Cyanidin-3-glucoside protects the photooxidative damage of retinal pigment epithelium cells by regulating sphingolipid signaling and inhibiting MAPK pathway
Tingting Liu,Wentao Qi,Wenting Peng,Jianan Zhang,Yong Wang,
a School of Health Science and Engineering, University of Shanghai for Science and Technology, Shanghai 200093, China
b Academy of National Food and Strategic Reserves Administration, Beijing 100037, China
c Department of Chemistry, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA
Keywords: Cyanidin-3-glucoside Ceramide MAPK pathway Mitochondria-dependent apoptosis Lipidomics analysis
ABSTRACT Cyanidin-3-glucoside (C3G) is the most common anthocyanin in dark grains and berries and is a food functional factor to improve visual health.However,the mechanisms of C3G on blue light-induced retinal pigment epithelial (RPE) cell photooxidative damage needs further exploration.We investigated the effects of C3G on blue light-irradiated A2E-containing RPE cells and explored whether sphingolipid,mitogen-activated protein kinase (MAPK),and mitochondria-mediated pathways are involved in this mechanism.Blue light irradiation led to mitochondria and lysosome damage in RPE cells,whereas C3G preserved mitochondrial morphology and function and maintained the lysosomal integrity.C3G suppressed the phosphorylation of JNK and p38 MAPK and mitochondria-mediated pathways to inhibit RPE cell apoptosis.Lipidomics data showed that C3G protected RPE cells against blue light-induced lipid peroxidation and apoptosis by maintaining sphingolipids balance.C3G significantly inhibited ceramide (Cer d18:0/15:0,Cer d18:0/16:0 and Cer d18:0/18:0) accumulation and elevated galactosylceramide (GalCer d18:1/15:0 and GalCer d18:1/16:0) levels in the irradiated A2E-containing RPE cells.Furthermore,C3G attenuated cell membrane damage by increasing phosphatidylcholine and phosphatidylserine levels.C3G inhibited apoptosis and preserved the structure of mitochondria and lysosome by regulating sphingolipid signaling and suppression of MAPK activation in RPE cells.Thus,dietary supplementation of C3G prevents retinal photooxidative damage.
1.Introduction
Blue light exposure has highly increased in recent years,because the use of mobile phones,computers,and other video devices has become a part of daily life.However,excessive exposure of blue light is a severe risk factor for eye health,because it can cause irreversible oxidative damage to the eye tissue[1].After retina exposure to blue light,inflammatory reaction and apoptosis could occur in retinal cells[2].N-retinylidene-N-retinyl-ethanolamine (A2E)is the main constituent of lipofuscin that accumulates in the lysosomal compartment of the retinal pigment epithelium (RPE) cells[3].A high percentage of polyunsaturated fatty acids is present in phospholipids from the membrane of photoreceptor cells.The neighboring RPE offers the vital function of phagocytosis of lipid-rich photoreceptor outer segments (OSs)[4].Overexposure to blue light increases the level of reactive oxygen species (ROS) and contributes to the generation of photooxidation products of A2E,which further release reactive aldehydes and lead the lipid peroxidation in RPE cells[5-6].Various lipid peroxidation products,such as malondialdehyde (MDA),4-hydroxynonenal (HNE),and 4-hydroxyhexenal (HHE),have been identif ied in the macula of age-related macular degeneration patients[7].
Blue light exposure increases the concentrations of ROS and lipid peroxidation products,as well as activates the mitochondria-dependent apoptosis and mitogen-activated protein kinase (MAPK) signaling pathways[8].Mitochondria-dependent apoptosis pathway plays an important role in blue light-induced RPE damage[9].Mitochondria are one of the primary target organelles in blue light-irradiated A2Econtaining RPE cells[10].Blue light disrupts calcium homeostasis in RPE cells,which decreases the mitochondrial membrane potential(MMP) and causes membrane lipid peroxidation[11].JNK and p38 MAPK are activated by environmental stresses,including proinflammatory cytokines,ultraviolet (UV) irradiation,and oxidative stress[12-13].Ceramide (Cer),which is the central molecule of sphingolipid metabolism,is an important modulator of cellular apoptosis and proliferation[14].Accumulation of lipofuscin A2E increases Cer in RPE cells,and dysregulation of Cer metabolism may cause retinal degeneration[15].Thus,targeting sphingolipid metabolism may be a novel strategy for preventing RPE cell damage.
Anthocyanins can improve visual health by reducing oxidative stress and lipid peroxidation[16-17].Cyanidin-3-glucoside (C3G) is among the most predominant anthocyanins;it is naturally found in black rice,black bean,and many dark-colored berries.C3G efficiently protects against light-induced retinal degeneration bothin vitroandin vivo[18-19].In our recent study,we found that C3G improved the barrier function of RPE cells by regulating endoplasmic reticulum (ER) stress-induced apoptosis[20].Besides reducing oxidative stress,C3G also inhibited the lipid peroxidation,such as the formation of HNE in the photoreceptor OSs,and attenuated HNE-induced apoptosis in RPE cells[21-22].Anthocyanins possess anti-inflammatory property and lipid peroxidation inhibitory effect,which are observed in the suppression of MAPK signaling in H2O2-induced RPE cells[23].Therefore,it is critical and promising to study the role of MAPK signaling pathway in the beneficial function of C3G in blue-light induced RPE damage.
The protective effect of C3G on retinal cells has already been reported,but the mechanisms of the action ofC3G in the regulation of sphingolipid signaling and MAPK pathway in blue light-induced RPE cell damage is still poorly understood.In the present study,we investigated the mechanisms of the role of C3G against blue light-induced RPE cells damage,especially on the MAPK and mitochondria-mediated pathways.Moreover,the lipidomic profile on sphingolipid levels of the effect of C3G in RPE cells was explored for the first time.
