Comparison of immune responses and intestinal f lora in epicutaneously sensitized BALB/c or C57BL/6 mouse models of food allergy
Gng Yu,Yuho Jing,Shuifeng Zhng,Pengpeng Liu,c,Shunyu Wng,Huong Sheng,Yno Wng,Qiozhi Zhng,,Linglin Fu,
a Food Safety Key Laboratory of Zhejiang Province, School of Food Science and Biotechnology, Zhejiang Gongshang University, Hangzhou 310018, China
b Zhejiang Fangyuan Test Group Co., Ltd., Hangzhou 310018, China
c Key Laboratory of Biosafety Detection for Zhejiang Market Regulation, Hangzhou 310018, China
d Zhejiang Liziyuan Food Co., Ltd, Jinhua 321031, China
Keywords: Food allergy Mouse models Epicutaneous sensitization Th2 response Gut microbiota
ABSTRACT Cutaneous exposure to food allergens through a disrupted skin barrier is recognized as an important cause of food allergy,and the cutaneous sensitized mouse model has been established to investigate relevant allergic disorders.However,the role of different genetic backgrounds of mice on immune responses to food allergens upon epicutaneous sensitization is largely unknown.In this study,two strains of mice,i.e.,the BALB/c and C57BL/6 mice,were epicutaneously sensitized with ovalbumin on atopic dermatitis (AD)-like skin lesions,followed by intragastric challenge to induce IgE-mediated food allergy.Allergic outcomes were measured as clinical signs,specif ic antibodies and cytokines,and immune cell subpopulations,as well as changes in intestinal barrier function and gut microbiota.Results showed that both strains of mice exhibited typical foodallergic symptoms with a Th2-skewed response.The C57BL/6 mice,rather than the BALB/c mice,were f itter for establishing an epicutaneously sensitized model of food allergy since a stronger Th2-biased response and severer disruptions in the intestinal barrier and gut homeostasis were observed.This study provides knowledge for selecting an appropriate mouse model to study food-allergic responses associated with AD-like skin lesions and highlights the role of genetic variations in the immune mechanism underlying pathogenesis of food allergy.
1.Introduction
Food allergy is an adverse reaction of the host immune system to otherwise harmless food components that usually are called allergens.Over the past decades,the incidence of food allergy is dramatically increased,especially in westernized countries.Over the world,about 8% of children and 5% of adults were affected by food allergies[1-2].Individuals with food allergies often exhibit itchy flushing skin,diarrhea,and/or vomiting,as well as respiratory distress,and are at the risk of anaphylactic shock after exposure to allergens[3].Many factors can affect the development of food allergies,such as genetic factors[2,4],environmental factors[2],dietary[1],and intestinal microbiota[5].
Recently,epidemiologic studies have demonstrated that food allergy is commonly related to cutaneous inflammation associated with atopic dermatitis (AD)[6-7].More recently,a systematic review verif ied that AD can contribute to the development of food allergy[8],and other studies revealed that compromised skin barriers promote a cutaneous sensitization that ultimately gives rise to food-related anaphylaxis[9-10].This indicated that the skin is another highly relevant site of allergen sensitization except for oral exposure.In this context,a few recent studies have used epicutaneously sensitized animal models to mimic and investigate the pathogenesis of food allergies associated with AD-like skin lesions.In doing this,cutaneous sensitization was commonly established by repeated exposure to the allergen via skin disrupted by tape stripping or pretreatment with MC903 (a vitamin D3analogue)[9-11].
Genetic background has been proven to affect susceptibility to some types of diseases.An example is allergic diseases,where studies have shown that patients and experimental animals with specific backgrounds appear to respond very strongly to allergens[12].Mice are commonly used experimental animals and are usually employed to investigate the cellular and molecular mechanisms of IgE-mediated allergic diseases[13].Among the many strains of mice,the strains BALB/c and C57BL/6 are often used for establishing murine models of food allergy[3,13].The BALB/c mouse is a nearcrossing high-IgE response strain and has been widely used in the study of hypersensitive diseases;the C57BL/6 mouse is usually used for studying the pathogenesis of allergic airway diseases and also attracts attention in the study of food allergy due to the advantage of enormous genetic variants available[3].Moreover,in murine models of allergic diseases,the BABL/c mice are deemed to be a strain with a Th2-dominant genetic background,conversely,C57BL/6 mice are the strain with a Th1-dominant genetic background[14].Liu and co-workers[15]intraperitoneally sensitized BABL/c mice with ovalbumin (OVA) and found that the levels of OVA-specific antibodies (IgE,IgG1,and IgG2a),anaphylactic mediators,and Th2 cytokines (interleukin (IL)-4 and IL-13) were significantly increased in sensitized mice.In addition to intraperitoneal sensitization,Noti et al.[9]epicutaneously sensitized BABL/c mice with OVA and demonstrated that epicutaneous sensitization significantly increased the levels of OVA-specific IgE antibodies,allergy score,and Th2 cytokines (IL-4,IL-5,and IL-13) in the jejunum.Kong et al.[16]recently established a peanut-induced allergic C5BL/6 mouse model via intragastric gavage and epicutaneous sensitization and illustrated the role of uric acid alarmins in activating dendritic cells (DC) in the local microenvironment of the host sensitized to peanuts.In this scenario,considering that both BALB/c and C57BL/6 strains can be used to establish mouse models of allergy,recently,several studies focused on phenotypic differences in the genetic background of mouse models in immune responses to anaphylactic diseases.Gueders and co-workers[17]developed mouse models of asthma using OVA to compare hyperresponsiveness in BALB/c mice and C57BL/6 mice and found that the BALB/c mice exhibited higher levels of IL-4,IL-5,and IL-13 in lung tissue,whereas,the C57BL/6 mice had higher levels of CCL11 and CCL5 in bronchoalveolar lavage fluid.Besides,Kodama et al.[18]investigated the strain-specific phenotypes of airway inflammation and bronchial hyperresponsiveness induced by epicutaneous OVA sensitization in BALB/c and C57BL/6 mice.Their results demonstrated that the levels of OVA-specific IgG1 and IgE were higher in BALB/c mice,while both eosinophilic airway inflammation and bronchial responsiveness to methacholine were more prominent in C57BL/6 mice[18].However,the effect of differences in genetic background among inbred mouse strains on immune responses related to food anaphylaxis mediated by epicutaneous sensitization is largely unknown.
