The in vitro digestion fates of diacylglycerol under different intestinal conditions: a potential lipid source for lipid indigestion patients
Qingqing Xu,Weifei Wng,Dongxio Sun-Wterhouse,c,Qin Zou,Menglei Yn,Xun Liu,Dongming Ln,,Yonghu Wng,d,
a School of Food Science and Engineering, South China University of Technology, Guangzhou 510640, China
b Sericultural and Agri-Food Research Institute, Guangdong Academy of Agricultural Sciences, Guangzhou 510610, China
c School of Chemical Sciences, The University of Auckland, Private Bag 92019, New Zealand
d Guangdong Youmei Institute of Intelligent Bio-manufacturing Co., Ltd., Foshan 528200, China
Keywords: Diacylglycerol In vitro digestion Lipolysis level Cholestatic Exocrine pancreatic insuff iciency
ABSTRACT The in vitro digestion models mimicking the gastrointestinal (GI) tract of general population and lipid indigestion patients (with lower levels of bile salts or pancreatic lipase) were selected to investigate whether diacylglycerols (DAGs) are potential good lipid sources for these patients.Linseed oil-based DAG (LD) and linseed oil (LT) were selected.LD-based emulsion ((83.74 ± 1.23)%) had higher lipolysis degree than LT-based emulsion ((74.47 ± 1.16)%) when monitoring the GI tract of normal population as previously reported.Indigestion conditions seriously decreased the digestive degree of LT-based emulsion((40.23 ± 2.48)%-(66.50 ± 3.70)%) while showed less inf luence on LD-based emulsion ((64.18 ± 2.41)%-(81.85 ± 3.45)%).As opposed to LT-based emulsion,LD-based emulsion exhibited preference for releasing unsaturated fatty acids (especially oleic acid and α-linolenic acid) due to their different glycerolipid compositions.LD-based emulsion showed potential for providing lipids and nutrients (including essential fatty acids) for lipid indigestion patients.*Corresponding authors at: School of Food Science and Engineering,South China University of Technology,Guangzhou 510640,China.
1.Introduction
In human daily diet,triacylglycerols (TAGs) have long been the major lipids for ingestion,whilst diacylglycerols (DAGs),monoacylglycerols (MAGs) and free fatty acids (FFAs) are normally ingested in small amounts.In humans,the digestion of dietary lipids mainly begins in the stomach,involving emulsification of lipids,and partial hydrolysis of TAGs to DAGs and FFAs by gastric lipase (10%-30% of overall TAG lipolysis)[1].The digestion of the remaining TAGs takes place in the duodenal lumen due to the actions of pancreatic lipase and colipase on TAG and DAG molecules,yielding mainly absorbable 2-MAGs and FFAs[2].
Bile salts are produced via synthesis from cholesterol in hepatocytes of the liver and stored in the gallbladder till bile salts are demanded for the digestion of macronutrients.Bile salts play an essential role in hepatobiliary and intestinal homeostasis,as well as the digestion,transport and absorption of dietary lipids and fatsoluble vitamins[3].Owing to their distinct chemical structure,bile salts possess surfactant/detergent-like properties and are important contributors to micellar solubilization of dietary lipids (mainly TAGs).The impairments of bile formation and secretion cause the reduction of bile salts secretion or obstruction of bile f low (which is called “cholestasis”)[4].Chronic cholestasis causes malabsorption of vital micronutrients[5].Cholestasis is usually diagnosed in newborns,small infants and pregnant women.It is estimated that neonatal cholestasis affects one out of 2 500 live births[6],and the incidence rate of obstetric cholestasis ranges from 0.7% to 5.0% worldwide[7].Furthermore,pancreatic exocrine insufficiency is a common disease that affects the normal digestion of lipids (mainly TAGs) due to the deficiency of exocrine pancreatic enzymes,causing maldigestion and malabsorption of macronutrients and micronutrients (especially fat-soluble vitamins)[8].When pancreatic exocrine insufficiency occurs,carbohydrate digestion is still maintained,with lipid digestion being greatly impaired and protein digestion being mildly impaired[9].Pancreatic exocrine insufficiency is a syndrome that is often found in patients with chronic pancreatitis[10].The nutritional status of patients with pancreatic exocrine insufficiency is poor,e.g.,decreased levels of lipid-soluble bio-actives (including vitamins),phosphorus,albumin and essential fatty acids[10].Accordingly,it is important to find lipids as alternatives to TAGs,which are health-promoting lipids for general populations and can be digested and absorbed well by patients with cholestasis or pancreatic exocrine insufficiency.
DAG-based edible oils have gained increasing research interest and rising popularity among consumers owing to their preliminarily confirmed health benefits and desirable physicochemical properties similar to their corresponding TAG-based oils[11].In particular,DAGs exhibit beneficial effects on insulin sensitivity and in the fight against obesity,diabetes,atherosclerosis and related cardiovascular diseases[11].Compared with TAGs ingestion,the transport of chylomicrons is delayed and less chylomicron remnants are retained in the serum after DAGs ingestion[12].Comparative studies on DAGs and TAGs involvinginvitrodigestion revealed that DAGs exhibited a higher hydrolysis rate and a greater final digestion extent than TAGs[2,13].Therefore,it is of high interest to examine whether DAG-based oils could be used as potential dietary lipids for patients who suffer to lipid indigestion (especially those experience insufficient secretion of bile salt or pancreatic lipase).To make a contribution to the understanding of this aspect,this study was set up to examine the changes in acylglycerol profiles,fatty acid compositions and glycerolipid compositions of linseed oil (LT) and its derived linseed oil-based DAGs (LD) before and after the 3-phasein vitrodigestion i.e.,oral,gastric and small intestinal digestion phases using simulated saliva fluid (SSF),simulated gastric fluid (SGF) and simulated intestine fluid (SIF).
2.Materials and methods
2.1 Materials
LT ((93.93 ± 0.76)% of TAGs) and its derived LD ((82.93 ± 0.80)%of DAGs) were kindly supplied by the Guangdong Yue-shan Special Nutrition Technology Co.,Ltd.(Guangdong,China).Soy lecithin(98% phosphatidylcholine) and bile salts were purchased from the Macklin Biochemical Co.,Ltd.(Shanghai,China).Rabbit gastric extract (RGE,measured lipase activity of (9.23 ± 0.31) U/mg,using tributyrin as substrate) was purchased from the Lipolytech Company(Marseille,France).Pancreatic lipase (measured lipase activity:(48.50 ± 0.54) U/mg,using tributyrin as substrate,determined after centrifugation with 4 000 r/min for 2 min),triolein,monoolein and 14% boron trifluoride-methanol were all purchased from the Sigma Aldrich (St.Louis,MO,USA).1,3-Diolein,1,2-diolein and 37 fatty acid methyl esters (FAMEs) were obtained from the AMPEL Laboratory Technologies Inc.(Shanghai,China).n-Hexane,formic acid,2-propanol and acetonitrile were of liquid chromatograph mass spectrometry (LC-MS) grade.All other reagents were of analytical grade.
2.2 Emulsion preparation
In this study,LD and LT were subjected to emulsification using a commercial soy lecithin.The emulsification process involved three steps.Firstly,LD or LT (final concentration in the mixture: 20%) was mixed with soy lecithin (final concentration in the mixture: 1%),and the resulting mixture was heated at 60 °C for 60 min.Then,the heated mixture and water was mixed at a weight ratio of 21:79,and the obtained mixture was stirred in a container seated in a water bath at 60 °C using a magnetic stirrer (150 r/min;3 min).Finally,the stirred mixture was further homogenized at 18 000 r/min for 3 min using a high shear homogenizer (power: 1 250 W,FJ200-SH,Huxi Industrial Co.,Ltd.,Shanghai,China).Fresh LD/LT emulsions were used forinvitrodigestion.