2.Materials and methods
2.1 Chemicals and reagents
Dulbecco’s modified Eagle’s/Ham’s F12 medium (DMEM/F12),0.05% trypsin-EDTA solution,MEM non-essential amino acid solution,penicillin-streptomycin solution,and phosphate-buffered saline (PBS) were purchased from Corning (Manassas,VA,USA).Fetal bovine serum (FBS) was obtained from Gibco Life Technologies(Grand Island,NY,USA).A2E was synthesized by incubating all-trans-retinal with ethanolamine and purified by HPLC[24].C3G was purchased from Nanjing Jingzhu Bio-technology,Ltd.(≥ 98% purity,Nanjing,Jiangsu,China).Protease and phosphatase inhibitors were obtained from Calbiochem (San Diego,CA,USA).SB203580,SP600125,RIPA lysis buffer,JC-1 probe kit,and Fluo-3 AM probe were purchased from Beyotime Biotechnology Co.,Ltd.(Shanghai,China).The ROS assay kit was purchased from Applygen Technologies (Beijing,China).Primary antibodies against p-NF-κB p65 (#3033),NF-κB p65 (#8242),p-p38 (#4511),p38(#8690),p-JNK1/2 (#9255),p-ERK1/2 (#4370),caspase-3 (#14220),Bax (#5023),Bcl-2 (#15071),and c-Jun (#9165) were purchased from Cell Signaling Technology (Danvers,MA,USA).Caspase-12(ab62484),p-c-Jun (ab32385),β-actin (ab179467),and a secondary antibody (ab205718) were obtained from Abcam (Cambridge,UK).JNK1/2 (sc-137019) and ERK1/2 (sc-514302) were purchased from Santa Cruz (Santa Cruz Biotechnology,Shanghai,China).Goat anti mouse IgG-HRP antibody was obtained from Gene-Protein Link(Beijing,China).
2.2 Cell culture and illumination
The ARPE-19 cells were obtained from the American Type Culture Collection (Manassas,VA,USA).Cells were cultured in DMEM/F12 containing 10% FBS,1% antibiotic mixture of penicillin (100 U/mL) and streptomycin (100 µg/mL),and 1% MEM non-essential amino acid solution in 5% CO2at 37 °C as our previous study[21].A2E (25 µmol/L) was incubated with the ARPE-19 monolayers for 3 days,thereby allowing A2E accumulation in the lysosomal compartment of the cells.Then,the cells were treated with C3G (0,10,and 25 µmol/L) or SB203580/SP600125 (10 µmol/L) for 24 h before exposure to blue light.The RPE cells were exposed to 430 nm irradiation (15 000 lx,40 min) to photooxidize A2E by using an illumination system[20].After light exposure,the RPE cells was continued for an additional 12 h or 24 h.
2.3 Measurements of lipid peroxidation products, antioxidant enzyme system, and inflammatory factors levels
The RPE cells were collected and washed twice with ice-cold PBS.According to the manufacturer’s instructions,the cells were frozen and thawed multiple times,and centrifuged at 3 000 ×gfor 20 min at 4 °C.The supernatants were collected,and the activities of superoxide dismutase (SOD),catalase (CAT),glutathione peroxidase(GSH-Px),and glutathione (GSH) were assessed using enzyme-linked immunosorbent assay (ELISA) kits (Chundubio,Wuhan,China).MDA,HNE,HHE,inducible nitric oxide synthase (iNOS),and cyclooxygenase-2 (COX-2) levels were measured using commercial ELISA kits (Jingmei Biotechnology Co.,Ltd.,Jiangsu,China).The total protein concentrations were determined with a BCA kit(Millipore,Bedford,MA,USA).Absorption was obtained at 450 nm on a Multimode Plate Reader (EnSpire 2300,PerkinElmer,Waltham,MA,USA).
2.4 Western blotting
After the treatment,the RPE cells were washed with ice-cold PBS and then harvested and transferred to a 1.5 mL centrifuge tube.Whole cell protein extracts were performed by lysis buffer containing phosphatase and protease inhibitor cocktails on ice.Then,the mixture was centrifuged at 12 000 ×gfor 15 min at 4 °C,and the protein concentration in the supernatant was measured by using a BCA kit.Protein samples were separated on a 10% SDS-PAGE gel and then transferred onto PVDF membranes (Millipore,Bedford,MA,USA).The membranes were blocked with 5% skim milk in Tris-buffered saline containing 0.05% Tween 20 for 1 h and incubated with the following primary antibodies overnight at 4 °C: anti-p-NF-κB p65 (1:1 000),anti-NF-κB p65 (1:1 000),anti-p-ERK1/2 (1:1 000),anti-ERK1/2 (1:1 000),anti-p-JNK1/2 (1:1 000),anti-JNK1/2 (1:1 000),anti-p-p38 (1:1 000),anti-p38 (1:1 000),anti-p-c-Jun (1:1 000),anti-c-Jun (1:1 000),anti-Bcl-2 (1:1 000),anti-Bax (1:1 000),anti-caspase-12 (1:1 000),anti-caspase-3 (1:1 000),and anti-β-actin(1:2 000).Membranes were incubated with secondary antibodies(1:2 000) for 2 h at room temperature,and protein bands were visualized using an ODYSSEY FC Imaging System (LI-COR Biosciences,Lincoln,NE,USA).
2.5 Mitochondrial membrane potential detection
MMP (Δψm) was detected by JC-1 probe[25].The RPE cells were seeded in glass-bottom culture dishes at a concentration of 4 × 105cells/mL.After treatment,the cells were fixed,permeabilized,and stained.Immunofluorescence images were obtained using a confocal laser scanning microscope (Olympus FV1200,Japan) at 60×magnification.
2.6 Measurement of intracellular calcium concentration
Ca2+concentration was assessed with a Fluo-3 AM probe kit following the manufacturer’s instructions.After treatment,RPE cells were washed with ice-cold PBS twice and incubated with 5 µmol/L Fluo-3 AM for 30 min at 37 °C.After washing thrice in PBS,the cells were immersed in 1 mL PBS for 20 min at 37 °C.Immunofluorescence images were obtained using a confocal laser scanning microscope at 60× magnification.
