydroxyl group is easy to be oxidized and has a strong ability to capture free radicals such as reactive oxygen species and reactive nitrogen,thus showing a strong antioxidant capacity[27-28].Yao et al.[29]showed that dihydromyricetin alleviated hyperglycemia in diabetic mice by regulating AMPK/Akt/GSK-3 signaling.Teng et al.[30]showed that raspberry anthocyanin could effectively prevent the release of ROS caused by oxidative damage.Lyu et al.[31]showed that the dihydromyricetin prevented hyperlipidemia in high-fat-diet mice mainly by affecting different pathways including glycerophospholipid metabolism,sphingolipid metabolism and pantothenate.Our previous study found thatM.micromalusMakino phenolic extract (MMPE) was a protective ingredient against Pb-induced liver and kidney damage in mice.Epicatechin (EC) is the highest content component in MMPE[15].EC is a natural polyphenol,with a variety of bioactive functions,such as anti-oxidation,chelating metal ions,and protecting the nervous system[32].
EC alleviated organ damage caused by Pb exposure (Table S2)and restored slow weight gain in Pb-damaged mice (Fig.S1).In our research,EC had an obvious improvement effect on Pb-induced learning and memory dysfunction in mice.Ca2+is a key signaling molecule for neural processes.Pb2+is a divalent cation with a similar structure and transport mechanism to Ca2+,which can interfere with Ca2+function as a second messenger[33].Pb induced an increase in intracellular Ca2+concentration by inhibiting the feedback regulation mechanism of Ca2+channels.At the same time,Pb exposure reduced the activity of AChE,causing changes in cholinergic function[34].Pb also affected the synthesis and release of Glu,resulting in glutamate excitotoxicity[35].The effects of Pb on learning and memory in mice were related to Ca2+,neurotransmitters (AChE,glutamate (Glu)),and energy supply.EC was able to modulate Pb-induced Ca2+levels,partially restore glutamate to normal levels,and restore AChE and creatine kinase activity.
The effect of oxidant/antioxidant imbalance is the main mechanism of organ or system poisoning caused by Pb[7].The nuclear factor erythroid-2-related factor 2 (Nrf2) signaling pathway is a key self-defense system in the body[36].Jiang et al.[37]showed that Pb exposure activated Nrf2/Keap1 pathway and aggravated oxidative stress.EC is a natural activator of Nrf2.EC is structurally composed of two aromatic rings and one oxygen-containing heterocyclic ring(Fig.1).The presence of multiple phenolic hydroxyl groups of EC enables direct or indirect scavenging of reactive oxygen species(ROS),which contributes to the potent anti-oxidant properties[38].EC increases the expression of antioxidant proteins and the nuclear accumulation of Nrf2,reducing brain tissue lesions and improving neurological dysfunction in mice[39].As a dietary antioxidant,EC effectively inhibited glutamate-induced neurotoxicity in HT22 cells[40].We found that EC relieved Pb-induced oxidative stress damage to mice erythrocytes (Fig.3),brain (Fig.4),liver,and kidney (Figs.S2 and S3),and restored their functions.
After entering the body,Pb first enters the blood,where it interacts with red blood cells and plasma proteins[4].Serum albumin was the most abundant protein in many biological circulatory systems and was also the transporter of Pb.EC could reduce the level of Pb in serum brain,liver and kidney and promote Pb excretion.(Table 1).This suggested that EC may antagonize Pb damage through other pathways besides its antioxidant effect.Polyphenols have various important biological properties in both plants and animals that can be divided into two main categories,with antioxidant and nonantioxidant function[38].We speculated that EC might affect the distribution of Pb in the body by interfering with the binding of Pb to carrier proteins in serum.
BSA was one of the most widely studied proteins due to its structural homology with human serum albumin (HSA)[41].To further study how EC affected the distribution of Pb in the body,BSA was selected as the protein model to explore.The presence of EC attenuated the quenching of BSA fluorescence caused by Pb2+and did not change the static quenching mechanism of the interaction between BSA and Pb2+.BSA fluorescence quenching is due to the transfer of excitation energy from BSA to the ligand[42].The results showed that EC increased the binding distance between BSA and Pb2+which was speculated that it may be due to the formation of a complex between Pb2+and EC,thereby reducing the binding affinity of Pb2+to BSA.The results showed that after chelating with EC,the effect of Pb2+-EC complex on the stability of BSA molecular structure was lower than the conformational change of BSA caused by the interaction between BSA and Pb2+[43].The existence of EC hindered the binding of Pb2+to BSA to some extent.In the BSA-(Pb2+-EC) system,the Pb2+-EC complex interacted with BSA,which weakened the polarity of the microenvironment of Trp and Tyr residues.Combined with the results in energy transfer,it is proved again that EC can chelate Pb2+and form a complex.