2.3 In vitro digestion
Theinvitrodigestion of the LD/LT emulsion or the unemulsified LD/LT was established according to the procedure reported by Giang et al.[14].The SSF,SGF and SIF were prepared on the day of digestion according to Brodkorb et al.[15].LD/LT emulsion (or unemulsified LD/LT and water,20:80,m/m) was mixed with SSF in a 1:1 (m/m)final ratio,and the pH of mixture was adjusted to 7.0 with 1 mol/L NaOH.There was no starch in this digestion system,so theα-amylase was not added or considered[15].The above mixture was incubated at a water bath (37 °C,2 min) for monitoring the oral digestion.Oral chyme was mixed with pre-warmed SGF to reach a final ratio of 1:1 (m/m).
The gastric phase was carried out by mixing the sample in the oral phase with SGF,and the mixture was pre-heated at 37 °C for 30 min.Afterwards,RGE solution (dissolved in SGF) was added to initiate the lipid hydrolysis (with gastric lipase activity of 60 U/mL).The gastric digestion was performed at a static pH of 3.0 with pH-stat method and the mixture was incubated in a thermostatic shaker (120 r/min) at 37 °C for 2 h.The volume of 0.1 mol/L NaOH used to neutralize the released FFAs during the gastric digestion process was recorded.The gastric digesta was mixed with SIF,bile salts (final concentration in the sample for digestion: 0.0,2.5,5.0 and 10.0 mg/mL) and calcium chloride (final concentration in the sample for digestion: 0.6 mol/L).The pH of the mixture was immediately adjusted to 7.0 by adding NaOH (1 mol/L).All the samples for digestion were pre-heated at 37 °C for 30 min before the adding of pancreatic lipase (final lipase concentration in intestinal digestion system: 0.25,0.50,1.50 and 3.00 mg/mL).The subsamples were withdrawn at different time points in intestine simulated digestion phase (i.e.,at the 5th,10th,15th,30th,60thand 120thmin).The digestion phase was terminated through chemical inhibition (200 µL of 1 mol/L 4-bromophenylboronic acid in methanol).The study protocol is shown in Fig.1.
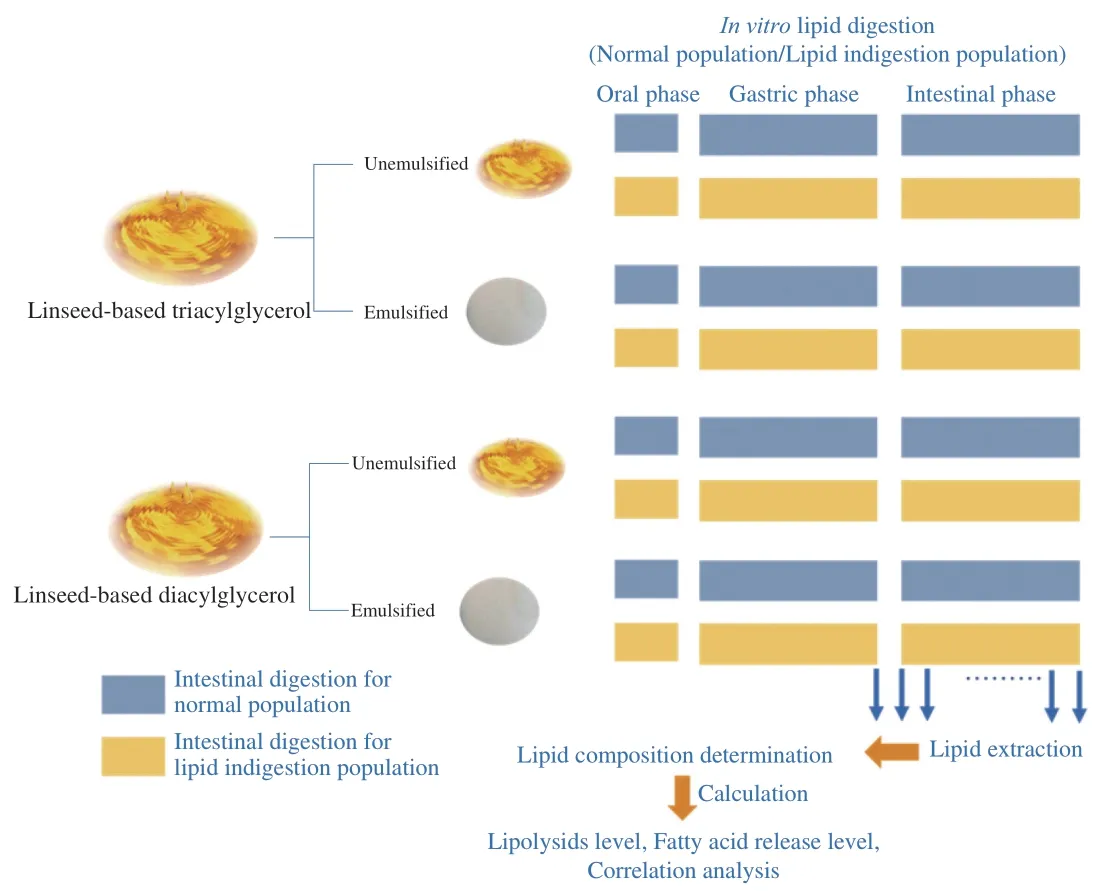
Fig.1 Study protocol.The digestion fates of the linseed-based diacylglycerol and linseed oil (with emulsified and unemulsified forms) in general population and lipid indigestion patients were investigated using the in vitro models.Subsamples were withdrawn at the end of gastric phase and during the intestinal phase (i.e.,at the 5th,10th,15th,30th,60th and 120th min).The lipid composition of raw materials and the lipids extracted from the subsamples were determined.The lipolysis degree,fatty acids release level and correlation analysis were further calculated.
2.4 Acylglycerol profile analysis by HPLC
The lipids in the LD emulsion,LT emulsion,unemulsified LD and unemulsified LT as well as their digested samples at different time points were extracted using hexane/methyl tertbutyl ether (50:50,V/V)following the procedure described by Martin et al.[2].The acylglycerol profiles of these lipids were determined by high performance liquid chromatography (HPLC) based on our published method[16].A HPLC system (Waters 2414,Milford,USA) was equipped with a refractive index detector (Waters 2414,Milford,USA) and a Phenomenex Luna silica column (250 mm × 4.6 mm,5 µm;maintained at 30 °C).Briefly,an aliquot (30 µL) of the lipid was mixed with 1.5 mL of the mobile phase (n-hexane,2-propanol and formic acid,18:1:0.003,V/V)and 1 g of anhydrous sodium sulfate.The mixture was subjected to centrifugation (6 744 g,4 °C,3 min).The obtained supernatant was filtered through a 0.22-µm pore size polyvinylidene fluoride(PVDF) membrane,and an aliquot (10 µL) of the filtered supernatant was injected into the HPLC system.The lipolysis percentage was calculated using Eq.(1).
wheremTAG0,mDAG0are the moles of TAGs and DAGs initially present in the vials;mTAGt,mDAGtare the moles of TAGs and DAGs at a specific digestion time point,t.
2.5 Fatty acid composition analysis by GC
The fatty acid composition was determined by gas chromatography (GC) using an 7890A system (Agilent Technologies,CA,USA) equipped with an Agilent HP-88 column (100 m × 0.25 mm inner diameter,0.2 µm) according to the method of Xu et al.[17].Each of the lipid obtained from section 2.4 was a mixture of FFAs,MAGs,DAGs and TAGs.The total amounts of individual fatty acids were determined according to the procedure reported by Tian et al.[18]with some modifications.Briefly,the lipid (50 mg) was mixed with 4 mL of methanolic sodium hydroxide solution (2 mol/L).The mixture was heated at 80 °C for 30 min,cooled down to room temperature with running water,and mixed with 4 mL of boron trifluoride-methanol(14%).The mixture was methylated at 80 °C for 8 min and then cooled down to room temperature,mixed withn-heptane (4 mL) and saturated sodium chloride solution (4 mL),vortexed and allowed standing till phases were separated.After the addition of anhydrous sodium sulfate to remove the water,the upper phase was used for injection into GC system.The fatty acid composition of total lipid was analyzed after normalization.