2.7 Transmission electron microscopy (TEM)
The RPE cells were collected by trypsinization.They were rinsed,fixed,dehydrated,embedded,and stained in accordance with our previous study[20].Morphological changes of mitochondria in the RPE cells were observed and photographed using a transmission electron microscope (FEI Tecnai Spirit 120 kV).
2.8 Measurement of caspase-3 activity using flow cytometry
RPE cells were seeded in 35 mm dishes and grown to confluence.After the aforementioned treatments,caspase-3 levels were examined by using GreenNucTMCaspase-3 Assay kit (Beyotime Biotechnology Co.,Ltd.) and analyzed using the NovoCyte Flow Cytometer(ACEA Biosciences Inc.,San Diego,CA,USA) with NovoExpress®software (1.2.5).
2.9 Lysosomal staining
RPE cells were plated in glass-bottom culture dishes at a concentration of 4 × 105cells/mL.After treatment with A2E and irradiation,the cells were washed with PBS twice and incubated in 5 µmol/L Acridine Orange (AO) at 37 °C for 15 min.Then,the cells were washed with PBS twice.All images were observed under confocal laser scanning microscope at 60× magnification.
2.10 Lipidomics analysis
After treatment,the RPE cells were collected and stored at -80 °C.The intracellular lipids were extracted in accordance with the method described by Song et al.[26].Upper aqueous phase containing the extracted cell pellet was dried in a SpeedVac under H2O mode.Total protein content was determined from the dried pellet using a BCA kit.
Lipidomic analyses were performed by the LipidALL Technologies(Changzhou,Jiangsu,China) using a Shimadzu Nexera 20AD HPLC coupled with Sciex QTRAP 6500 Plus,as reported previously[27].The polar lipids were separated by normal phase-HPLC with a TUP-HB silica column (i.d.150 × 2.1 mm,3 µm) under the following chromatographic conditions: mobile phase A (chloroform:methanol: ammonium hydroxide,89.5:10:0.5) and mobile phase B(chloroform: methanol: ammonium hydroxide: water,55:39:0.5:5.5).Multiple reaction monitoring (MRM) transitions were established for the comparative analysis of various polar lipids.Individual lipid species were quantified using spiked internal standards.d9-PC32:0(16:0/16:0),d9-PC36:1p(18:0p/18:1),d7-PE33:1(15:0/18:1),d9-PE36:1p(18:0p/18:1),d31-PS(d31-16:0/18:1),d7-PA33:1(15:0/18:1),d7-PG33:1(15:0/18:1),d7-PI33:1(15:0/18:1),C14-BMP,d3-16:0-carnitine,C17-SL,d5-CL72:8(18:2)4,Cer d18:1/15:0-d7,d9-SM d18:1/18:1,C8-GluCer,C8-GalCer,d3-LacCer d18:1/16:0,Gb3-d18:1/17:0,d7-LPC18:1,d7-LPE18:1,LPI-C17:1,LPA-C17:0,LPS-C17:1,LPG-C17:1,Sph-d17:1,and S1P-d17:1 were obtained from Avanti Polar Lipids.GM3-d18:1/18:0-d3was purchased from Matreya LLC.Free fatty acids were quantitated using d31-16:0(Sigma-Aldrich) and d8-20:4 (Cayman Chemicals).A modified reverse-phase HPLC/MRM method was used to quantify the glycerol lipids,including diacylglycerols (DAG) and triacylglycerols(TAG)[26].Free cholesterols and cholesteryl esters (CE) were analyzed using atmospheric pressure chemical ionization (APCI)mode on a Jasper HPLC coupled to Sciex 4500 MD,as previously described with the corresponding d6-cholesterol and d6-C18:0 cholesteryl ester (CE) (CDN isotopes) as internal standards[28].
2.11 Statistical analysis
Statistical analyses were conducted by GraphPad Prism 5.0 statistical software.Data were presented throughout as the mean ± SD.The differences between the groups were analyzed using one-way ANOVA followed by Tukey’s multiple comparison test.P< 0.05 indicated significant differences.
3.Results
3.1 C3G alleviated ROS levels and lipid peroxidation
Intracellular ROS levels were significantly increased in the irradiated A2E-containing cell group compared with the control group(P< 0.05;Fig.1A),but 10 and 25 µmol/L C3G treatment significantly reduced ROS levels (P< 0.05).ROS production could induce lipid peroxidation.So,the lipid peroxidation products MDA,HNE,and HHE levels were determined.MDA,HNE,and HHE levels were significantly increased after A2E and irradiation treatment (P< 0.05;Figs.1B-D),whereas 10 and 25 µmol/L C3G treatments effectively reduced the levels of these lipid peroxidation products (P< 0.05).The levels of MDA,HNE and HHE did not significantly differ between the control and negative control groups (P> 0.05).
3.2 C3G improved mitochondrial dysfunction
As shown in Fig.2A,the control and negative control groups showed a deep red fluorescence intensity and a weak green fluorescence intensity.After A2E and irradiation treatment,the ratio of red/green fluorescence intensity was significantly decreased(P< 0.05),which indicated the loss of the MMP and mitochondrial dysfunction in RPE cells (Fig.2C).However,the 10 and 25 µmol/L C3G treatment effectively reversed the fluorescence intensity change and inhibited mitochondrial depolarization (P< 0.05).
The fluorescence intensity of Ca2+had a noticeable increase in irradiated A2E-containing RPE cell group compared with the control group (P< 0.05;Figs.2B,D),which accelerated the loss of the MMP and triggered the mitochondrial apoptosis pathway.Nevertheless,C3G (10 and 25 µmol/L) significantly attenuated the Ca2+concentration and maintained calcium homeostasis in RPE cells(P< 0.05).
