Trimethylamine N-oxide generation process was inf luenced by the proportion and source of macronutrients in the diet
Chengheng Wng,Xuefeng Dun,Xioyue Li,Jinyue Yng,Chnghu Xue,b,Teruyoshi Yngit,Tintin Zhng,,Yuming Wng,b,
a College of Food Science and Engineering, Ocean University of China, Qingdao 266003, China
b Laboratory for Marine Drugs and Bioproducts, Pilot National Laboratory for Marine Science and Technology (Qingdao), Qingdao 266237, China
c Laboratory of Nutrition Biochemistry, Department of Applied Biochemistry and Food Science, Saga University, Saga 840-8502, Japan
Keywords: Trimethylamine N-oxide (TMAO)Trimethylamine (TMA)Dietary composition Macronutrients Gut microbiota
ABSTRACT Trimethylamine N-oxide (TMAO) is a risk factor of various chronic diseases,which was produced by metabolism from precursors to trimethylamine (TMA) in gut and the oxidation from TMA in liver.The TMA generation was influenced by diet,mainly due to the rich TMAO precursors in diet.However,it was still unclear that the effects of different proportion and source of macronutrients in different dietary pattern on the production process of TMAO.Here,the generation of TMA from precursors and TMAO from TMA was determined after single oral choline chloride and intraperitoneal injection TMA,respectively,in mice fed with carbohydrates,proteins and fats in different proportion and sources.The results suggested that the generation of TMAO was increased by low non-meat protein and high fat via enhancing the production of TMAO from TMA,and decreased by plant protein and ref ined sugar via reducing TMA production from precursors in gut and TMAO transformation from TMA in liver.The high fat and high sugar diets accelerating the development of atherosclerosis did not increase the production of TMAO,the risk factor for atherosclerosis,which indicated that the dietary compositions rather than the elevated TMAO level might be a more key risk factor for atherosclerosis.
1.Introduction
TrimethylamineN-oxide (TMAO) is a risk factor of various chronic diseases,especially arteriosclerosis[1].It has been reported that dietary choline andL-carnitine increased the circulating level of TMAO,which promoted atherosclerosis[2].The precursors of TMAO,such as choline,L-carnitine and betaine,produces trimethylamine(TMA) by intestinal microbiota metabolism,which is further metabolized to TMAO by f lavin-containing monooxygenases (FMOs)in liver.Therefore,reducing precursors intake,transformation by gut microbiota and the production by FMOs in liver are the effective ways to reduce the level of circulating TMAO.
Our previous study has reported that TMAO generation ability of different TMAO precursors is closely associated with the molecular structure.Choline,L-carnitine and betaine had different ability to produce TMAO[3].In addition,the conversion efficiency of choline salts to TMAO was higher than that of organic form phosphatidylcholine[4].Furthermore,the number and type of fatty acids in phosphatidylcholine affected its TMAO generation ability[4-5].Gut microbiota is necessary for TMA production from precursors,since the TMAO level in circulation of mice fed withL-carnitine would not increase when gut microbiota was inhibited[1-2].Studies revealed that TMAO levels in circulation could be regulated by diets attributed to the changed intake of precursors[6-7].In addition,it has been widely reported that the composition and abundance of gut microbiota were significantly regulated by diets[8-9].However,it remained unclear how the production of TMAO was influenced by different proportion and source of macronutrients in different dietary pattern by changing gut microbiota,besides precursors intake.Moreover,the TMAO levels in circulation were also affected by some natural active ingredients,such as fish oil,via regulation of FMOs activity[10].However,it is still unclear whether the FMOs activity in liver was regulated by the different proportion and source of macronutrients in different pattern,simultaneously,the production of TMAO was regulated.In addition,the generation,metabolism,and excretion of TMAO and TMA maintained dynamic balancein vivo,therefore,it is necessary to study the production process of TMAO,including TMA production from precursors in gut and TMAO production from TMA in liver.
Hence,in order to investigate the effects of dietary composition on TMAO production,it was comparatively studied in the present study that the effects of different diets containing macronutrients in different proportions (high protein,high carbohydrate/low protein,and high fat) and sources (corn starchvsrefined sugar,caseinvsplant protein,plant oilvsanimal fat) on the TMAO level in serum and its generation process,including TMA generation from precursors in gut and TMAO generation from TMA in liver.Further,the composition of gut microbiota and FMOs activity involved in the producing process of TMAO were assayed.
2.Materials and methods
2.1 Materials
Choline chloride (purity > 99%) was obtained from Ruitaibio Technology Co.,Ltd.(Beijing,China).D9-TMA (purity > 99%)and D9-TMAO (purity > 99%) were purchased from Merck Ltd.(Darmstadt,Germany).Reduced coenzyme II tetrasodium salt(NADPH-Na4) (purity > 98%) was obtained from Solarbio Science &Technology Co.,Ltd.(Beijing,China).Primary antibody against flavin-containing monooxygenases 3 (FMO3) was obtained from Cell Signalling Technology Inc.(Danvers,Massachusetts,USA).