The composition of fatty acids in an esterified form of the lipid was determined by the transesterification method according to Chinese standard GB 5009.168-2016.Briefly,4 mL of isooctane was added into a 20-mL screw top glass tube to dissolve 40 mg of the lipid.The resulting mixture was mixed with 200 µL of methanolic sodium hydroxide solution (2 mol/L),vortexed using a vortex mixer for 30 s,and allowed standing for 10 min.Then,1 g of sodium bisulfate anhydrous was added to remove excess sodium hydroxide,and the resulting mixture was vortexed for 1 min and allows standing for 30 min.The upper organic phase was collected and used for injection into the GC system.
The release level of individual fatty acid was calculated using Eq.(2).
where TFAiis the total amount (moles) of the individual fatty acid;EFAiis the amount (moles) of individual fatty acid in the esterified form.
2.6 Glyceride analysis by UPLC-Q-TOF-MS
An ultra-performance liquid chromatography (UPLC,Waters,Milford,USA) system equipped with a Acquity UPLC CSH C18column (2.1 mm × 50 mm,1.7 µm,maintained at 60 °C) was coupled online to a quadrupole time of flight mass spectrometry (Q-TOF-MS,Waters,Milford,USA) for glyceride analysis[19].Eluent A and eluent B were water/acetonitrile (40:60,V/V) and isopropanol/ acetonitrile(90:10,V/V),respectively,both contained 0.1‰ (volume ratio) of formic acid and 10 mmol/L of ammonium acetate.The flow rate was 0.5 mL/min and the injection volume was 5 µL.The elution gradient was as follows: 0 min,15% B;0-2 min,30% B;2-2.5 min,48% B;2.5-11 min,72% B;11-12 min,99% B;12-15 min,15% B.
Identification of glycerides was performed in the positive electrospray ionization mode under the following MS conditions:capillary voltage,3.0 kV;cone voltage,30 V;source block temperature,120 °C;cone gas flow,50 L/h;desolvation gas (N2)flow rate,800 L/h;desolvation temperature,500 °C.The mass range was fromm/z100 tom/z2 000 with a scan duration of 0.1 s.MS data were collected in the model of Q-TOF-MSEand were analyzed by UNIF software.
2.7 Statistical analyses
The experiments were carried out for at least three times,and data are presented as means ± standard deviations.Differences were considered to be significant atP< 0.05,which were determined by the Turkey’s test using IBM SPSS 23.0 (IBM Corp.,Chicago,USA).The graphs were constructed using Origin 2018 (OriginLab,MA,USA) and open-source software R Studio version 1.4.1106(Integrated Development for R.RStudio,PBC,Boston,MA,USA).Pearson’s and Spearman’s correlations were also analyzed using IBM SPSS 23.0 at the level ofP< 0.05 for significant difference.
3.Results and discussion
3.1 Lipid compositions of the experimental lipids
Lipid compositions of LD and LT are summarized in Fig.2,and their proportions are all based on molar ratios.As shown in Fig.2A,LD contained (11.33 ± 0.77)% of TAGs,(0.30 ± 0.08)% of FFAs,(58.60 ± 0.70)% of 1,3-DAGs,(27.33 ± 0.30)% of 1,2-DAGs,(2.21 ± 0.12)% of 1-MAGs and (0.22 ± 0.19)% of 2-MAGs.Fresh whole LT was largely composed of TAGs ((86.64 ± 1.94)%),however,there was also minor contents of DAGs ((2.15 ± 0.06)% of 1,3-DAGs and(1.34 ± 0.05)% of 1,2-DAGs) and FFAs ((9.87 ± 1.92)%).
Fig.2B depicts the fatty acid compositions of LD and LT.The predominant fatty acid of LD wasα-linolenic acid (ALA,C18:3,(51.22 ± 0.28)%),followed with oleic acid (C18:1,(20.30 ± 0.36)%),linoleic acid (C18:2,(15.78 ± 0.04)%),palmitic acid (C18:0,(7.59 ± 0.02)%) and stearic acid (C16:0,(5.11 ± 0.01)%).LT showed similar fatty acid composition with LD (P> 0.05).Wang et al.[20]also observed the similarity between the fatty acid composition of DAG and the corresponding TAG.Furthermore,the glyceride composition was analyzed.The main DAGs in LD were LnLn,LLn and OLn (Ln,α-linolenic acid;L,linoleic acid;O,oleic acid),accounting for (25.04 ± 0.86)%,(23.07 ± 1.24)% and (21.13 ±1.11)% of total DAGs.In LT,the top four TAGs were OLnLn (or LLLn),LnLnLn,LLnLn,and OOLn (or OLL),accounting for (15.84 ± 0.40)%,(13.76 ± 0.51)%,(10.69 ± 0.37)%and (9.16 ± 0.64)% of total TAGs,respectively (Figs.2C-D).
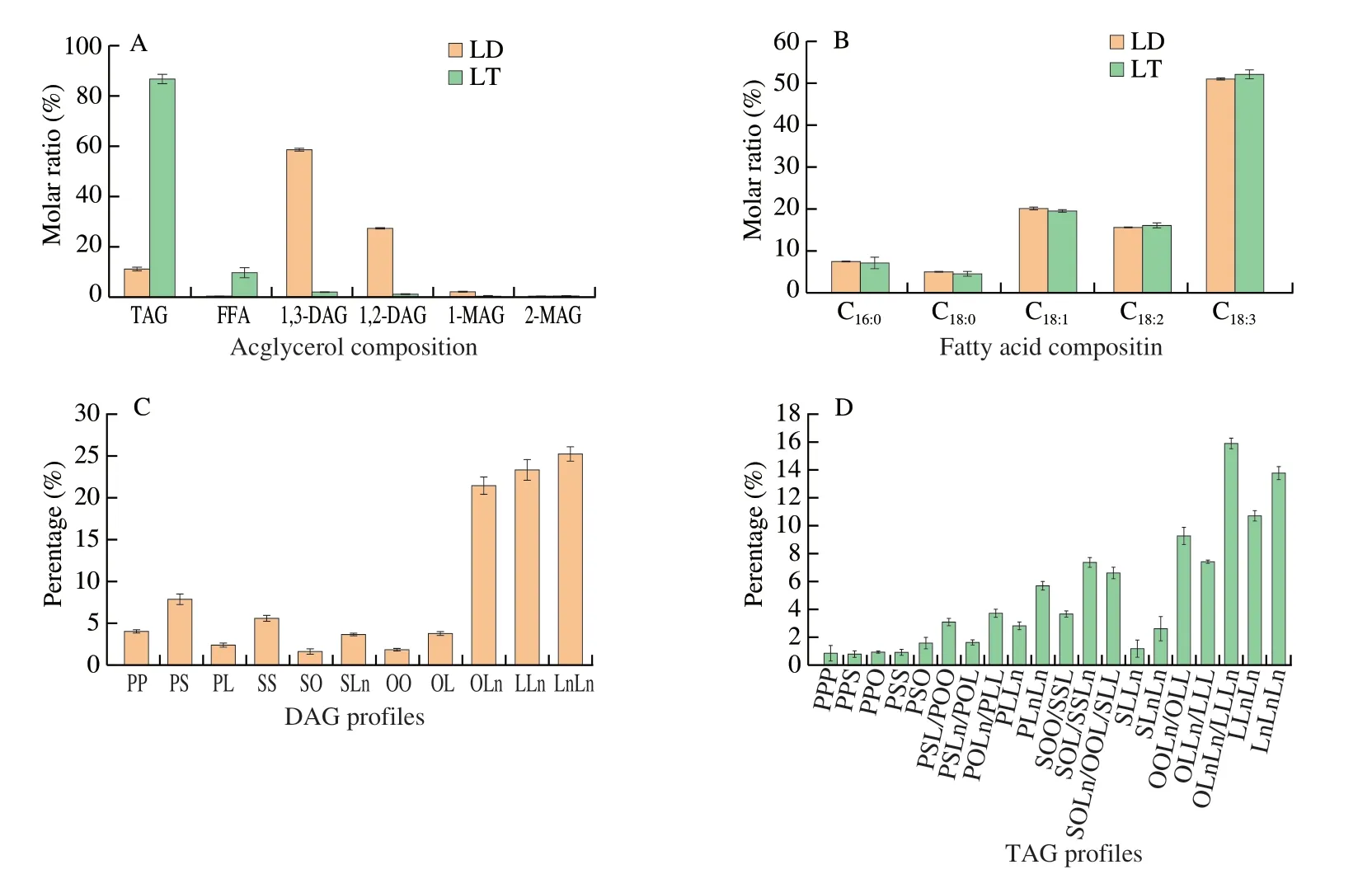
Fig.2 Lipid compositions of LD and LT.(A) Acylglycerol profiles,(B) fatty acid compositions and (C-D) glyceride compositions (C for DAG profiles and D for TAG profiles).P,C16:0 (palmitic acid);S,C18:0 (stearic acid);O,C18:1 (oleic acid);L,C18:2 (linoleic acid);Ln,C18:3 (α-linolenic acid).PP,PS,PL,SS,SO,SLn,OO,OL,OLn,LLn,and LnLn represent the diacylglycerol molecules with different fatty acids;PPP,PPS,PPO,PSS,PSO,PSL,POO,PSLn,POL,POLn,PLL,PLLn,PLnLn,SOO,SSL,SOL,SSLn,SOLn,OOL,SLL,SLLn,SLnLn,OOLn,OLL,OLLn,LLL,OLnLn,LLLn,LLnLn,and LnLnLn represent the triacylglycerol molecules with different fatty acids.The same below.
3.2 Analysis of lipolysis species and lipolysis level during in vitro digestion