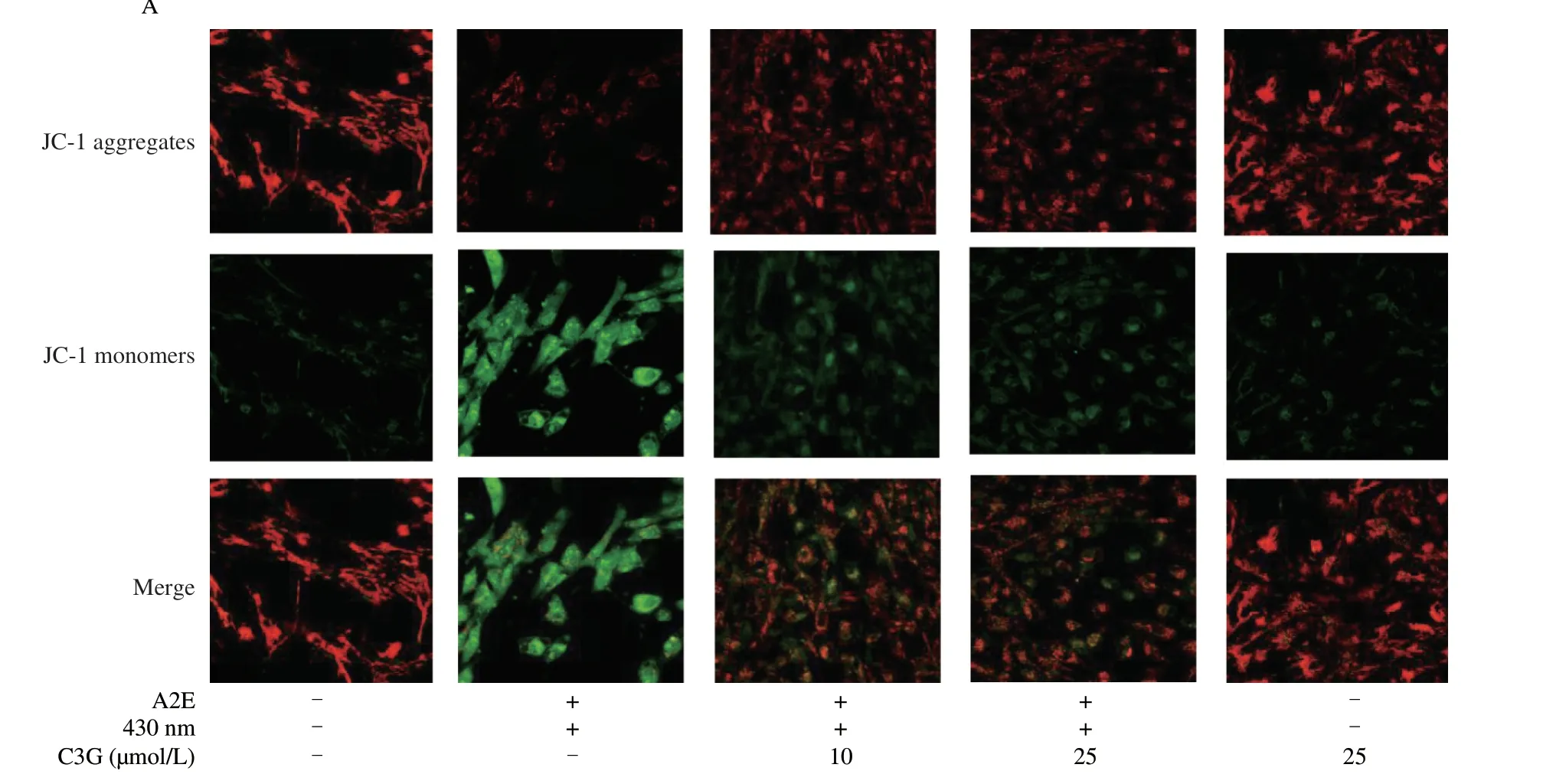
Fig.2 The effect of C3G on mitochondrial function in RPE cells.(A) Evaluation of MMP in RPE cells by a JC-1 probe.Immunofluorescence images show the levels of JC-1 aggregates (red),JC-1 monomers (green),and the overlap of red and green fluorescence (magnification 60 ×).(B) Immunofluorescence analysis of Ca2+ concentration using Fluo-3 AM probe (magnification 60 ×).(C) Quantitation of MMP.(D) Quantitation of Ca2+ concentration.(E) The effect of C3G on mitochondrial ultrastructure in RPE cells using a transmission electron microscope.The blue arrows indicate normal mitochondrial structure,whereas the red arrows indicate abnormal mitochondrial structure.Scale bar: 1 μm.Results are mean ± standard deviation (n=3).#P < 0.05 compared with untreated RPE cells;*P < 0.05 compared with blue light-irradiated A2E-containing RPE cells.+,treated;-,not treated.
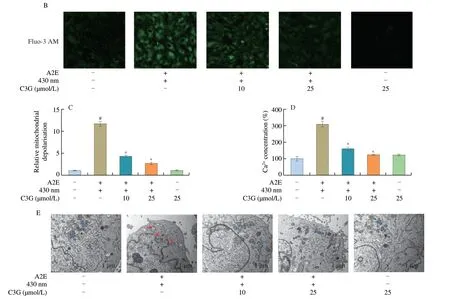
Fig.2 (Continued)
3.3 C3G protected mitochondrial morphology
TEM results showed that normal mitochondrial ultrastructure was observed in the control and negative control groups (Fig.2E).Conversely,the mitochondria appeared to be in a swollen state with disrupted membranes and the inner cristae disappeared in irradiated A2E-containing RPE cell group.Thus,with the loss of MMP and the increase of Ca2+level,A2E photooxidation also induced considerable disturbance of mitochondrial morphology in RPE cells.However,the changes observed in mitochondrial ultrastructural morphology were reversed after 10 and 25 µmol/L C3G treatment,which showed that C3G preserved the structure and function of RPE mitochondria.