In this study,two strains of mice (e.g.,the BALB/c and C57BL/6 mice) were used to establish OVA-mediated food allergy models by epicutaneous sensitization and intragastrical challenge.The comparison of allergic manifestations and immune responses of the two mouse strains was performed based on indexes including clinical symptom signs,specific antibody isotypes,lymphocyte subpopulation,and related cytokines.Additionally,the gut homeostasis of different mouse models was also compared according to the outcomes of the intestinal barrier integrity and fecal microbiota.
2.Materials and methods
2.1 Chemicals
OVA (≥ 98%,cat# A5503) and MC903 (calcipotriol,a vitamin D3analogue,≥ 98%,cat# C4369) were purchased from Sigma-Aldrich (St.Louis,MO,USA).The AIN-93G purified chow diet was obtained from Nanjing Xietong Bioengineering Co.,Ltd.(Nanjing,China).The IFN-γ (cat# 88-7314),IL-4 (cat# 88-7044),IL-5 (cat#88-7054),and IL-13 (cat# BMS6015) mouse ELISA kits were purchased from Thermo Fisher Scientific (Waltham,MA,USA).A histamine mouse ELISA kit (cat# H171) was bought from Nanjing Jiancheng Bioengineering Institute (Nanjing,China).A Total RNA Extraction Kit (cat# R1200) was purchased from Solarbio Science &Technology Co.,Ltd.(Beijing,China).The Hifair® III cDNA Synthesis SuperMix qPCR kit (cat# 11141ES60) and the Hieff UNICON® Universal Blue SYBR qRT-PCR kit (cat# 11184ES60)were bought from Yeasen Bio-Technologies Co.,Ltd.(Shanghai,China).The eBioscience™ Cell Stimulation Cocktail (cat# 00-4975)was purchased from Thermo Fisher Scientific (Waltham,MA,USA).All antibodies and fixation/permeabilization reagents used for flow cytometry were products from BD Biosciences (NJ,USA).Ultrapure water purified from a Millipore Milli-Q Advantage A10 Water Purification System (Bedford,MA,USA) was used throughout.All other chemicals and reagents used were of analytical grade unless otherwise noted.
2.2 Epicutaneous sensitization and oral challenge of mice
Six-week-old female BALB/c or C57BL/6 mice were housed in specific pathogen-free conditions at the Laboratory Animal Research Center of Zhejiang Academy of Medical Sciences (Zhejiang,China).The protocol was approved by the Institutional Animal Care and Use Committee of the Zhejiang Academy of Medical Sciences.The housing conditions were controlled as follows: an environmental temperature of (22 ± 1) °C,a relative humidity of (55 ± 10)%,and a light-dark cycle of 12/12 h.A standard diet (AIN-93G) was fed to the mice with ad libitum intake of distill-water throughout the study.The mice were randomly divided into 4 groups (n=5/group),i.e.,the BALB/c mice control group (B-CON),BALB/c mice OVA-treated group (B-OVA),C57BL/6 mice control group (C-CON),and C57BL/6 mice OVA-treated group (C-OVA).The protocols of sensitization,challenge,and sampling were presented in Fig.1A.In brief,after one-week adaptation,mice in the B-OVA and C-OVA groups were smeared daily with 2 nmol of MC903 (dissolved in 20 µL of ethanol) and 100 μg of OVA (dissolved in 10 µL of PBS (10 mmol/L,pH=7.4)) on both ears for 14 consecutive days.As respective controls,the same volume of ethanol (without MC903) and OVA solution was applied to the ears of mice in the B-CON and C-CON groups.On day 21,the ear thickness of each mouse was measured.On days 22 and 25.5,all mice were challenged intragastrically with 50 mg of OVA (dissolved in PBS).After the last oral challenge,the rectal temperature of each mouse was recorded within 30 min,and the allergic symptom score was assessed within 30-60 min (scoring details listed in Supplementary Table 1).The final clinical allergy score was calculated with the following equation:
whereSs,Sb,Sp,andSstpresented the score of scratching,behavior,physical appearance,and stool consistency,respectively.