It must be noted that during theinvitrogastric digestion of LD/LT emulsion or the unemulsified LD/LT with pH-stat method,almost no fatty acids were released.The high level of polyunsaturated fatty acids in LD/LT and the preference of gastric lipase on short-and medium-chain fatty acids[21]might be related with this phenomenon.However,this result is not totally in alignment with previous study which reported 6.2% of fatty acids were released during the gastric digestion of LT[22].This might be explained by the different emulsifiers[23]and gastric lipase used in the digestion process.Therefore,the subsequent description of theinvitrodigestion refers to theinvitrointestinal digestion unless otherwise specified.
3.2.1 Effects of bile salts content
The contents of bile salts in the small intestine depends on a large number of factors,especially the heathy status of an individual[24].In this study,theinvitrodigestion with bile salts concentrations of 5.0 and 10.0 mg/mL was used as a digestion model for general population based on the reported literatures[15,25].Theinvitrodigestion with lower bile salts contents (0.0 and 2.5 mg/mL) was chosen to monitor the gastrointestinal (GI) tract of the patients with cholestasis.
The acylglycerol profiles of the LD/LT emulsion or the unemulsified LD/LT during the digestion process with the different levels of bile salts (0.0-10.0 mg/mL,and 1.5 mg/mL of pancreatic lipase) all followed a similar trend (Figs.3 and 4).In general,there was a decrease in the content of initial substates (mainly DAGs or TAGs) and an increase in the content of lipolytic products (mainly FFAs for all investigated samples).Similar general pattern of lipid-class composition was also acquired in the rat small intestine[26].The digestion rate at the beginning time was faster and gradually decreased after 20 min.Similar phenomena have been reported earlier[2,13]and was explained by the fact that the surface-active MAGs and FFAs produced during the digestion process can regulate the fat digestion.Especially,the 2-MAGs with high concentration were found to shortly dominant the surface which push the lipase into the aqueous phase[27].Lipolysis was therefore slowed down because the contact between lipid droplets and pancreatic lipase was restricted[2].Meanwhile,the concentration of substrates which can be hydrolyzed also gradually decreased.

Fig.3 Time dependency of acylglycerol profiles during the in vitro intestinal digestion process of LD emulsion (A) and LT emulsion (B) when stimulating the gastrointestinal tract of general population (1.5 mg/mL of pancreatic lipase and 5.0 mg/mL of bile salts).
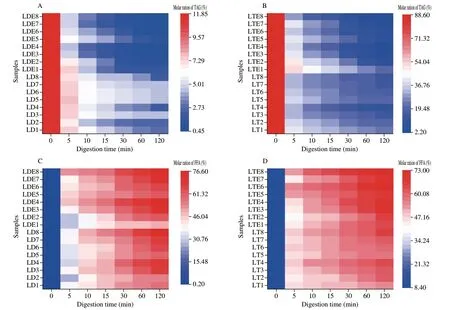
Fig.4 Effects of bile salts content (0.0,2.5,5.0 and 10.0 mg/mL) and pancreatic lipase content (0.25,0.50,1.50 and 3.00 mg/mL) on the changes of acylglycerol profiles (A,B: TAG level;C,D: FFA;E,F: 1,3-DAG;G,H: 1,2-DAG;I,J: 1-MAG;K,L: 2-MAG) of unemulsified LD/LT and LD/LT emulsions during the in vitro intestinal digestion process.LD1-4: unemulsified LD digested in the presence of 0.0,2.5,5.0 and 10.0 mg/mL of bile salts,respectively;LT1-4:unemulsified LT digested in the presence of 0.0,2.5,5.0 and 10.0 mg/mL of bile salts,respectively;LDE1-4: LD-based emulsion digested in the presence of 0.0,2.5,5.0 and 10.0 mg/mL of bile salts,respectively;LTE1-4: LT-based emulsion digested in the presence of 0.0,2.5,5.0 and 10.0 mg/mL of bile salts,respectively;LD5-8: unemulsified LD digested in the presence of 0.25,0.50,1.50 and 3.00 mg/mL of pancreatic lipase,respectively;LT5-8: unemulsified LT digested in the presence of 0.25,0.50,1.50 and 3.00 mg/mL of pancreatic lipase,respectively;LDE5-8: LD-based emulsion digested in the presence of 0.25,0.50,1.50 and 3.00 mg/mL of pancreatic lipase,respectively;LTE5-8: LT-based emulsion digested in the presence of 0.25,0.50,1.50 and 3.00 mg/mL of pancreatic lipase,respectively.In fact,the digestion conditions for 3 and 7 were the same which could all monitor the gastrointestinal tract of general population.The same below.

Fig.4 (Continued)
Despite the acylglycerol profiles,the lipolysis level was important indicator for evaluating the extent of lipid digestion[28].The first thing to say is that,the lipolysis level at 120 min was in decreasing order when monitoring the digestion of the general population: LD emulsion((83.74 ± 1.23)%) > LT emulsion ((74.47 ± 1.16)%) > unemulsified LD ((58.58 ± 2.20)%) > unemulsified LT ((46.10 ± 1.60)%,Fig.5A).In general,the lower the esterification of the glyceride the higher extent of lipolysis[13].Furthermore,it is known that the emulsification process with suitable emulsifiers could improve the digestion extent[2].The emulsification process can provide larger contact area.Moreover,bile salts are good at replacing the adsorbed material (mainly protein and ionic polar lipids) from the oil droplets and preparing the interface for pancreatic lipase.The lipolysis levels of emulsified lipids were therefore higher than that of unemulsified ones[29].It can be concluded that the unemulsified LD/LT was not suitable for lipid indigestion individuals due to their low digestion extent even under the normal condition.