2.2 Animals and treatments
Male C57BL/6J mice (6-8 weeks old) were maintained in a room with standard condition and provided with food and waterad libitum.After adaptation for 1 week,feces were collected and added in the diet of mice every day to create the same gut microbiota composition for 2 weeks.Then,the mice were randomly divided into 7 groups:control group (carbohydrate:protein:fat=65% corn starch,20%casein,5% corn oil),high casein group (HC,carbohydrates:protein:fat=45% corn starch,40% casein,5% corn oil),low casein group(LC,carbohydrate:protein:fat=75% corn starch,10% casein,5%corn oil),high plant oil group (HPO,carbohydrate:protein:fat=45% corn starch,20% casein,25% corn oil),high animal fat group(HAF,carbohydrate:protein:fat=45% corn starch,20% casein,25% lard),refined sugar group (RS,carbohydrate:protein:fat=65%sucrose,20% casein,5% corn oil) and plant protein group (PP,carbohydrate:protein:fat=65% corn starch,20% soybean protein,5% corn oil).The ratio of protein to oil in diet was controlled and carbohydrate was used for fixing the weight of diet.HC (45:40:5)and LC (75:10:5) groups were compared to the control group(65:20:5) under the equivalent oil to compare the effects of diets containing protein in different proportions,while HPO (45:20:25)group was compared to the control group (65:20:5) under the equivalent protein to compare the effects of diets containing fat in different proportions.In order to compare the effects of different diets containing macronutrients in different sources,corn starch(control) and refined sugar (RS),casein (control) and soybean protein (plant protein,PP),corn oil (high plant oil,HPO) and lard(high animal fat,HAF) were added into diets,respectively.The mice were fed with modified diets of AIN-93G for 2 weeks (Table 1).Then,fresh feces were collected for gut microbiota identification by 16S rRNA gene sequence analysis,and the blood was collected through the caudal vein and the level of TMAO,TMA and choline in serum were measured.Subsequently,the single oral choline chloride test was carried out immediately and the single intraperitoneal injection TMA test was performed 1 week later.Another week later,the mice were sacrificed,and the liver was collected,frozen in liquid nitrogen and stored at -80 °C until used.
2.3 Single oral choline chloride test
After feeding for 2 weeks,a single oral choline chloride test was carried out for studying the effects of dietary composition on TMA generation from precursors.The mice were gavaged with 340 mg/kg body weight choline chloride.Blood was taken from the caudal vein at 0,3,4.5,5.5,7 and 9 h after gavage for determining the levels of TMA and TMAO.
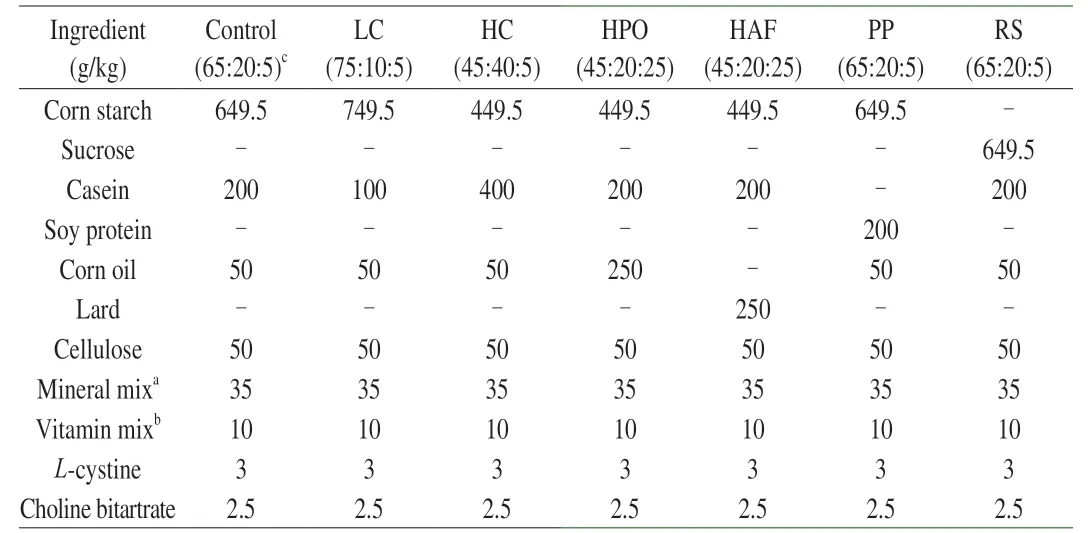
Table 1 Ingredient compositions of experimental diets.
2.4 Single intraperitoneal injection TMA test
One week after single oral choline chloride test,the mice were intraperitoneal injected 144 mg/kg body weight TMA for studying the effects of dietary composition on TMAO generation from TMA.Blood was taken from the caudal vein at 0,0.5,1,2 and 3 h after injection for determining the levels of TMAO.
2.5 Determination of choline, TMA and TMAO by LC-MS/MS
The serum was mixed with cold methanol and placed at -40 °C for 1 h,followed by centrifugation at 12 000 ×g,4 °C for 15 min.The supernatant was filtered by 0.22 μm organic membrane for determination.The levels of choline,TMA and TMAO in serum were detected by Agilent 1200 Series LC system coupled to a triple-quadrupole mass spectrometer (AB Sciex,Darmstadt,Germany).An Agilent ZORBAX SB-Phenyl column (2.1 mm × 100 mm,3.5 μm) was employed for liquid chromatography.The liquid chromatography and mass spectrometer condition were performed according to the previous description[3].