Twelve hours after the last oral challenge,all mice were anesthetized and killed by cervical dislocation.The blood was collected to prepare serum fraction,and the spleen,small intestinal tissue,and colon content were collected and snap-frozen with liquid nitrogen before storage at -80 °C.
2.3 Serum OVA-specific antibodies and cytokines level
The levels of OVA-specific antibodies,namely,IgE,IgG1,and IgG2a,in the serum were detected by indirect ELISA as previously indicated[15].Besides,the serum contents of IFN-γ,IL-4,IL-5,IL-13,and histamine were measured by commercially available ELISA kits according to the manufacturer’s instructions.
2.4 Flow cytometry
The spleen collected post-challenge from individual mouse was prepared for single-cell suspension as previously indicated[19].The flow cytometry staining protocol was performed as recommended by the provider of antibodies (BD Biosciences,NJ,USA).The cells were stimulated for 6 h with eBioscience™ Cell Stimulation Cocktail(containing phorbol 12-myristate 13-acetate,ionomycin,brefeldin A,and monensin).After stimulation,cells were first stained with cellsurface antigens (FITC-conjugated anti-mouse CD4 antibody)and then,after fixation and permeabilization with BD fixation/permeabilization reagent,stained with PC5.5-conjugated anti-mouse IFN-γ antibody (intracellular marker) and PE-conjugated anti-mouse IL-4 antibody (intracellular marker).Analysis of labeled cells was conducted with a CytoFLEX Flow cytometer (Beckman Coulter,Inc.,Brea,CA,USA),and the data were analyzed by CytExpert software(Version 2.0,Beckman Coulter,Inc.,Brea,CA,USA).
2.5 Analysis of gene expression by real-time quantitative PCR (qRT-PCR)
Total RNA of the spleen or intestinal tissue was extracted by a Total RNA Extraction Kit.5 mg of total RNA from each sample were reversely transcribed into cDNA by using the Hifair® III cDNA Synthesis SuperMix qPCR kit per the manufacturer’s protocol.Specific primers (Table 1) were designed and synthesized by Sangon Biotech.Co.,Ltd.(Shanghai,China).After pre-treating with the Hieff UNICON®Universal Blue SYBR qRT-PCR kit,qRT-PCR was conducted by the LightCyler® 480 II qRT-PCR system (Roche,Switzerland).Data were normalized to the housekeeping genes of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and analyzed with the ΔΔ cycle threshold method as described previously[20].
2.6 Morphological analyses of jejunum tissue
To analyze the integrity of the jejunum villi,the jejunum of each mouse was isolated,fixed,dehydrated,and dried,and then observations of the dried jejunum were performed by scanning electron microscope (SEM) according to a previous study[15].
2.7 Bacterial DNA extraction and 16S rRNA gene sequencing
Feces were collected post-challenge from individual mouse and snap-frozen with liquid nitrogen before storage at -80 °C.These samples were analyzed for 16S rRNA gene sequencing by Novogene Bioinformatics Technology Co.,Ltd.(Beijing,China) using standard protocols.In brief,the total genome DNA was extracted by the CTAB/SDS-based method and analyzed by 1% agarose gel electrophoresis to monitor the concentration and purity of DNA.The extracted DNA was further diluted to 1 ng/µL with UP water.Afterward,the V3-V4 hypervariable regions of bacterial 16S rRNA were amplified with the forward primer 341F (5’-CCTAYGGGRBGCASCAG-3’)and reverse primer 806R (5’-GGACTACNNGGGTATCTAAT-3’).After quantification and purification,the PCR amplicons were mixed depending on the amount of data required for each sample.Sequencing was performed on an Illumina NovaSeq platform by using a 300 bp paired-end model.
2.8 Bioinformatics analysis
Paired-end reads were merged using FLASH (version 1.2.11) and the splicing sequences were called Raw Tag.Quality filtering on the raw tags was implemented using Fastp (Version 0.20.0) software to obtain high-quality Clean Tags.After comparing with the reference database (Silva database https://www.arb-silva.de/ for 16S/18S)using Vsearch (Version 2.15.0),the chimera sequences were detected from Clean Tags.The Effective Tags were obtained by removing the chimera sequences from Clean Tags and applied for subsequent bioinformatics analysis.Using the QIIME2 software (Version QIIME2-202006),all Effective Tags of each sample were denoised,filtered,annotated based on the Silva database,and finally clustered into operational taxonomic units (OTUs) based on 97% sequence similarity.Forα-diversity analysis,a rarefaction index curve was generated,and the indexes of Chao1,Shannon,and Simpson were calculated using QIIME2 software.