Furthermore,LD emulsions ((64.18 ± 2.41)%-(88.53 ± 1.84)%)showed higher lipolysis levels than LT emulsion ((40.23 ± 2.48)%-(78.59 ± 5.62)%) in the presence of any level of bile salts (0-10.0 mg/mL,Fig.5B).The 1,3-regiospecificity of pancreatic lipase caused higher 2-MAGs level during the lipolysis process of LT emulsion[30].The high surfactantcy of 2-MAGs could inhibit the action of pancreatic lipase which was also declared above[27].Although lipolysis of LD emulsion presented certain level of 1-MAGs with high interfacial activity,1-MAGs could be quickly hydrolyzed by the action of 1,3-sepcific lipase (pancreatic lipase)[2].The production of 1-MAGs therefore exhibited low impacts on the lipid digestion.In addition to this,the amphipathic property of LD is thought to be important which could solubilize partial phospholipids and leading to totally different interface with LT emulsion[31].This means the interface of LD emulsion was consisted with LD and phospholipids which leading to a decrease of the emulsion stability when compared with LT emulsion[31].According to Ye et al.[1],the low emulsion stability could help bile salts to better prepare the interface for pancreatic lipase,and therefore improve the lipolysis level.
Bile salts play a crucial role in digestion by replacing other compounds on the interface for the adsorption of pancreatic lipase and instigate the lipolysis[29].In belief,bile salts are responsible for majority of lipid lipolysis during the digestion.According to the principle,as the bile salts content decreases,the hydrolysis level decreases for lipids[29].This phenomenon was heavily reported,but only for TAG-rich lipids[1,29].In this study,the digestion of LT emulsion was consistent with previously reported studies[1,32].The lipolysis level of LT emulsion decreased quickly from (78.59 ± 5.62)% to(40.23 ± 2.48)% with decreasing of bile salt contents from 10.0 mg/mL to 0 mg/mL (P< 0.01,Fig.5B).Moreover,when bile salts were absent or in a particular low level,phospholipids could dominant the interface for LT emulsion.Since pancreatic lipase exhibited a reduced affinity for the oil droplets when phospholipids were present in the interface[33],the lipase activity was inhibited[34].In contrast,in the system of LD emulsion,the main interfacial active components were soy lecithin and LD.The presence of the hydroxyl group in the DAG molecule can lead to relatively high polarity,which resulting in more distribution of phospholipids in the oil phase[31].This phenomenon contributed to a decrease in phospholipid content at the interface,and more DAG molecules was distributed on the interface and directly exposed to pancreatic lipase[31].Meanwhile,the produced 1-MAGs showed low capacity on occupying the interface which showed low impacts on digestion as described above[2].These interfacial behaviors led to largely different results for the digestion of LD emulsion with different bile salts contents.The bile salts contents did influence the lipolysis level of LD emulsion with a smaller degree,and the lipolysis level decreased from (88.53 ± 1.84)% to (64.18 ± 2.41)% when the bile salts level decreased (Fig.5B).Good digestibility of LD emulsion was observed even under the extremely poor bile salts content.It could be concluded that LD emulsion might be a potential good lipid source for cholestasis patients.
3.2.2 Effects of pancreatic lipase content
Though 2 000 U/mL of pancreatic lipase was suggested by Brodkorb et al.[15]forinvitrodigestion process,a range of 63-550 U/mL digest were more frequently used in the reported literatures[32,35].In this paper,intestinal phase with 1.5 and 3.0 mg/mL of pancreatic lipase was used to stimulate the GI tract of general population.Furthermore,invitrodigestion model with lower pancreatic lipase content (0.25 and 0.5 mg/mL) was used to monitor the intestinal environment of patients suffered pancreatic exocrine insufficiency.
The change of acylglycerol profiles of LD/LT emulsion or the unemulsified LD/LT during the digestion process in the presence of different pancreatic lipase contents (0.25-3.0 mg/mL,with bile salts of 5 mg/mL) as a function of digestion time are displayed in Figs.3 and 4.The changes of TAGs,1,3-DAGs,1,2-DAGs,1-MAGs,2-MAGs and FFAs concentrations during intestinal digestion showed similar trends with the section 3.2.1.
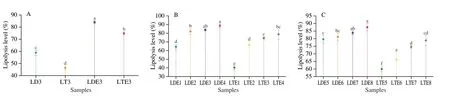
Fig.5 Lipolysis level at 120 min of (A) unemulsified LD/LT and LD/LT emulsion when stimulating the gastrointestinal tract of general population (1.5 mg/mL of pancreatic lipase and 5.0 mg/mL of bile salt);(B) LD/LT emulsion affected by the level of bile salts;(C) LD/LT emulsion affected by the level of pancreatic lipase.LD3: unemulsified LD digested when stimulating the gastrointestinal tract of general population;LT3: unemulsified LT digested when stimulating the gastrointestinal tract of general population.Different letters represent the significant differences between different samples (P < 0.05).
Initially,the unemulsified LD/LT exhibited low lipolysis level((58.58 ± 2.20)% and (46.10 ± 1.60)%,respectively,Figs.5A and 6)even when monitoring theinvitrodigestion of general population,thus,unemulsified LD/LT might not suitable for patient with exocrine pancreatic dysfunction.This result was also confirmed by Couëdelo et al.[36],who reported that the absence of emulsifiers could delay the GI lipolysis.
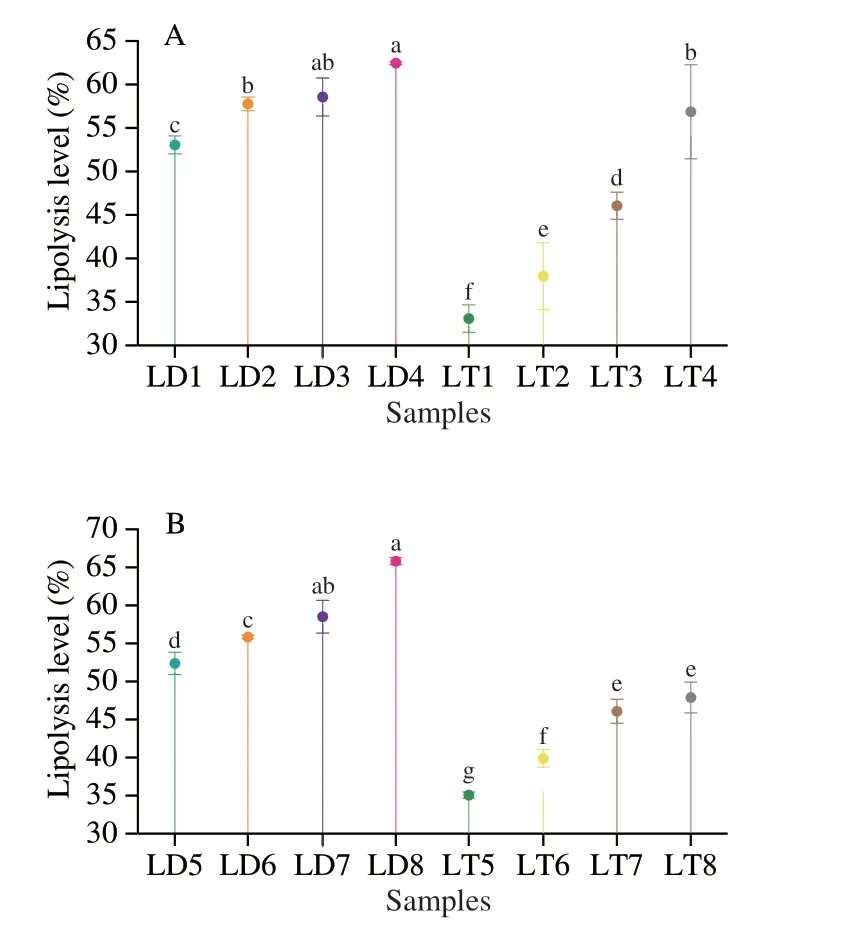
Fig.6 Lipolysis level at 120 min of unemulsified LD/LT affected by the level of (A) bile salts and (B) pancreatic lipase.Different letters represent the significant differences between different samples (P < 0.05).