2.6 Fecal DNA extraction and 16S rRNA gene sequence analysis
Total genome DNA of gut microbiota was extracted from fresh feces by the fecal genome DNA isolation kit (TIANGE,Beijing,China) according to the manufacturer’s instructions.The V4 to V5 regions of 16S rRNA was amplified by polymerase chain reaction(PCR).The 16S rRNA gene was sequenced and analyzed using Illumina Hiseq (Novogene Bioinformatics Technology Co.,Ltd.,Tianjin,China).After read splicing and filtering and operational taxonomic units (OTUs) clustering,the species annotation and abundance were analyzed.Subsequently,OTUs related to TMAO generation was retrieved and analyzed for relative abundance.
2.7 Determination of FMOs enzyme activity in liver
Liver tissue was homogenized in cooled Tris-HCl buffer containing 1 mmol/L EDTA,0.25 mol/L sucrose,10 mmol/L Tris,pH 7.4,followed by centrifugation at 750 ×g,4 °C for 15 min.The supernatant was centrifuged at 12 000 ×g,4 °Cfor 20 min,and then the supernatant was collected in new tube.The new supernatant was centrifuged at 125 000 ×gfor 50 min,and the precipitate was collected as microsome component.The microsome component was washed and resuspended by buffer.The liver FMOs enzyme activity was measured according to the reported method[11].In brief,the 1 mL reaction system contained 10 mmol/L Tris,pH 7.4,100 mmol/L D9-TMA,100 mmol/L NADPH,and microsomes containing 5 mg protein.After 8 h,the reaction was stopped by 0.2 mL formic acid,and the reaction solution was filtered by 0.22 μm organic membrane for determining D9-TMAO by LC-MS/MS.The enzyme activity of liver FMOs was calculated according to the following formula.
FMOs enzyme activity=D9-TMAO concentration × Reaction solution volume/Reaction time/5 mg protein content
2.8 Protein extraction and Western blotting assay
Protein extraction and Western blotting assay were performed as described previously[12].In brief,the total protein was extracted by RIPA buffer,and equal amount of protein was separated on 10% SDS-PAGE gels and transferred to PVDF membrane.After blocked in 5% BSA,the blots on PVDF membrane were incubated with primary antibodies against FMO3 at 4 °C overnight,followed by secondary antibodies for 2 h.The blots were visualized using enhanced chemiluminescence (ECL) substrate.
2.9 Statistical analysis
All data was expressed as mean ± SEM (standard error of the mean,indicated by error bars),and the significant differences were assessed by Student’st-test.*Indicate significant differences whenP< 0.05.
3.Results
3.1 Effects of dietary composition on TMAO level in serum
In order to study the effect of different dietary composition on circulating TMAO level,the concentrations of TMAO and TMA in serum were determined after feeding the mice with diets consisting different proportion and source of macronutrients for 2 weeks.The different proportion of macronutrients was showed in Fig.1A,and the results suggested that the level of TMAO in serum was significantly increased in diet with low protein (10% casein,LC),while it was significantly decreased in diet with high protein (40% casein,HC),compared with control (20% casein) (Fig.1B).The similar level of TMAO in serum was found in high plant oil (HPO) and control diets(Fig.1B).In addition,the level of TMA in serum was significantly increased in diet with LC (Fig.1C),while the level of choline was not significantly changed (Fig.1D).The above results revealed that the levels TMAO in circulation was significantly affected by the proportion of protein,in which the level of TMAO in circulation was negatively correlated with protein intake.

Fig.1 Effects of dietary macronutrients on TMAO level in serum.(A) The proportion of dietary macronutrients.The effects of proportion of dietary macronutrients on the concentrations of (B) TMAO,(C) TMA and (D) choline in serum.The effects of carbohydrates from different sources (corn starch in Control and sucrose in RS) on the concentrations of (E) TMAO,(F) TMA and (G) choline in serum.The effects of proteins from different sources (casein in Control and soy protein in PP) on the concentrations of (H) TMAO,(I) TMA and (J) choline in serum.The effects of fats from different sources (corn oil in HPO and lard in HAF) on the concentrations of (K) TMAO,(L) TMA and (M) choline in serum.Data is presented as mean ± SEM.*indicates significant difference in control vs HC,control vs LC,control vs HPO,control vs RS,control vs PP and HPO vs HAF,at P < 0.05 by t-test.