3.4 C3G maintained lysosomal integrity
More than 90% A2E accumulated in the lysosomes of RPE cells,and the changes of lysosomal membrane permeability might result in the release of A2E from lysosome into the cytosol and stimulate cell apoptosis[29].We used AO to determine the lysosomal damage,and increased green fluorescence represented the loss of lysosomal integrity.Higher green fluorescence intensity and lower red fluorescence intensity were shown in irradiated A2E-containing RPE cell group compared with the control group (P< 0.05;Fig.3),which demonstrated that A2E photooxidation could induce loss of lysosomal integrity.As expected,C3G (10 and 25 µmol/L) effectively enhanced the red fluorescence intensity (P< 0.05),indicating that C3G maintained lysosomal integrity.
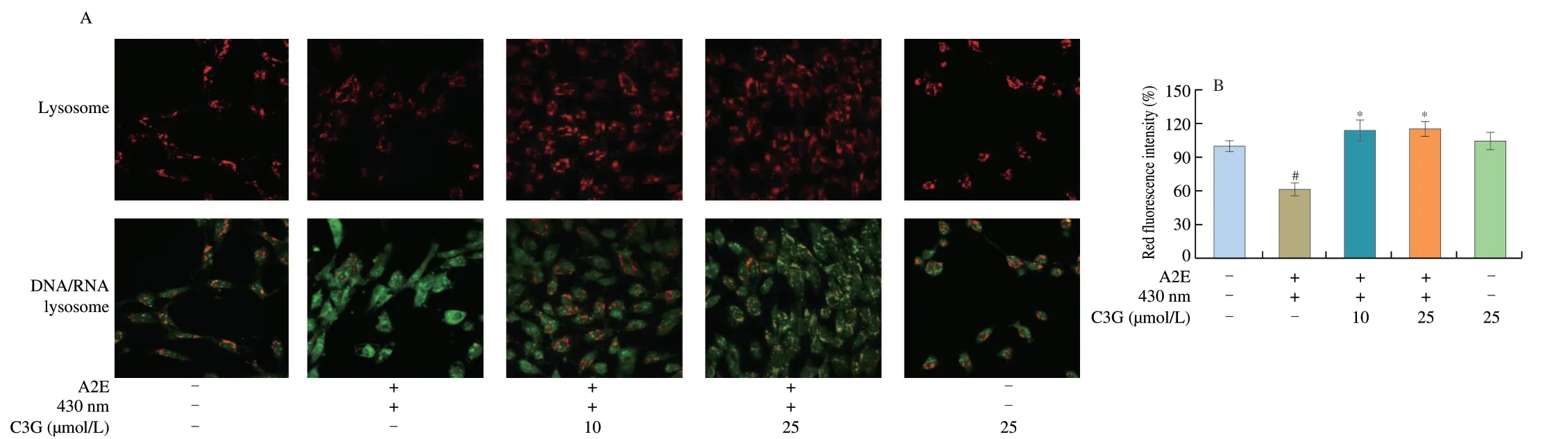
Fig.3 The effect of C3G on lysosomal integrity in RPE cells.(A) Fluorescence microscope images of Acridine Orange (AO) sequestered in intact lysosomes (red) or bound to DNA,RNA,and other cell constituents (green) (magnification 60 ×).(B) Red fluorescence intensity of AO-stained lysosomes in RPE cells.Results are mean ± standard deviation(n=3).#P < 0.05 compared with untreated RPE cells;*P < 0.05 compared with blue light-irradiated A2E-containing RPE cells.+,treated;-,not treated.
3.5 C3G suppressed apoptosis
The balance between anti-apoptotic protein Bcl-2 and proapoptotic protein Bax plays a critical role in the phrase of cell apoptosis.Western blot analysis shows that the protein levels of cleaved caspase-12,caspase-3,and Bax were significantly increased in the irradiated A2E-containing RPE cell group (P< 0.05;Figs.4A-D).C3G treatment inhibited RPE cell apoptosis;it significantly decreased the protein expression of cleaved caspase-12 and caspase-3,increased the ratio of Bcl-2/Bax,and decreased the activation of caspase-3 (P< 0.05;Fig.4).
3.6 C3G inhibited MAPKs pathway
JNK1/2,c-Jun,and p38 phosphorylation were significantly increased in irradiated A2E-containing RPE cell group compared with the control group (P< 0.05;Figs.5A-D).The phosphorylation level of ERK1/2 was not significantly changed among all groups (P> 0.05;Fig.5E).C3G treatment significantly decreased the phosphorylation levels of JNK1/2,c-Jun,and p38 (P< 0.05).Furthermore,Bax protein level was significantly decreased after treating the inhibitor of JNK and p38 (P< 0.05;Figs.5F-G).Simultaneously,C3G treatment significantly reduced Bax expression,which showed a similar trend in the inhibition of JNK or p38 expression.Therefore,MAPK pathway was involved in the regulation of mitochondrial apoptosis in irradiated A2E-containing RPE cells,and C3G prevented RPE apoptosis by inhibiting JNK and p38 MAPK pathways.
3.7 C3G improved antioxidant capacity and attenuated inflammation
The levels of SOD,CAT,GSH-Px,and GSH were significantly decreased in blue light irradiated A2E-containing RPE cells (P< 0.05;Fig.S1).A2E photooxidation decreased antioxidant defense system in RPE cells by decreasing the antioxidant enzyme activities.However,10 and 25 µmol/L C3G treatment significantly increased SOD,CAT,and GSH-Px activities and GSH level in RPE cells (P< 0.05).The phosphorylation levels of nuclear factor-kappa B (NF-κB) p65 and protein levels of iNOS and COX-2 in irradiated A2E-containing RPE cell group were significantly increased when compared with the control group (P< 0.05;Fig.S2).However,10 and 25 µmol/L C3G treatment could remarkably suppress the phosphorylation of NF-κB p65 and decrease the protein levels of iNOS and COX-2 (P< 0.05).