Furthermore,the lipolysis level of LT emulsioninvitrodigestion decreased obviously (from (78.67 ± 3.41)% to (59.85 ± 1.83)%,P< 0.001,Fig.5C) with the decreasing of pancreatic lipase contents(from 3.0 mg/mL to 0.25 mg/mL).This phenomenon was consistent with a previous study[24].The decrease in pancreatic lipase content inevitably led to less substrate or interface in contact with lipase,which causing to slower and lower lipolysis.Meanwhile,the main product,2-MAGs,during the digestion of LT emulsion could further inhibit the contact between the lipase and substrate[37].Thus,in recent years,2-MAGs were used to reduce the calorie uptake without cutting down the intake[38].Moreover,excess of undigested lipids in colon can cause some side effects such as steatorrhea and fecal incontinence[34].It can be therefore assumed that LT emulsion was not suitable for the patients lack of pancreatic lipase.Further,50%-60% of dietary TAG-rich lipids for these patients were not absorbed as previously reported[39-40].This similar digestibility to this experimental result further confirmed that our choice for lipase content was suitable for mimicking the intestinal environment of patients with exocrine pancreatic dysfunction.
In contrast,LD emulsion exhibited higher lipolysis level((79.46 ± 2.67)% when pancreatic lipase was 0.25 mg/mL) which were not so largely affected by pancreatic lipase level (from 3.0 mg/mL to 0.25 mg/mL,P< 0.05,Fig.5C).The higher affinity of pancreatic lipase to interface of LD emulsion described above could explain this result.This guaranteed the contact area even under pretty low level of pancreatic lipase.Furthermore,the lower 2-MAG/1-MAG ratio during the digestion process of LD emulsion than LT emulsion showed low impacts for lipid digestion[2](Fig.7).Above all,it can be concluded that the LD emulsion might be a potential lipid source for patients with exocrine pancreatic dysfunction.
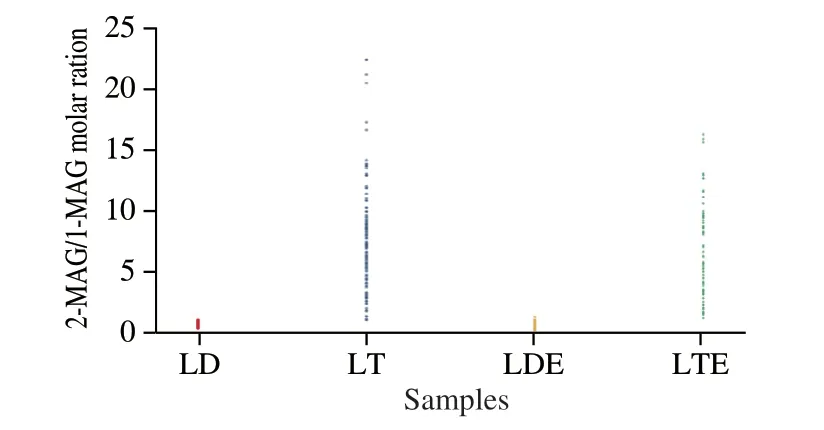
Fig.7 2-MAG/1-MAG molar ratios of unemulsified LD/LT and LD/LT emulsion during the whole in vitro intestinal digestion process in the presence of different levels of bile salts (0.0,2.5,5.0 and 10.0 mg/mL) and pancreatic lipase (0.25,0.50,1.50 and 3.00 mg/mL).
3.3 Release profile of individual fatty acid
More importantly,according to Abdel-Ghaffar et al.[41],the infants and children with lipid indigestion showed great risk of essential fatty acids deficiency which could lead to growth disturbances and poor development of neurological,visual and psychomotor.Thus,the individual fatty acid release preference of LD/LT emulsion was also compared.The release profiles of individual fatty acid during the digestion of LD/LT emulsion are shown in Figs.8 and 9.The individual fatty acid released rapidly in the first 20 min and gradually reached to a plateau.This phenomenon was consistent with the change of lipolysis level described above.Similar results were also reported by Ye et al.[28].
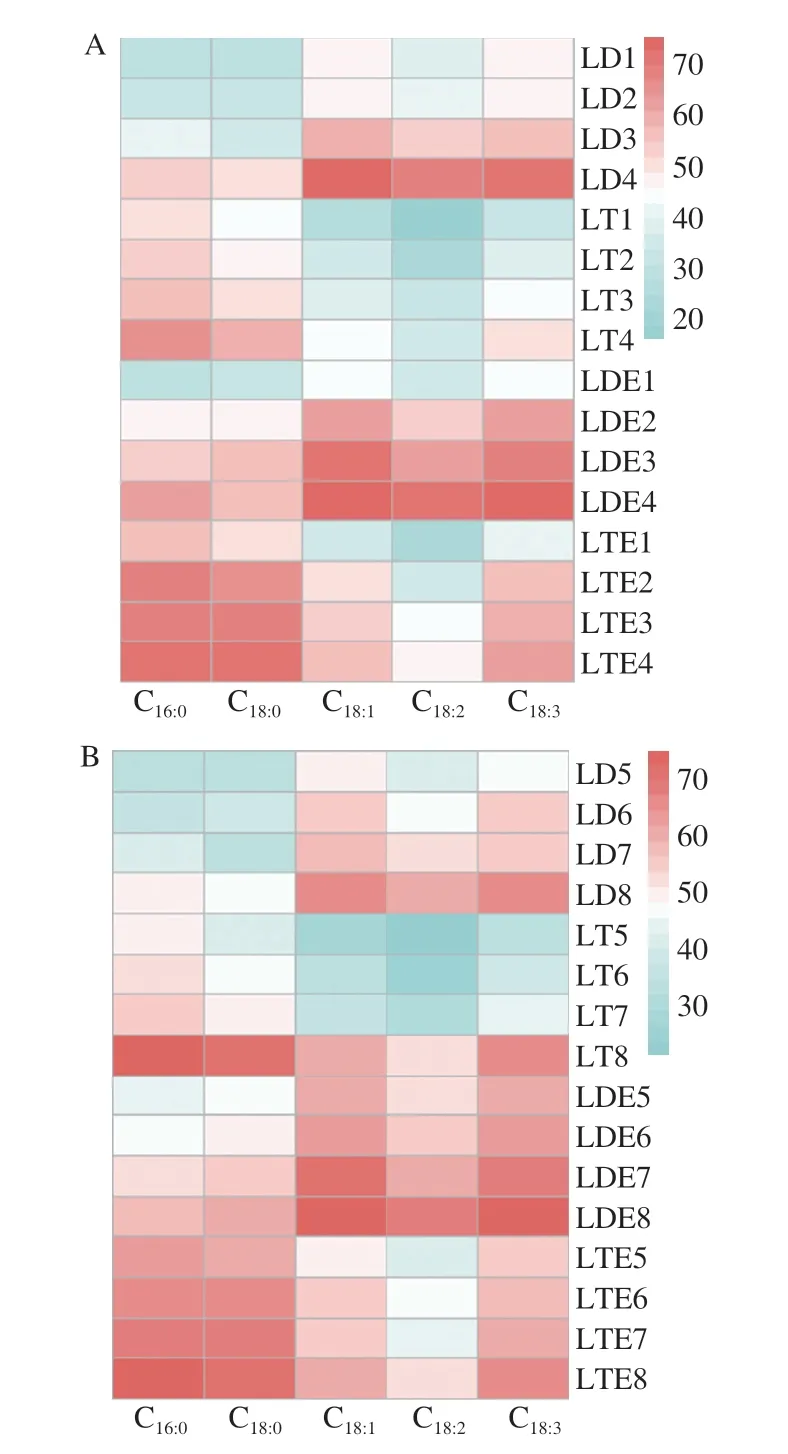
Fig.8 Individual fatty acids release level from unemulsified LD/LT and LD/LT emulsion after the in vitro intestinal digestion (at 120 min) was affected by (A) bile salts content and (B) pancreatic lipase content.