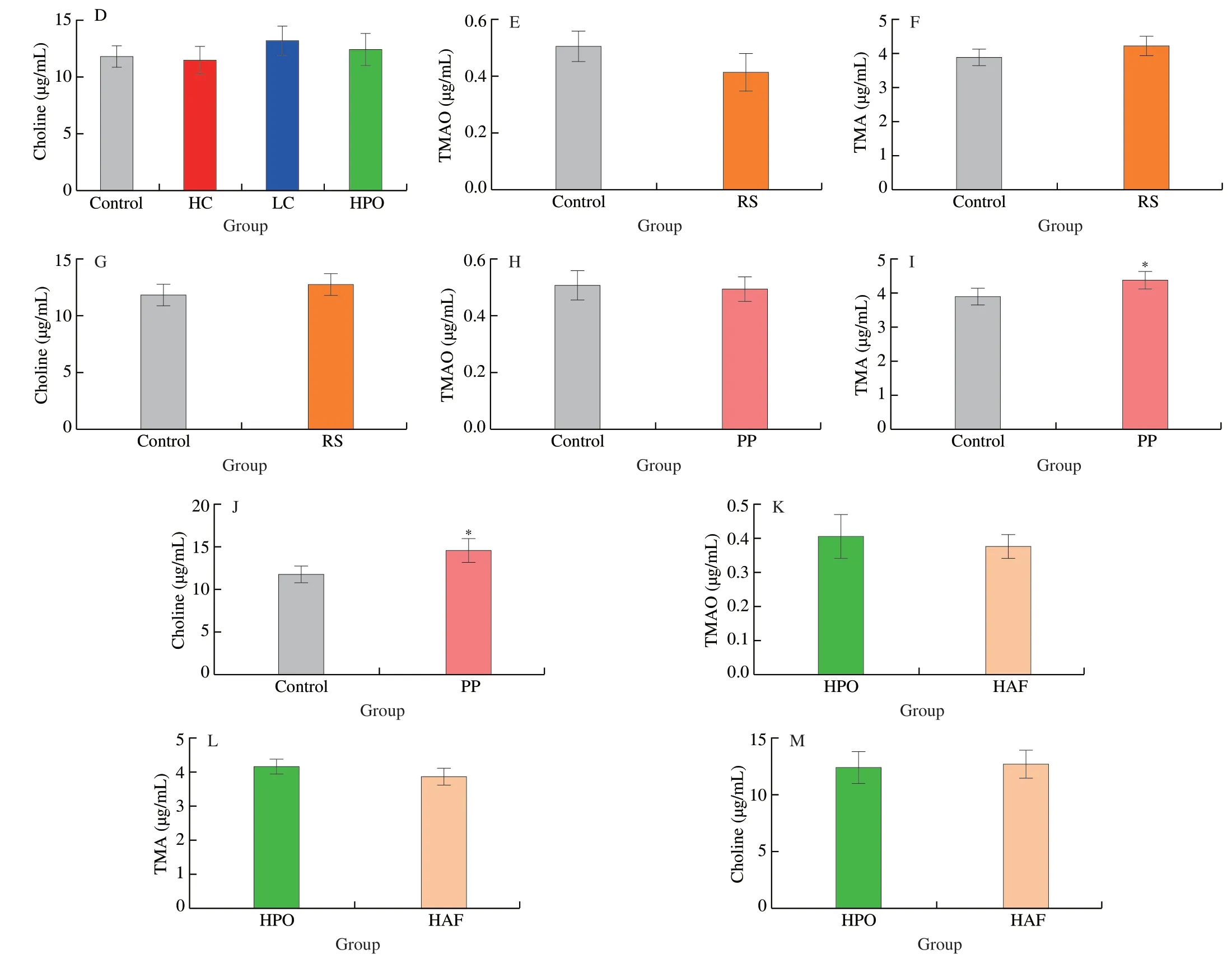
Fig.1 (Continued)
In addition,the effects of different source of carbohydrates,proteins and fats on the levels of TMAO,TMA and choline in serum were compared in the present study,respectively.The results suggested that there were no significant differences between corn starch (control) and refined sugar (RS) group in the levels of TMAO,TMA and choline (Figs.1E-G).The levels of TMA and choline were significantly increased by the diet with soybean protein (plant protein,PP),though the level of TMAO was not significantly changed,compared with animal protein casein (control) (Figs.1H-J).In order to investigate the effects of diets containing fat in different sources,the diets with 25% corn oil (high plant oil,HPO) and 25% lard (high animal fat,HAF) were compared.The results suggested that the significant differences between HPO and HAF group were not found in the levels of TMAO,TMA and choline (Figs.1K-M).The results showed that the source of protein affected the levels of TMA and choline in circulation.
3.2 Effects of the proportion of dietary macronutrients on TMA generation
TMA was produced from the precursors of TMAO containing the trimethylamine moiety,such as choline,L-carnitine and betaine by gut microbiota,which was further metabolized to TMAO by FMOs in liver.In order to study the effects of the proportion of dietary macronutrients on TMA generation by gut microbiota,the concentrations of TMA and TMAO in serum were determined after single oral choline chloride.The results suggested that the levels of TMA at 3rdhour was significantly increased by LC compared with Control,revealing the generation rate of TMA was significantly increased by LC,although there were no significant differences in the area under curve (AUC) of TMA and TMAO among the different proportion of dietary macronutrients groups (Fig.2).
3.3 Effects of the source of dietary macronutrients on TMA generation
It was compared that the effects of the diets with different source of carbohydrates,proteins,and fats on TMA generation after single oral choline chloride.The results suggested that the levels of TMA and TMAO were significantly decreased by the diets with RS and PP,compared with control (Figs.3A-H),which revealed inhibitory effects of refined sugar and plant protein on the formation of TMA.The levels of TMA and TMAO were significantly increased by the diets with HAF,compared with HPO,suggesting a promoting effect of animal fat on TMA generation compared with plant oil (Figs.3I-L).