3.8 C3G modulated lipids metabolism
To further explore the changes of lipids metabolism under photooxidative conditions,a lipidomics analysis was used to detect the total lipids classes.Based on the significant differences of 38 classes of lipids in different groups,PCA score plot revealed that the control,A2E,and A2E+C3G groups were well separated (Fig.6A).This finding confirmed our hypothesis that A2E photooxidation induced disorders of the lipidomics profiles in RPE cells,but C3G significantly regulated the cellular lipometabolism,thereby exerting protective effects.The volcano plot showed the results of the comparison between pairwise groups,where the upregulated lipid metabolites were far less than the downregulated lipid metabolites (Figs.6B-D).When compared with the control group,287 lipids metabolites were significantly downregulated and 90 lipids metabolites were significantly upregulated in the A2E and irradiation treatment groups (P< 0.05;Fig.6B).There were 21 lipid metabolites that were significantly downregulated and 8 lipids metabolites that were significantly upregulated in the C3G treatment group compared with the A2E and irradiation treatment groups(P< 0.05;Fig.6C).Heatmap of all lipid classes was shown in Fig.6E,in which lyso-phosphatidylcholines (LPC),sphingosine(Sph),Cer,phosphatidylserines (PS),lyso-phosphatidylserines (LPS),and cardiolipins (CL) were the lipids classes with the most significant differences (Fig.S3).
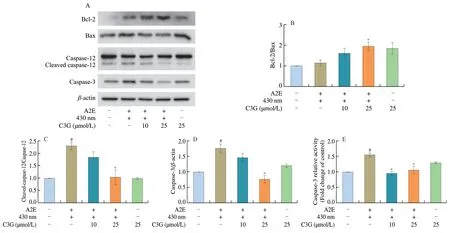
Fig.4 The effect of C3G on apoptotic proteins expression in RPE cells.(A) Western blot bands of Bcl-2,Bax,cleaved caspase-12,caspase-12,and caspase-3 in RPE cells.(B-D) Relative protein levels of Bcl-2/Bax,cleaved caspase-12/caspase-12,and caspase-3 were quantified and normalized to the control group.(E) Quantitation analysis of caspase-3 activation using flow cytometry.Results are mean ± standard deviation (n=3).#P < 0.05 compared with untreated RPE cells;*P < 0.05 compared with blue light-irradiated A2E-containing RPE cells.+,treated;-,not treated.

Fig.5 The effect of C3G on MAPK pathway in RPE cells.(A) Western blot bands of p-JNK1/2,JNK1/2,p-c-Jun,c-Jun,p-p38,p38,p-ERK1/2,and ERK1/2.(B-E) Relative protein levels of p-JNK1/2/JNK1/2,p-c-Jun/c-Jun,p-p38/p38,and p-ERK1/2/ERK1/2 were quantified and normalized to the control group.(F-G) The protein level of Bax was detected by Western blot in RPE cells after SB203580 and SP600125 treatments.Results are mean ± standard deviation (n=3).#P < 0.05 compared with untreated RPE cells;*P < 0.05 compared with blue light-irradiated A2E-containing RPE cells.+,treated;-,not treated.
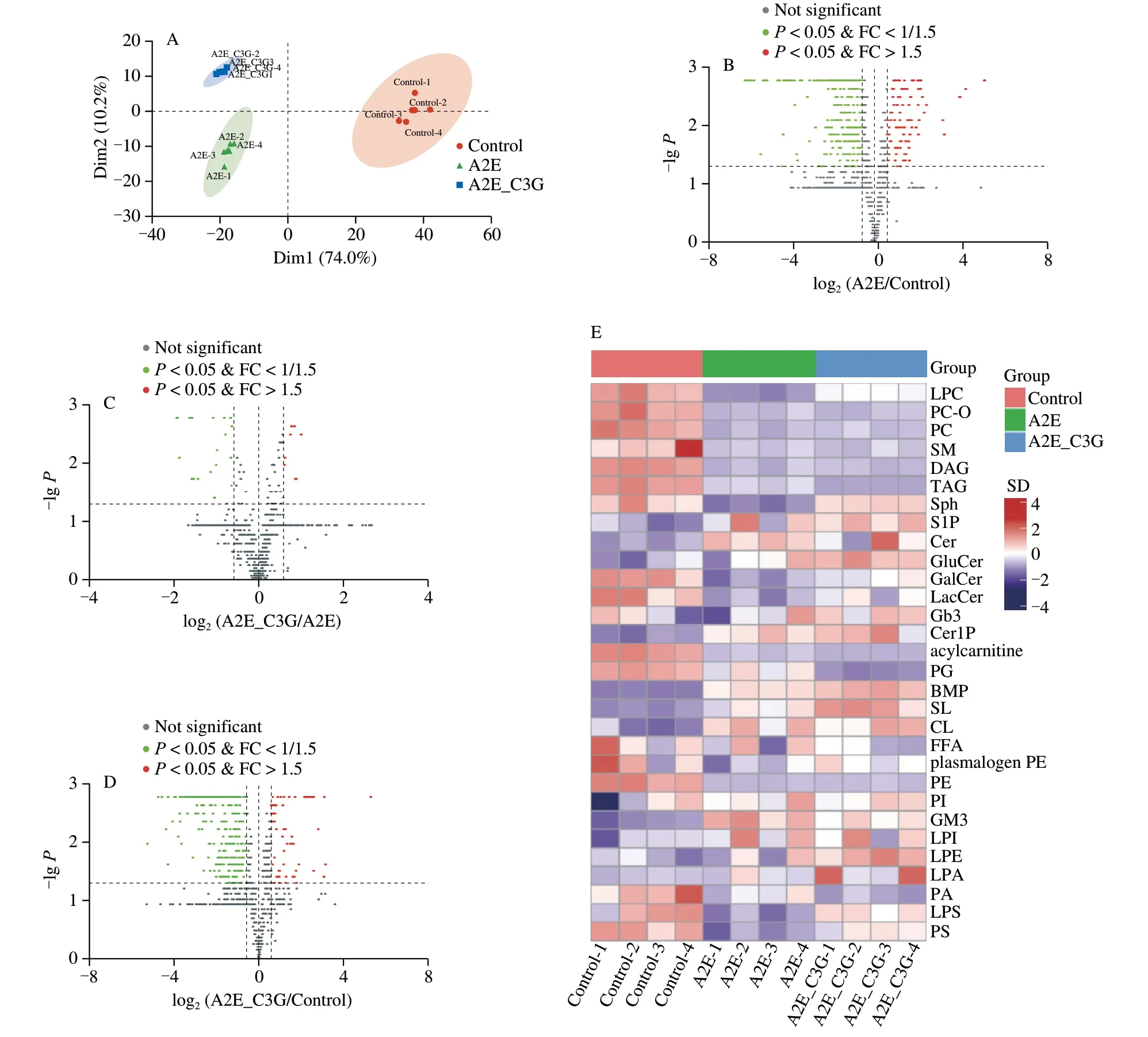
Fig.6 The effect of C3G on cellular content of lipid species in RPE cells.(A) Principal component analysis of total comparison of RPE cells sample.(B-D) Volcano of pairwise comparison of RPE cells sample.(E) Heatmaps of lipid classes.