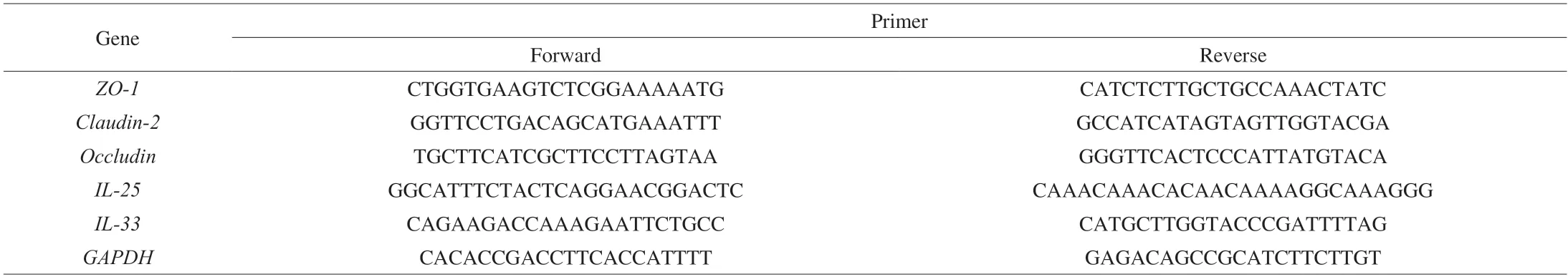
Table 1 Primers used for qRT-PCR in the study.
Theβ-diversity was calculated based on weighted and unweighted Unifrac distances using QIIME2.Significant differences in phylotypes among groups were identified by a taxonomy-based analysis.Based on the Mann-Whitney test,only the taxa with an average abundance > 1%,P-value < 0.05,andqvalue (risk of false discovery) < 0.1 were marked significant.Linear discriminant analysis effect size(LEfSe) analysis was used to analyze the biomarkers among groups based on the following criteria: (a) theαvalue for the factorial Kruskal-Wallis test among the taxonomic classes was < 0.05 and (b)the threshold on the logarithmic linear discriminant analysis (LDA)score for the discriminative features was > 3.0.
2.9 Statistical analysis
All experiments were completed thricely at least,and the results were shown as the mean ± standard error of the mean (SEM).Differences between two groups were analyzed with Student’st-test or Mann-Whitney test.TheP-value significance threshold was set at 0.05.
3.Results
3.1 Differences in allergic symptoms in two strains of mice
Six-week-old female BALB/c and C57BL/6 mice were housed in the same conditions feeding the same chow diet.After oneweek adaption,mice in the B-OVA and C-OVA groups were epicutaneously sensitized to OVA on AD-like skin lesions.During the sensitization period,as expected,mice in the B-OVA and C-OVA groups showed a significant reduction in average body weight(P< 0.05),when compared to the respective controls (Fig.1B).Additionally,the thickness of ears in sensitized mice all showed a significant incrassation when compared to that of the unimmunized controls (P< 0.001,Fig.1C).
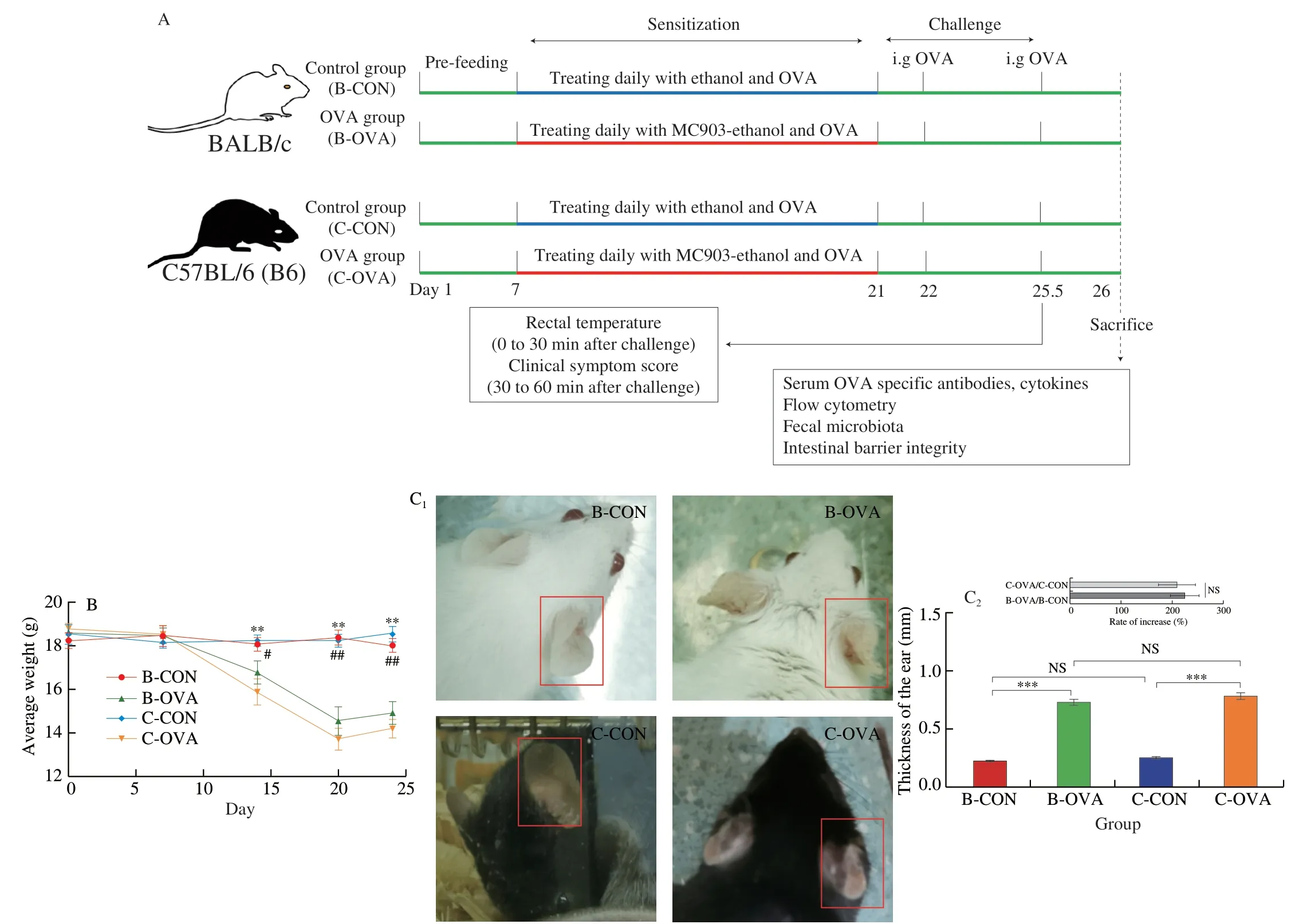
Fig.1 (A) The immunization protocol of BALB/c or C57BL/6 mouse food allergy model;changes in the (B) average weight during the experiment (#,significant differences between B-CON and B-OVA,#P < 0.05,##P < 0.01;*,significant differences between C-CON and C-OVA,*P < 0.05,**P < 0.01),(C) representative images (left) and thickness (right) of ears of mice,(D) reduction in rectal temperature,and (E) clinical allergy score of mice upon the last oral challenge.Serum levels of (F) histamine and OVA-specific (G) IgE,(H) IgG1,and (I) IgG2a.Data are expressed as the mean ± SEM (n=5).