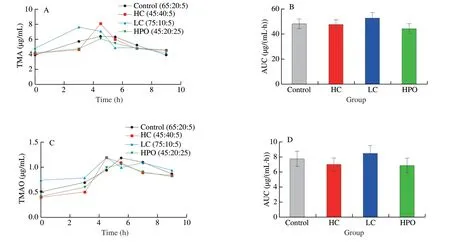
Fig.2 Effects of dietary macronutrients proportion on TMA generation after single oral choline chloride.(A) The concentration curve and (B) its AUC of TMA after single oral choline chloride.(C) The concentration curve and its (D) AUC of TMAO after single oral choline chloride.Data is presented as mean ± SEM.*indicates significant difference in control vs HC,control vs LC and control vs HPO at P < 0.05 by t-test.

Fig.3 Effects of dietary macronutrients source on TMA generation after single oral choline chloride.(A) The effects of carbohydrates from different sources on the concentration curve and (B) its AUC of TMA after single oral choline chloride.(C) The effects of carbohydrates from different sources on the concentration curve and (D) its AUC of TMAO after single oral choline chloride.(E) The effects of proteins from different sources on the concentration curve and (F) its AUC of TMA after single oral choline chloride.(G) The effects of proteins from different sources on the concentration curve and (H) its AUC of TMAO after single oral choline chloride.(I) The effects of fats from different sources on the concentration curve and (J) its AUC of TMA after single oral choline chloride.(K) The effects of fats from different sources on the concentration curve and (L) its AUC of TMAO after single oral choline chloride.Data is presented as mean ± SEM.*indicates significant difference at P < 0.05 by t-test.
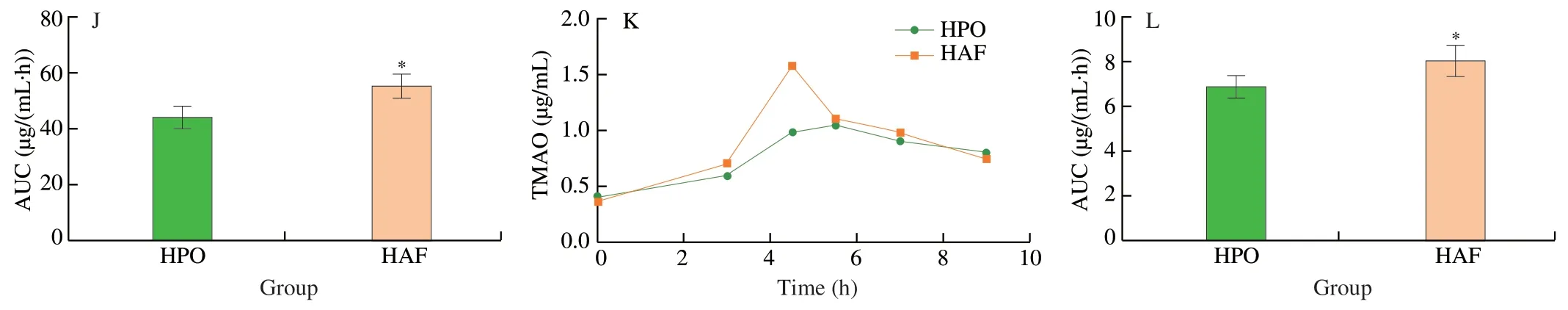
Fig.3 (Continued)
3.4 Effects of dietary composition on gut microbiota related to TMAO generation
Further,the gut microbiota related to TMAO generation was assayed,since the magnitude of the production of TMA in gut was influenced by the composition of the microbiota.The results suggested that the Firmicutes/Bacteroidetes ratio was elevated by the diet with HPO,but reduced by the diet with HC (Figs.4A and B).Indeed,the increased TMAO and TMA level were associated with higher activity of bacterial members of the phylum Firmicutes.Therefore,the increased Firmicutes/Bacteroidetes ratio might led to the elevated levels of TMAO and TMA in serum due to the inability of Bacteroidetes to produce TMA[13-14].However,the increased Firmicutes/Bacteroidetesratio was not consistent with the level of TMA in serum after single oral choline chloride,which might be due to that only a minor fraction of gut microbiota exhibited the genes required for TMA production[15].Therefore,the representative gut microbiota related to TMAO generation was further analyzed.The results showed that the abundance of some gut microbiota in LC was higher than that in control group,such as Enterococcaceae,Enterobacteriales,Escherichia_coli,Klebsiellaand some lineages ofClostridiumfrom Ruminococcaceae and Lachnospiraceae (Fig.4C),which was consistent with the increased generation rate of TMA.

Fig.4 Effects of dietary macronutrients on gut microbiota related to TMAO generation.The effects of macronutrients proportion in the diet on (A) the gut microbiota in phylum level,(B) Firmicutes/Bacteroidetes ratio,(C) representative gut microbiota related to TMAO generation.The effects of macronutrients source in the diet on (D) the gut microbiota in phylum level,(E) Firmicutes/Bacteroidetes ratio,(F) representative gut microbiota related to TMAO generation.Data is presented as mean ± SEM.*indicates significant difference in control vs HC,control vs LC,control vs HPO,control vs RS,control vs PP and HPO vs HAF,at P < 0.05 by t-test.