Fig.9 Individual fatty acids release level from unemulsified LD/LT and LD/LT emulsions was affected by bile salts content during the in vitro intestinal digestion.(A-D) LD1-4;(E-H): LT1-4;(I-L): LDE1-4;(M-P): LTE1-4.
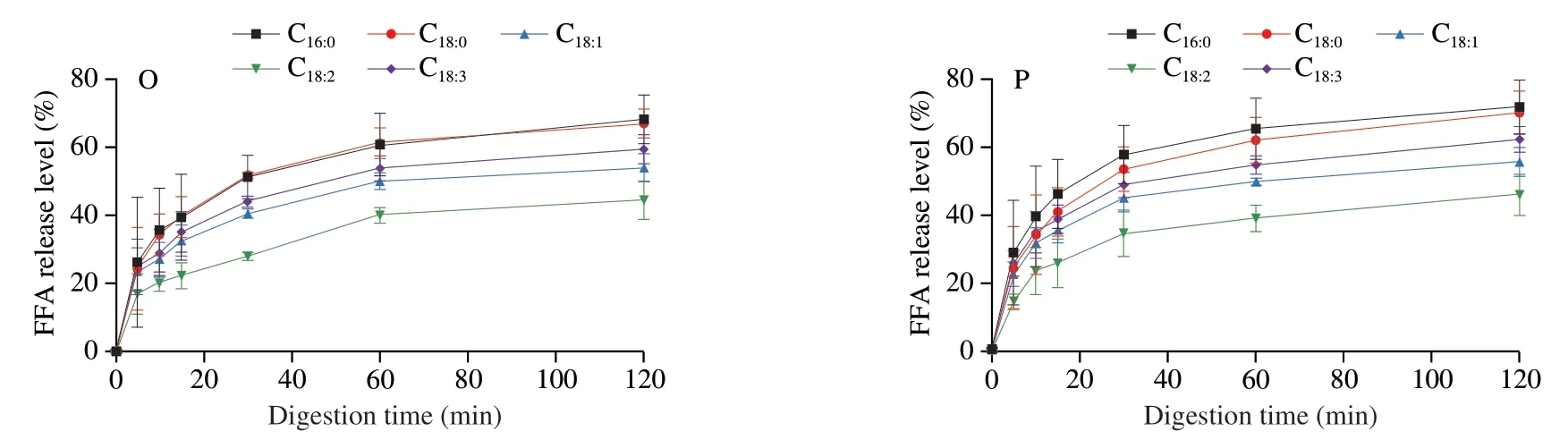
Fig.9 (Continued)
The release levels of C16:0,C18:0,C18:1,C18:2and C18:3at 120 min,in digestion system of LT emulsion were (68.21 ± 7.15)%,(67.02 ± 4.21)%,(54.06 ± 4.09)%,(44.54 ± 5.61)% and (59.50 ± 4.29)% when monitoring the condition of general population,respectively,with the following order: C18:0≈ C16:0> C18:3> C18:1> C18:2(Figs.8A and 9O).It should be noted that this rule hold at any sampling time.This order was consistent with the report of Ye et al.[28]who explored the digestion fate of LT and declared that the saturated fatty acids were released faster than unsaturated ones[42-43]and poly-unsaturated fatty acids seemed difficult to be released[28].Surprisingly,the digestion of LD emulsion showed great differences.The release percentage of individual fatty acid decreased in the following order:C18:1≈ C18:3> C18:2> C18:0≈ C16:0(Figs.8A and 9K).The LD emulsion showed great priority on releasing unsaturated fatty acids(including essential fatty acids).This result demonstrated that the DAG structure showed great influence on the fatty acid release.This might be related with the different position of these fatty acids between the TAGs and DAGs,and this should be further studied.Based on this phenomenon,it could be concluded that the LD emulsion could better improve human health by providing unsaturated fatty acids (mainly focused on the essential fatty acids).Similar pattern was also observed for theinvitrodigestion process with different levels of bile salts and pancreatic lipase (Figs.8,9 and 10).
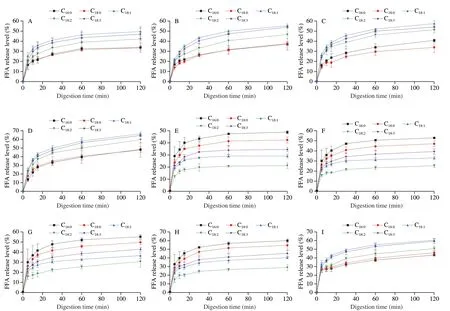
Fig.10 Individual fatty acids release level from unemulsified LD/LT and LD/LT emulsions was affected by pancreatic lipase content during the in vitro intestinal digestion.(A-D) LD5-8;(E-H): LT5-8;(I-L): LDE5-8;(M-P): LTE5-8.