We focused on the most significant differences among these lipids and presented the diagrams (Fig.7).Levels of LPC 14:0e,LPC 16:1,LPC 16:0,LPC 18:1,and LPC 18:0 were significantly reduced in the A2E photooxidation group,but these were significantly upregulated after C3G treatment (P< 0.05).Similarly,the levels of LPS,total CE,Sph,and part of PS were significantly decreased in the irradiated A2E-containing RPE cell group,but they were significantly increased in the C3G treatment group (P< 0.05).Levels of CL 66:4 (16:1),CL 68:6 (16:1),CL 68:5 (16:1),and CL 68:5 (18:2) were significantly decreased after being treated with A2E and irradiation and significantly increased after C3G treatment(P< 0.05).Compared with the control group,Cer d18:0/15:0,Cer d18:0/16:0,and Cer d18:0/18:0 levels were significantly upregulated in the A2E photooxidation group;their upregulation was effectively prevented by C3G treatment (P< 0.05).In addition,GalCer d18:1/15:0 and GalCer d18:1/16:0 levels were significantly reduced in A2E photooxidation group.C3G treatment obviously elevated these GalCer levels (P< 0.05).The above results further revealed the changes of lipid metabolism under photooxidation and demonstrated that C3G protected the RPE cells through the regulation of sphingolipid signaling.Based on the lipid metabolic pathways identified by KEGG,the lipids of particular interest are listed in Fig.8.
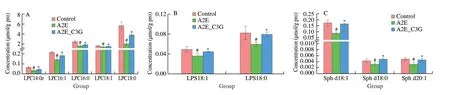
Fig.7 The effect of C3G on intracellular contents of (A) LPC,(B) LPS,(C) Sph,(D) CL,(E) PS,(F) total CE,(G) Cer,and (H) GalCer in RPE.Results are mean ± standard deviation (n=4).#P < 0.05 compared with untreated RPE cells;*P < 0.05 compared with blue light-irradiated A2E-containing RPE cells.
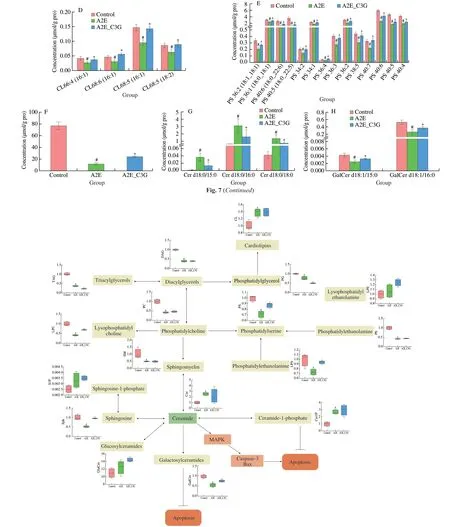
Fig.8 Changes of lipid metabolism in RPE cells after C3G treatment and intracellular A2E photooxidation.Red,green,and blue indicate control,A2E photooxidation,and C3G treatment and A2E photooxidation groups,respectively.n=4 per group.#P < 0.05 compared with untreated RPE cells;*P < 0.05 compared with blue light-irradiated A2E-containing RPE cells.
4.Discussion
C3G is the most common anthocyanins in dark grains and berries.It has great beneficial potential for protecting retinal cells.In this study,we investigated the effects of C3G on blue light irradiated A2E-containing RPE cells.Our findings are as follows,1) Blue light irradiation led to mitochondria and lysosome damage in RPE cells,but C3G preserved the mitochondrial structure and function and maintained the lysosomal integrity.2) C3G suppressed the phosphorylation of JNK and p38 MAPK and mitochondria-mediated pathways to inhibit RPE cell apoptosis.3) C3G protected against A2E photooxidation and induced lipid peroxidation by maintaining sphingolipids balance in RPE cells.Thus,we elucidated the molecular mechanisms by which C3G guards against blue light-induced RPE cell damage (Fig.9).
The ROS production by A2E photosensitization can initiate lipid peroxidation[3].Proteins modified by lipid peroxidation products,such as HNE and MDA,were detected in A2E-laden RPE cells exposed to blue light[30].In our previous study,C3G reduced HNE release in rod OSs incubated with all-trans-retinal followed by 430 nm irradiation[21].In addition,HNE can be detoxified by formation of HNE-GSH conjugate;C3G protected GSH from the reaction with photo-oxidized A2E in a cell-free assay[21].In this study,C3G protected RPE cells against blue light-induced damage by inhibiting lipid peroxidation and improving the antioxidant system.Our result on the scavenging ROS effects of C3G is also consistent with other findings that fucoxanthin decreased the ROS level in retinal cells under UVB irradiation without clear dose dependence[31].