Moreover,the gut microbiota influenced by the diets with different source of carbohydrates,proteins and fats was analyzed,and the results suggested that Firmicutes/Bacteroidetes ratio was decreased by the diet with PP,compared with control group.In addition,Firmicutes/Bacteroidetes ratio in the diet with HAF was elevated compared with HPO (Figs.4D-E).Further,the diet with RS decreased the abundance of Coriobacteriales,Gammaproteobacteriaand so on,and increased the abundance ofClostridiaand several lineages ofClostridiumfrom Ruminococcaceae and Lachnospiraceae,compared with control(corn starch) group (Fig.4F).The abundances of Coriobacteriales and Gammaproteobacteria were decreased by the diet with PP,compared with control (casein) group.Some lineages ofClostridiumfrom Ruminococcaceae and Lachnospiraceae were decreased while others were increased by the diet with PP (Fig.4F).The above results suggested that the decreased generation of TMA in the diet with RS and PP might be related to decreased Coriobacteriales and Gammaproteobacteria.The abundance of some gut microbiota in HAF group was increased compared with HPO group,such as Clostridia and some lineages ofClostridiumfrom Ruminococcaceae and Lachnospiraceae(Fig.4F),which was consistent with the increased generation of TMA.
3.5 Effects of the proportion of dietary macronutrients on TMAO generation
The TMA produced in gut was absorbed and transported to the liver through blood circulation.In liver,TMA was oxidized to TMAO by FMOs,particularly FMO3[16].In order to investigate the effects of the proportion of dietary macronutrients on TMAO generation in liver,the level of TMAO was determined after single intraperitoneal injection TMA.The results suggested that the diets with HPO significantly increased the level of TMAO in serum compared with control diet.Moreover,the diets with LC significantly increased the AUC of TMAO in serum after single intraperitoneal injection TMA,though the lower peak was found compared with control diet,while the diets with HC was found to have a lower peak,though no significant difference was found in AUC compared with control diet (Fig.5).The results revealed that the diets with low protein and high fat aggravated the generation of TMAO in liver.
3.6 Effects of the source of dietary macronutrients on TMAO generation
Further,it was compared that the effects of the diets with different source of carbohydrates,proteins,and fats on TMAO generation after single intraperitoneal injection TMA.The results suggested that the levels of TMAO were significantly decreased by the diets with RS and PP,compared with control (Figs.6A-D),which revealed inhibitory effects of refined sugar and plant protein on the formation of TMAO.The level of TMAO was significantly decreased by the diets with HAF,compared with HPO,suggesting an inhibitory effect of animal fat on TMA generation compared with plant oil (Figs.6E-F).
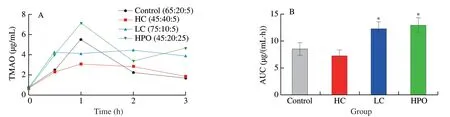
Fig.5 Effects of dietary macronutrients proportion on TMAO generation after single intraperitoneal injection TMA.(A) The concentration curve and(B) AUC of TMAO after single intraperitoneal injection TMA.Data is presented as mean ± SEM.*indicates significant difference in control vs HC,control vs LC and control vs HPO at P < 0.05 by t-test.
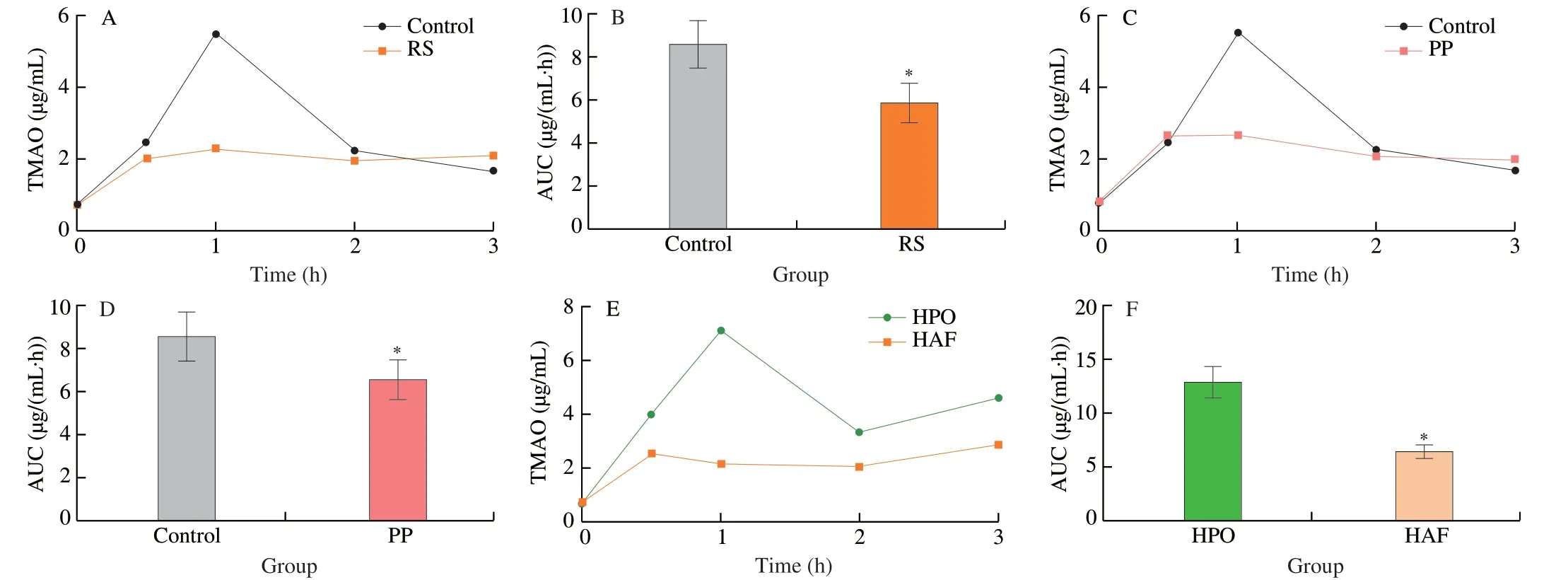
Fig.6 Effects of dietary macronutrients source on TMAO generation after single intraperitoneal injection TMA.The effects of carbohydrates from different sources on (A) the concentration curve and (B) its AUC of TMAO after single intraperitoneal injection TMA.The effects of proteins from different sources on(C) the concentration curve and (D) its AUC of TMAO after single intraperitoneal injection TMA.The effects of fats from different sources on (E) the concentration curve and (F) its AUC of TMAO after single intraperitoneal injection TMA.Data is presented as mean ± SEM.*indicates significant difference at P < 0.05 by t-test.