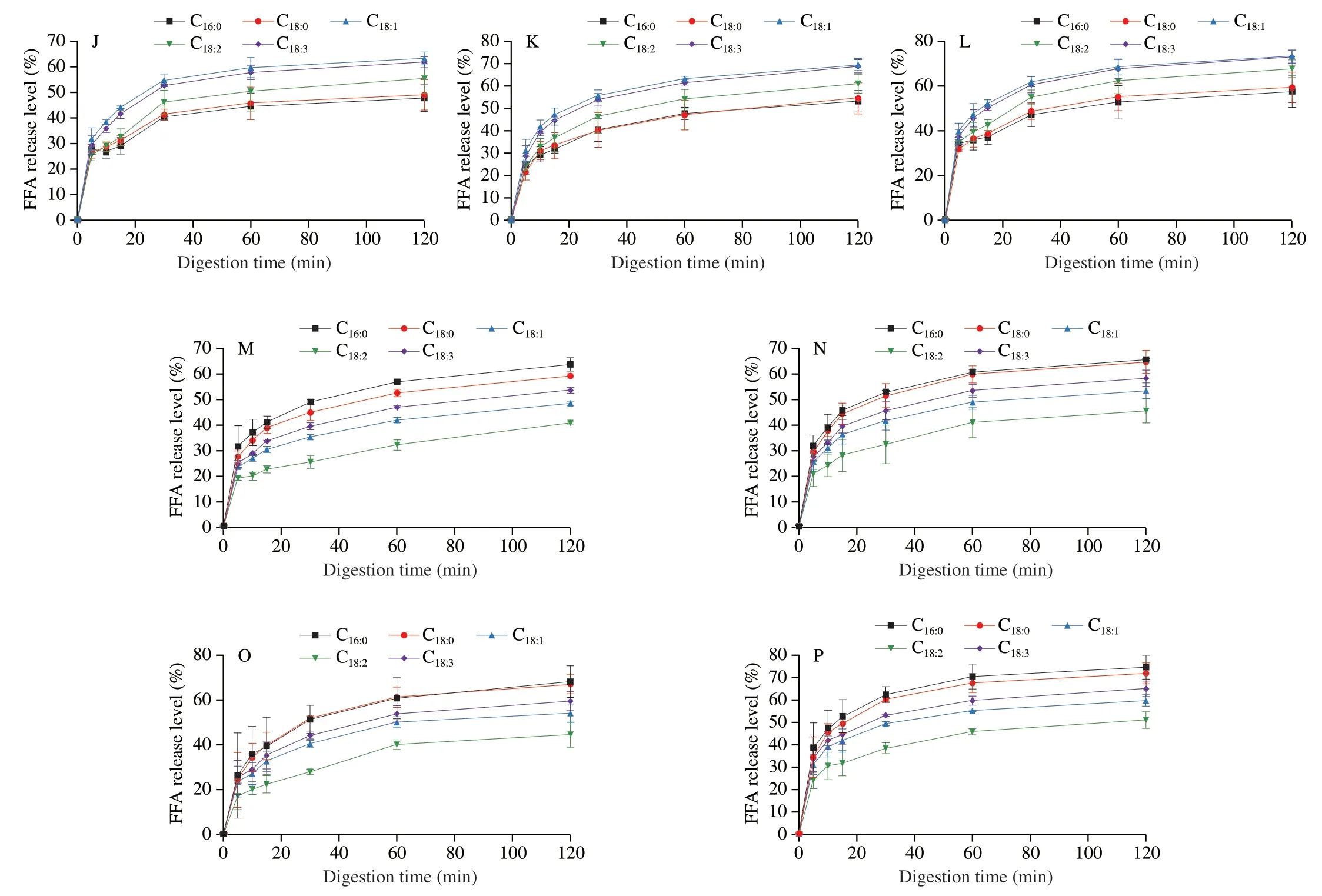
Fig.10 (Continued)
3.4 Evolution of the TAG or DAG profiles during lipolysis
The percentages of TAG or DAG molecular species are reported and summarized in Figs.11 and 12.The percentages of UU and UUU (diunsaturated-DAG and triunsaturated-TAG) were decreased from (75.13 ± 1.06)% to (24.80 ±3.62)% and (55.86 ± 1.36)% to(31.08 ± 1.51)% during the digestion process of LD and LT emulsion when monitoring theinvitrodigestion of general population,respectively (Fig.11).This result induced the quickly digested property of UU and UUU.Other than that,a delayed hydrolyzed of SS,SSS,SSU (desaturated-DAG,trisaturated-TAG and disaturatedmonounsaturated-TAG) was inferred by the rise of the percentages of these TAGs or DAGs during the digestion process (Fig.11).That was related with the higher melting points of SS,SSS and SSU and lower melting points of UU and UUU[30,44].It could be concluded that the saturation degree of DAG or TAG molecules played a significant role on the ease or difficulty of the lipolysis process.This was also evidenced by Ren et al.[21],who reported that the lipids with high melting points have the potential to delay the lipid release.It should be noticed that the main DAGs (OLn,LLn,LnLn) in LD were all UU (accounting for more than 70%,Figs.2C-D),which further improved the digestibility of LD emulsion.Similar phenomenon was also observed when the levels of bile salts and pancreatic lipase were changed (Fig.12).
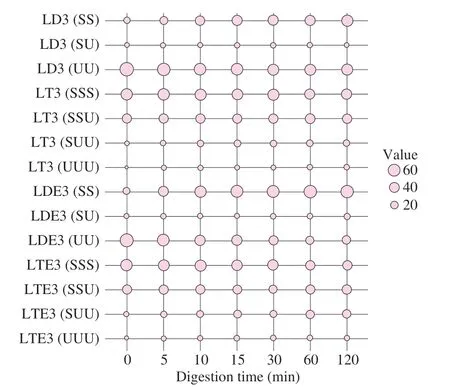
Fig.11 Time dependency of DAG or TAG profiles during the in vitro intestinal digestion process of LD/LT emulsion when stimulating the gastrointestinal tract of general population (1.5 mg/mL of pancreatic lipase and 5.0 mg/mL of bile salts).SS: desaturated-DAG;SU: monosaturatedmonounsaturated-DAG;UU: diunsaturated-DAG;SSS: trisaturated-TAG;SSU:disaturated-monounsaturated-TAG;SUU: monosaturated-diunsaturated-TAG;UUU: triunsaturated-TAG;LDE3: LD-based emulsion digested when stimulating the gastrointestinal tract of general population;LTE3: LT-based emulsion digested when stimulating the gastrointestinal tract of general population.
3.5 Pearson and Spearman correlations analysis
Pearson and Spearman correlations between emulsification state(emulsified or unemulsified),lipid structure (TAG or DAG),bile salts content and pancreatic lipase content with lipolysis level are shown in Table 1.The strength of association between two variables is described by Pearson’s correlation coefficients (r).The correlation coefficient and the degree of correlation are defined as follows:weak correlation,r< 0.5;moderate correlation,0.5 <r< 0.7;strong correlation,0.7 <r< 0.9;perfect correlation,r> 0.9.
In this study,pancreatic lipase showed no correlation with lipolysis level (P> 0.05).Meanwhile,Pearson correlation coefficient analysis revealed that emulsification state,lipid structure and bile salts content were correlated with the lipolysis level after the digestion process,and with the following order: emulsification state (P=0.000,r=0.724) > lipid structure (P=0.000,r=0.439) > bile salts content(P=0.000,r=0.376).
First of all,it was strange that the pancreatic lipase and bile salts levels showed no or low correlations with the lipolysis level after the digestion process.This can be explained by the huge influence of lipid structure and emulsification state of the lipids on the lipolysis level,which overshadowing the role of pancreatic lipase and bile salts.Furthermore,it can be concluded that replacing the lipid and improve the emulsification state were good ways to alleviate malnutrition in patients with lipid indigestion due to the strong influence of lipid structure and emulsification state on lipolysis level.
4.Conclusion
In this study,theinvitrodigestion behaviors of LD/LT emulsion were examined at the standard and poor digestion conditions.The digestion process of LT emulsion was significantly delayed with the decreasing of bile salts or pancreatic lipase level.In contrast,LD emulsion showed good digestibility even under the extremely poor digestion conditions.In contrast to LT-based digestion system,LD-based one showed a preference on releasing unsaturated fatty acids(mainly C18:1and C18:3).This also implied that the LD emulsion might be more beneficial to the adsorption and utilization of unsaturated fatty acids in human body.Besides,based on the HPLC-MS analysis,it could be noticed that the UU,UUU could be digested quickly.As it happens,LD mainly consisted with UU (accounting for more than 70%) which might further enhance the digestion of LD emulsion.In conclusion,LD emulsion can be considered as a good nature lipid source for patients with cholestasis and pancreatic exocrine insufficiency.In this study,animal and clinical experiments were not carried out,and these will be the focus of follow-up research.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
National Key R&D Program of China (2022YFC2805100),National Science Fund for Key Program of National Natural Science Foundation of China (31930084),National Science Fund for Distinguished Young Scholars of China (31725022),China Agriculture Research System (CARS-18-ZJ0503),Guangdong Provincial Key R&D Programme (2022B0202010002),Science and Technology Innovation Project of Foshan City (FS0AAKJ919-4402-0013).
- 食品科学与人类健康(英文)的其它文章
- Protective effects of oleic acid and polyphenols in extra virgin olive oil on cardiovascular diseases
- Diet and physical activity inf luence the composition of gut microbiota,benef it on Alzheimer’s disease
- Inf luence of nitrogen status on fermentation performances ofnon-Saccharomyces yeasts: a review
- Ganoderma lucidum: a comprehensive review of phytochemistry,eff icacy,safety and clinical study
- Resveratrol combats chronic diseases through enhancing mitochondrial quality
- Demonstration of safety characteristics and effects on gut microbiota of Lactobacillus gasseri HMV18