Mitochondria exert important functions in modulating cell survival and apoptosis.A2E photooxidation by blue light decreased MMP,increased intracellular Ca2+levels,altered mitochondrial morphology,and enhanced Bax and caspase-3 activities in RPE cells[32].Thus,mitochondria may be an important target for the protection of RPE cells.Considering that A2E accumulates in lysosomes,the primary site of blue light-induced lipid peroxidation is the lysosomal membrane in RPE cells[33].In this study,C3G preserved MMP,lowered intracellular Ca2+,and maintained the mitochondrial and lysosomal structures in irradiated A2E-containing RPE cells.
As a transcription factor,the phosphorylation of NF-κB p65 is the key to the initiation of inflammation,and Cer-induced activation of NF-κB leads to apoptosis in RPE[34].The Bcl-2 family played a critical role in the regulation of the mitochondria-mediated apoptosis,and blue light attenuated the ratio of Bcl-2/Bax and activated caspase-3 by destruction of MMP,activation of ER stress,and p38 MAPK phosphorylation in RPE cells.C3G attenuated inflammatory response through the suppression of NF-κB pathway and inhibited the mitochondrial apoptosis pathway in RPE cells.
The photooxidation of A2E activated p38 and JNK MAPK pathways,which could further phosphorylate c-Jun and c-Fos in RPE cells[35].JNK pathway was involved in ER stress-dependent apoptosis in retinal cells[36].Bilberry anthocyanins containing delphinidin and cyanidin inhibited the blue light-induced activation of p38 MAPK in photoreceptor cells[37].In this study,C3G exerted protective effects against blue light-induced apoptosis of RPE cells through the inhibition of p38 and JNK/c-Jun activation.
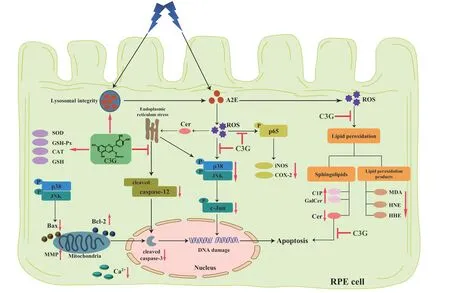
Fig.9 Diagram of the protective effect and potential mechanism of C3G against blue light-induced damage in RPE cells.C3G prevented A2E photooxidationinduced mitochondrial apoptosis by maintaining sphingolipid balance and inhibiting MAPK pathway in RPE cells.
The phospholipid bilayers are major components of cell membranes and are among the main targets of ROS,thereby leading to lipid peroxidation[38-39].RPE cells can generate Cer,which induces cell apoptosis.They can also produce GalCer,ceramide-1-phosphate (Cer1P),glucosylceramides (GluCer),and sphingosine-1-phosphate (S1P),which effectively inhibit cell apoptosis[40].C3G is more likely to inhibit apoptosis by increasing the levels of GalCer,Cer1P,GluCer,and S1P.
Cer participates in initiating RPE cell apoptosis by triggering JNK and p38 MAPK pathways[41-42].Furthermore,Cer cooperates with Bax to increase caspase-3 activation,to inactivate the anti-apoptotic protein Bcl-2,and to trigger the loss of MMP,which causes mitochondrial apoptosis in RPE[43].UV and hydrogen peroxide induced Cer production to promote RPE cell apoptosis[44].Meanwhile,the inhibition of Cer biosynthesis can protect retinal cells from light-induced degeneration[45].Anthocyanin supplementation reduced plasma Cer in human and rodent[46-47].In this study,C3G maintained sphingolipids balance;it significantly decreased Cer d18:0/15:0,Cer d18:0/16:0,and Cer d18:0/18:0 in the irradiated A2E-containing cells (Fig.7G),indicating that C3G prevents RPE cell apoptosis by inhibiting Cer accumulation.
PS and LPC are the major components of biological membranes.Intracellular supplementation of PS regulates immune responses by inhibiting NF-kB and p38 MAPK activation[48].In this study,A2E photooxidation by blue light significantly decreased LPC and PS,but C3G treatment attenuated cell membrane damage by increasing LPC and PS levels.Phosphatidylglycerol (PG) is a component of the mitochondrial inner membrane,and PG generates CL,which plays a central role in mitochondrial function and dynamics[49].CL is highly susceptible to free radical lipid peroxidation and generation of bioactive lipids,such as HNE[50].C3G effectively regulated CL level and maintained the normal physiological function of mitochondria in RPE cells.
5.Conclusions
C3G protected the mitochondrial morphology and function,and maintained the lysosomal integrity in blue light-irradiated A2E-containing RPE cells.C3G alleviated blue light-induced RPE cell lipid peroxidation and mitochondrial apoptosis by inhibiting MAPK pathway and maintaining sphingolipid balance.In particular,it attenuated ceramide accumulation.We elucidated the key targets for the protective effect of C3G against photooxidative damage in RPE cells,which provided scientific evidence for C3G as functional food factors to maintain vision health.
Conflict of interest
Yong Wang is an editorial board member forFood Science and Human Wellnessand was not involved in the editorial review or the decision to publish this article.The authors declare no competing financial interest.
Acknowledgments
This work was funded by the National Natural Science Foundation of China (31901698) and Young Elite Scientists Sponsorship Program by the China Association for Science and Technology(2019QNRC001).
Appendix A.Supplementary data
Supplementary data associated with this article can be found,in the online version,at http://doi.org/10.26599/FSHW.2022.9250053.
- 食品科学与人类健康(英文)的其它文章
- Protective effects of oleic acid and polyphenols in extra virgin olive oil on cardiovascular diseases
- Diet and physical activity inf luence the composition of gut microbiota,benef it on Alzheimer’s disease
- Inf luence of nitrogen status on fermentation performances ofnon-Saccharomyces yeasts: a review
- Ganoderma lucidum: a comprehensive review of phytochemistry,eff icacy,safety and clinical study
- Resveratrol combats chronic diseases through enhancing mitochondrial quality
- Demonstration of safety characteristics and effects on gut microbiota of Lactobacillus gasseri HMV18