*P < 0.05,**P < 0.01,***P< 0.001,compared to the indicated control;NS,no significant differences,Student’s t-test or Mann-Whitney test.

Fig.1 (Continued)
On days 22 and 25.5,all mice were intragastrically challenged with OVA,and 30 min after the last challenge,the rectal temperature of each mouse was recorded.As shown in Figs.1D-E,the two strains of mice immunized by OVA both exhibited a significant reduction in rectal temperature and a statistical increase in clinical allergy score,in comparison to their respective controls (P< 0.001).Additionally,sensitized mice in the B-OVA and C-OVA groups not only showed scratching skin as a hallmark of AD[21](Fig.S1A),but also exhibited typical food-allergic symptoms including decreases in rectal temperature (Fig 1D) and increased diarrhea rates (Fig.S1B).This indicated that both mouse models of food allergy immunized by epicutaneous exposure were successfully established.Furthermore,histamine,a bio-activator released by mast cells and basophil cells in the effective phase of food allergy was also determined.As shown in Fig.1F,all epicutaneously sensitized mice had a significantly higher level of histamine than that of their control counterparts (P< 0.001).The rate of increase in histamine levels in sensitized C57BL/6 mice was statistically lower than that of the immunized BALB/c mice(P< 0.01,insert of Fig.1F).Interestingly,it was noted that even mice in the C-CON group showed a higher level of histamine when compared to mice in the B-OVA group (P< 0.001,data not shown).
3.2 Comparison of specific antibodies and cytokine levels

Fig.2 Serum concentrations of cytokines in different strains of mice epicutaneously sensitized by OVA: (A) IFN-γ;(B) IL-4;(C) IL-5;(D) IL-13.Data are expressed as the mean ± SEM (n=5).*P < 0.05,**P < 0.01,***P < 0.001,compared to the indicated control;NS,no significant differences,Student’s t-test or Mann-Whitney test.