3.7 Effects of dietary composition on FMO3 activity
The FMOs,particularly FMO3 in liver,were the core metabolic enzyme for TMA to generate TMAO.Therefore,the protein level of FMO3 and the activity of FMOs were determined in the present study,and the results suggested that the diets with LC and HPO significantly increased the activity of FMOs compared with control,which was consistent with the level of TMAO in serum after single intraperitoneal injection TMA,though the protein level of FMO3 was not increased (Figs.7A-C).The diet with HC significantly decreased the activity of FMOs and the protein level of FMO3 compared with control (Figs.7A-C).In addition.The activity of FMOs and the protein level of FMO3 were significantly reduced by the diets with RS and PP compared with control (Figs.7A,B,D and E),while the activity of FMOs was significantly reduced by the diets with HAF compared with HPO,though the protein level of FMO3 was not changed (Figs.7A,B and F).
4.Discussion
TMAOin vivois derived from directly ingested TMAO or the transformation of its precursors.The transformation of TMAOin vivorequires the joint action of gut microbiota and liver FMOs.Diet not only determines the intake of TMAO and its precursors,but also affects the composition of gut microbiota and even liver function,which influenced the level of TMAO in serum[17].Western diet was characterized by high consumption of animal proteins and fats,and especially animal products such as liver,pork meat,and eggs.These animal products contained large amounts of choline andL-carnitine[18]and increased TMAO level in serum after consumption,which mainly revealed the efficiency of the intake of TMAO and its precursors.In order to investigate the effects of dietary pattern on TMAO production,it was comparatively studied in the present study that the effects of different dietary composition and the sources of carbohydrates,proteins,and fats on the production process of TMAO.The level of TMAO in serum after administration of different diets suggested that the level of TMAO in circulation was negatively correlated with protein intake but not affected by the different source of carbohydrates,proteins,and fats (Fig.1).It has been reported that chronic dietary red meat increased the systemic TMAO levels by enhancing dietary precursors,increasing microbial TMA/TMAO production fromL-carnitine,and reducing renal TMAO excretion,compared with white meat and non-meat protein[7],which was due to the carnitine rich in red meat.In order to avoid the interference ofL-carnitine in exploring the effect of protein on TMAO generation,the non-meat protein derived from animal (casein) with different proportions was compared with non-meat protein derived from plants(soy protein) in the present study.The results suggested that the level of TMAO in circulation was negatively correlated with non-meat protein (casein) intake.There was no significant difference in TMAO levels between casein and plant protein,though TMA levels were increased by plant protein compared with casein (Fig.1).
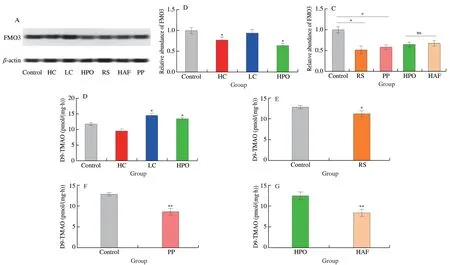
Fig.7 Effects of dietary macronutrients on the protein level of FMO3 and the activity of FMOs.(A) The representative bands of FMO3 determined by western blotting.(B) The effect of dietary macronutrients proportion on the relative protein level of FMO3.(C) The effect of dietary macronutrients source on the relative protein level of FMO3.(D) The activity of FMOs in different proportion of dietary macronutrients.(E) The activity of FMOs affected by carbohydrates from different sources.(F) The activity of FMOs affected by proteins from different sources.(G) The activity of FMOs affected by fats from different sources.Data is presented as mean ± SEM.*and **indicate significant differences in control vs HC,control vs LC,control vs HPO,control vs RS,control vs PP and HPO vs HAF,at P < 0.05 and P < 0.01 by t-test,respectively.
In addition,several studies reported that diets rich in saturated fat cause gut microbiota profile changes related to TMA production[19],which might increase the level of TMAO in serum.In the present study,the increased level of TMAO was not found in diets rich with high plant oil and high animal saturated fat,which might be due to the timeliness of production and excretion of TMAO,as well as the low intake of precursor choline on an empty stomach.TMAO,TMA and their precursors maintained the dynamic balance of generation,metabolism,and excretion,therefore,it is necessary for clarifying the effect of diet on TMAO production that studying the production process of TMAO,including TMA production from precursors in gut and TMAO production from TMA in liver.