It was found that Pb2+has two binding regions (site I and site II)on BSA,and the binding site of EC (site III) might be different from that of Pb2+.For the BSA-Pb2+-EC system,the non-competitive binding of EC and Pb2+at different sites of BSA formed a new ternary complex (Fig.7).For the BSA-(Pb2+-EC) system,Pb2+and EC formed a Pb2+-EC complex (lgK=9.89) with a complex ratio of 1:1.Complexation reaction is the main reaction between heavy metals and catechol derivatives.It indicated that the complex had strong stability,which was conducive to the excretion of heavy metal ions.The molecular docking results were consistent with the conclusion of site competition and reported studies[44].For the ternary system,the Pb2+-EC complex formed hydrogen bonds with Gln32,Asp107,Gln220,Glu339,Val342,Lys439,Cys447 and formed hydrophobic interactions with Lys465,Pro446,and Ala341 at two interaction sites.It was speculated that the Pb2+-EC complex shifted the binding sites of Pb2+on BSA and changed their interaction mode,thus having less effect on the structure of BSA.
We speculated that this is related to the chelating properties of EC.Compared with other flavanols,the existence of epigallate and hydroxyl,as well as the number and position of double bonds,contribute to the unique pharmacological properties of EC[45].The structural formula of EC is shown in the Fig.1.EC has a catechol structure,and the hydrogen atom in the phenolic hydroxyl group is more easily substituted or exchanged by some metal ions to form a five-membered ring chelate[46].
After entering the body through the digestive tract or other channels,Pb first combined with the blood cells and albumin in the blood system,then traveled to various tissues in the body with the blood[47].When the concentration of Pb in blood increased,Pb2+can antagonize with Ca2+.Pb would accumulate in bones,which had the highest amount of Ca2+[33].When the concentration of Pb in the body was reduced,the Pb in the bones was redistributed and released back into the circulatory system.Pb was reabsorbed into the small intestine,making it difficult to be excreted[48].Catechol moieties and combinations of hydroxyl and carbonyl groups present in polyphenols are centers of high affinity for metal ions[49].EC could chelate with Pb2+due to its special catechol structure[9].In this study,we found that EC altered the distribution of Pb2+and reduced the accumulation of Pb in blood and brain.It might be partly resulted from interference effect of EC on the binding process about Pb and albumin in blood,which had been simulated and explored in this paper by spectroscopic analysis and docking studiesinvitro.On the other hand,Pb got into brain and caused oxidative stress.EC had stable biological activity and exerted its antioxidant activity after entering tissues and organs[50].From all,it might speculate that EC significantly alleviated Pb induced oxidative stress in brain via suppressing Pb accumulation and activating Nrf2 signaling pathway.
5.Conclusion
In conclusion,EC significantly alleviated Pb-induced oxidative stress by activating the Nrf2/ARE pathway.EC can improve the learning and memory dysfunction induced by Pb in mice.EC interfered with the interaction between Pb and serum albumin in plasma.EC affected the distribution and metabolism of Pb in the body,which could reduce Pb damage to the body.This study provided research ideas for food-derived phenolic compounds to antagonize the hazard of Pb.
With the development of modern medical and biotechnology research,the mechanisms of action associated with EC toward various chronic diseases are becoming more apparent,and the pharmacological development and utilization of EC in food has been increasingly clarified.This study demonstrated the antagonistic effect of epicatechin on Pb exposure and provided research ideas for the antagonistic effect of foodborne phenolic compounds on heavy metal hazards.More studies are needed on the application of epicatechin in food and its availability in humans.
Conflicts of interest
No conflict of interest exists in the submission of this manuscript.
Acknowledgements
This work was supported by the National Key Research and Development Program of China under Grant (2022YFF1102800);the Graduate Scientific Research Innovation Project of Tianji(2022SKY109);the Project of Tianjin Science and Technology Program (22JCYBJC00360) and the Project of Tianjin Science and Technology Program (21ZYJDJC00060).
Appendix A.Supplementary data
Supplementary data associated with this article can be found,in the online version,at http://doi.org/10.26599/FSHW.2022.9250092.
- 食品科学与人类健康(英文)的其它文章
- Protective effects of oleic acid and polyphenols in extra virgin olive oil on cardiovascular diseases
- Diet and physical activity inf luence the composition of gut microbiota,benef it on Alzheimer’s disease
- Inf luence of nitrogen status on fermentation performances ofnon-Saccharomyces yeasts: a review
- Ganoderma lucidum: a comprehensive review of phytochemistry,eff icacy,safety and clinical study
- Resveratrol combats chronic diseases through enhancing mitochondrial quality
- Demonstration of safety characteristics and effects on gut microbiota of Lactobacillus gasseri HMV18