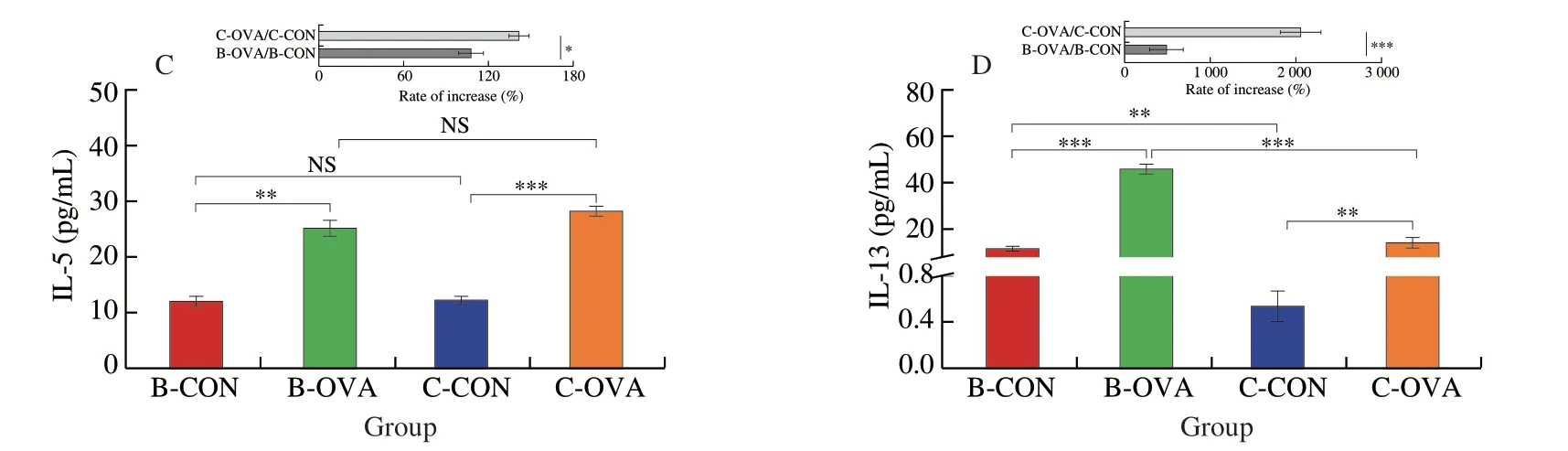
Fig.2 (Continued)
The serum levels of OVA-specific antibodies,namely IgE,IgG1,and IgG2a,were further analyzed and the results are given in Figs.1G-I.Compared to respective control mice,mice in the B-OVA and C-OVA groups both showed significantly higher levels of OVA-specific IgE,IgG1,and IgG2a (P< 0.01),in line with findings in previous reports[22-26].In addition,the C57BL/6 mice had a higher rate of increase in specific IgE (Fig.1G),while with a lower rate of increase in specific IgG2a (Fig.1I) when compared to the BALB/c mice (P< 0.05).
Furthermore,serum concentrations of immune cytokines,namely,IFN-γ,IL-4,IL-5,and IL-13 were detected.As shown in Fig.2,the two strains of mice sensitized with OVA both had significantly higher levels of IL-4,IL-5,and IL-13,while with a statistically lower level of IFN-γ when compared to their respective controls (P< 0.01),in agreement with previous publications[27-28].Of note,mice in the C-OVA group had a higher rate of reduction/increase in the contents of IFN-γ,IL-5,and IL-13 when compared to mice in the B-OVA group (P< 0.05) (Figs.2A,C-D).These results suggested that the C57BL/6 mice epicutaneously treated with OVA possessed a stronger Th2-type response than that of the BALB/c mice,which kept in agreement with the results of specific IgE levels.
3.3 Comparison of splenic T lymphocyte cell subsets
The subpopulation of T lymphocyte cells in the spleen was analyzed by flow cytometry and the results are shown in Fig.3.The proportion of CD4+IFN-γ+Th1 cells in BALB/c mice was significantly reduced when epicutaneously sensitized by OVA(14.40%vs11.43%) (P< 0.01) (Fig.3A).Previously,after being sensitized by OVA,the proportion of Th1 in spleen of the BABL/c mice reduced from 15% to 8.5%,which was in accordance with our present study[15].In comparison,a statistical higher subset of Th1 cells was observed in sensitized C57BL/6 mice (15.35%vs20.01%) (P< 0.01).In parallel,mice in the B-OVA and C-OVA groups both possessed a statistically higher proportion of CD4+IL-4+Th2 than their unimmunized controls (P< 0.01).The two mouse strains had a similar rate of increase in the population of Th2 cells (P> 0.05) (Fig.3B).
3.4 Gut homeostasis
3.4.1 Changes in gut barrier integrity
The morphological structure of jejunum tissue was observed using SEM.As shown in Fig.4A,the two strains of immunized mice both exhibited varying degrees of damage to the jejunum villi when compared to their control counterparts,which typically showed smooth and intact jejunum villi.Moreover,the mRNA expression levels of tight junction-related (TJ) proteins,includingZO-1,claudin-2,andoccludin,in the intestinal tissue were measured to evaluate the integrity of the intestinal barrier.After being epicutaneously sensitized by OVA,the two strains of mice both showed significantly down-regulated expression levels ofZO-1(Fig.4B) andclaudin-2(Fig.4C) in the intestine (P< 0.01).In comparison,a significantly lower expression level ofoccludinwas observed in sensitized C57BL/6 mice (P< 0.01),but not in sensitized BALB/c mice when compared to their respective controls (P> 0.05)(Fig.4D).Besides,alarmins,such as IL-25 and IL-33 were secreted by damaged epithelial cells of the intestine,which can induce group 2 innate lymphoid cells (ILC2s) activation and secretion of Th2-type cytokines[29-30].As shown in Figs.4E-F,the two strains of mice both had significantly up-regulated expression levels of IL-25 and IL-33 in the intestine when compared to their respective controls(P< 0.01).Of note,when compared to the sensitized BABL/c mice,the sensitized C57BL/6 mice showed statistically higher expression levels of IL-25 and IL-33 (P< 0.05).

Fig.3 Subpopulations of T lymphocytes in the spleen of different strains of mice epicutaneously immunized by OVA: (A) CD4+ IFN-γ+ Th1 cells;(B) CD4+IL-4+ Th2 cells.Data are expressed as the mean ± SEM (n=5).*P < 0.05,**P < 0.01,***P < 0.001,compared to the indicated control;NS,no significant differences,Student’s t-test or Mann-Whitney test.