Further,the generation of TMA from choline chloride and the production of TMAO from TMA were determined after single oral its precursor choline chloride and single intraperitoneal injection TMA,respectively.The results suggested that the generation of TMA from choline chloride and the production of TMAO from TMA was aggravated by the diets with low protein (Figs.2 and 5),which was consistent with the increased level of TMAO in serum (Fig.1B).Moreover,the generation of TMA from choline chloride and the production of TMAO from TMA were inhibited by plant protein compared with control (casein)(Figs.3 and 6),which revealed the TMAO production was influenced by the proportion and source of proteins.
It has been reported that diets rich in high saturated fat promoted an increase in plasma TMAO level in mice[20-21].Consistently,the generation of TMA from choline chloride was promoted by animal fat compared with plant oil in the present study (Fig.3).Furthermore,it was also found that the generation of TMAO from TMA in liver was increased by high plant oil in the present study (Fig.5).In addition,several studies suggested that diets with high resistant starch increased the level of TMAO in plasma in adults,however,other studies revealed that lower resistant starch intake did not decrease the serum TMAO concentrations even increased in male and female[22-25].The differences between the effects of starch and resistant starch on TMAO level in serum was still controversial,and it was still unclear that the differences between that of starch and refined sugar which was widely consumed in modern diets.In the present study,the generation of TMA from choline chloride was inhibited by refined sugar compared with control (starch),and the generation of TMAO from TMA was also inhibited by refined sugar (Fig.6).
Gut microbiota plays an important role in diets influencing the generation of TMAO.It has been reported that the Firmicutes/Bacteroidetes ratio was increased by high-fat diet,which was consistent with the present study[26].The bacterial members in the phylum Firmicutes were involved in the generation of TMAO and TMA.In addition,it has been reported that FMOs expressions and activities were associated with the composition of gut microbiota[27-28],and lipid homeostasis and glucose metabolism in liver which was influenced by diet[29].The expressions and activities of FMOs were up-regulated in livers of high fat diet (HFD)-fed mice,which might be related to endoplasmic reticulum (ER) where they located in,since ER stress could be cause by HFD-induced lipid metabolism disrupts[29-31].
There was a debate for the relationship between TMAO level in serum and the risk of various chronic diseases.Many studies reported that dietary choline,L-carnitine and TMAO exacerbated the atherosclerosis process[1,32-34].However,some studies suggested that the plaque area of atherosclerosis was not increased by dietary choline and TMAO in Apoe-/-or Ldlr-/-mice,though the level of TMAO in serum was elevated[35].These contradictory results might be caused by the different proportions and sources of macronutrients,since our previous study suggested that dietary TMAO exacerbated atherosclerosis under a low-fat rather than high-fat diet[36].Therefore,the proportions and sources of macronutrients rather than the elevated TMAO level might be a more important risk factor for various diseases,since foods rich in precursor choline andL-carnitine tend to be high in fat and cholesterol which is a more important factor in atherosclerosis.In addition,the present study suggested that the TMAO production was exacerbated by low protein intake rather than high animal fats and refined sugars,though it has been reported that atherosclerosis was aggravated by high-fat and high-sugar diet rather than low protein[37].The effects of dietary compositions on TMAO production in the present study indicated that the dietary compositions rather than the elevated TMAO level might be a more important risk factor for various diseases,such as atherosclerosis.
In the present study,the different proportions and sources of non-meat protein was compared in TMAO generation process for avoiding the interference ofL-carnitine.The comparative analysis with non-meat protein,and meat protein without TMAO and its precursor,on the generation of TMAO is open to discussion.In addition,representative animal fat and plant oil were compared in the present study.Future studies are recommended to understand other fats from different sources,especially the fats with differentn-3/n-6 polyunsaturated fatty acids (PUFA) ratio,since it was found in our previous study that fish oil rich inn-3 PUFA ameliorated TMAO-exacerbated glucose intolerance in mice fed a high fat diet[38-39].
In conclusion,our study demonstrated that TMAO generation process was influenced by the proportion and source of dietary macronutrients.The generation process of TMAO was enhanced by low non-meat protein and high fat via promoting the transformation of TMAO from TMAin vivo.For the source of dietary macronutrients,the generation process of TMAO was suppressed by plant protein and refined sugar via attenuating precursors producing TMA in gut and TMA producing TMAO in liver.In addition,animal fat promoted the production of TMA form precursors in gut but suppressed the transformation of TMAO from TMA in liver compared with plant oil.
Conflict of interests
Yuming Wang is an editorial board member forFood Science and Human Wellnessand was not involved in the editorial review or the decision to publish this article.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (32072145).
- 食品科学与人类健康(英文)的其它文章
- Protective effects of oleic acid and polyphenols in extra virgin olive oil on cardiovascular diseases
- Diet and physical activity inf luence the composition of gut microbiota,benef it on Alzheimer’s disease
- Inf luence of nitrogen status on fermentation performances ofnon-Saccharomyces yeasts: a review
- Ganoderma lucidum: a comprehensive review of phytochemistry,eff icacy,safety and clinical study
- Resveratrol combats chronic diseases through enhancing mitochondrial quality
- Demonstration of safety characteristics and effects on gut microbiota of Lactobacillus gasseri HMV18