Fig.3 (Continued)

Fig.4 The pathological morphology of jejunum villi as assessed by (A) SEM and the expression levels of genes related to intestinal barrier integrity in the intestine: (B) ZO-1,(C) claudin-2,and (D) occludin.The expression levels of (E) IL-25 and (F) IL-33 in the intestine tissue.Data are expressed as the mean ± SEM(n=5).*P < 0.05,**P < 0.01,***P < 0.001,compared to the indicated control;NS,no significant differences,Student’s t-test or Mann-Whitney test.
3.4.2 Changes in the gut microbiota
The rarefaction curves of observed_OTUs and Shannon index suggested that the depth of sequencing was sufficient to reflect biological information,and the data of sequencing was reliable (Fig.S2).The indexes of observed_OTUs,Chao1,Shannon,and Simpson were used to evaluate theα-diversity of gut microbiota among the four groups of mice (Fig.5).In BALB/c mice,no significant difference in theα-diversity was observed after epicutaneous sensitization.In comparison,drastic decreases in the observed_OTUs,Chao1,Shannon,and Simpson indexes were observed in sensitized C57BL/6 mice when compared to the unimmunized control (P< 0.05).
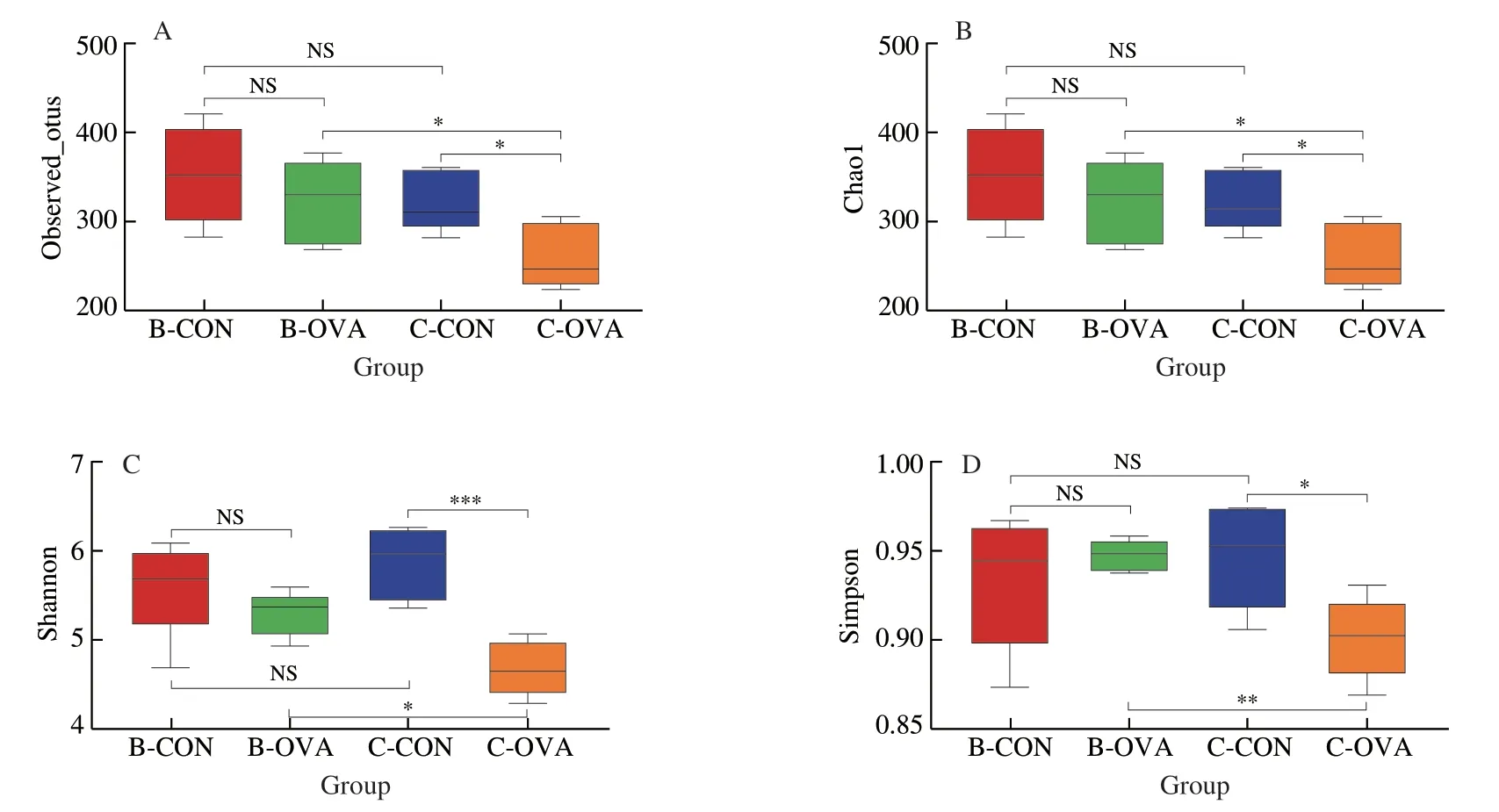
Fig.5 Analysis of the α-diversity of fecal microbiota in BALB/c or C57BL/6 mice epicutaneously sensitized by OVA.α-Diversity indices were assessed by(A) Observed_OTUs,(B) Chao1,(C) Shannon,and (D) Simpson indexes.Data are expressed as the mean ± SEM (n=5).*P < 0.05,**P < 0.01,***P < 0.001,compared to the indicated control;NS,no significant differences,Student’s t-test or Mann-Whitney test.
Furthermore,theβ-diversity was evaluated by the principal coordinates analysis (PCoA) based on unweighted and weighted unifrac distances,and the results are shown in Figs.6A-B.According to the unweighted_unifrac PCoA,we observed a moderate separation in microbiota members between the immunized and control groups in both strains of mice (Anosim,P< 0.05) (Fig.6A).Similarly,the weighted_unifrac PCoA showed that both the B-OVA and C-OVA groups can be separated from their respective control groups in the overall microbiota structure (Anosim,P< 0.01) (Fig.6B).
The taxonomic distributions of gut microbiota at the levels of phylum,family,and genus are shown in Figs.6C-E,respectively.At the phylum level (Fig.6C),Bacteroidetes was the most dominant phylum among all the samples,followed by Firmicutes and Desulfobacterota,the total relative abundance of which composed at least 90% of the total bacterial communities.In the BALB/c stain,epicutaneous sensitization with OVA significantly increased the relative abundance of Bacteroidetes while reduced the relative abundance of Firmicutes (P< 0.05;Fig.7A).By contrast,the immunized C57BL/6 mice displayed a significantly lower abundance of Desulfobacterota when compared to the control mice (P< 0.05;Fig.7D).At the family level,mice in the B-OVA group had a statistically higher abundance of Bacteroidaceae,Tannerellaceae,and Akkermansiaceae,with a lower abundance of Lachnospiraceae,Marinifilaceae and Oscillospiraceae than the mice in the B-CON group (P< 0.05) (Fig.7B).In comparison,mice in the C-OVA group showed a higher abundance of Bacteroidaceae,Lactobacilliaceae,and Tannerellaceae,with a lower abundance of Muribaculacea,Rikenellaceae,Desulfovbrionacea,Marinifilaceae,and Oscillospiraceae than the mice in the C-CON group (P< 0.05;Fig.7E).Accordingly,at the genus level,after epicutaneous sensitization,a series of genera including theBacteroides,Parabacteroides,andPrevotellawere either up-regulated or down-regulated in the BALB/c strain (P< 0.05;Fig.7C);in C57BL/6 mice,5 genera (i.e.,Bacteroides,Lactobacillus,Parabacteroides,Clostridium_innocuum_group,andNegativibacillus) were significantly enriched while the other 8 genera were statistically depleted in C-OVA group when compared to the C-CON group (P< 0.05;Fig.7H).

Fig.7 Significantly different (A) phyla,(B) families,and (C) genera between the B-CON and B-OVA groups,and significantly different (D) phyla,(E) families,and (F) genera between the C-CON and C-OVA groups.LEfSe analysis shows the species that best explain differences between (G) the B-CON and B-OVA groups and (H) the C-CON and C-OVA groups.Taxa with LDA scores greater than 3 are shown.
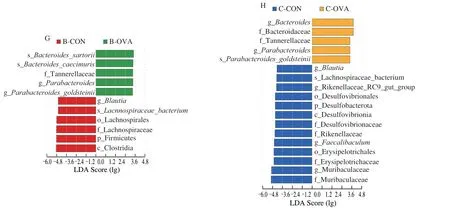
Fig.7 (Continued)
Finally,significantly different bacterial taxonomic biomarkers between the control and epicutaneously sensitized groups were determined by the LEfSe method.As shown in Fig.7G,the LEfSe analysis demonstrated 11 significantly discriminative features between the B-CON and B-OVA groups.Firmicutes spp.(includingClostridia,Lachnospiraceae,andBlautia) were more abundant in the B-CON group,whileBacteroidesandParabacteroidesspp.were more enriched in the B-OVA group.In comparison,18 significantly different features were identified between the C-CON and C-OVA groups (Fig.7H).BacteroidesandParabacteroidesspp.were also found to be enriched in the C-OVA group.Whereas,Desulfobacterota,Erysipelotrichales(includingFaecalibaculum),Muribaculaceae,Rikenellaceae,and Lachnospiraceae spp.were more abundant in the C-CON group.
4.Discussions
AD is a known risk factor for the development of food allergies[8,31].In the context of an AD-like skin lesion,repeated exposure to food allergens through the skin may override tolerizing oral exposure and lead to the incidence of food anaphylaxis[11,21].Currently,there is no report that studies the influence of genetic backgrounds on the establishment of food allergic responses in mouse models associated with AD-like skin lesions.The present study evolves three important conclusions according to the comparative analysis of immune responses and intestinal gut homeostasis in epicutaneously sensitized BALB/c or C57BL/6 mice.Firstly,we found that both BALB/c and C57BL/6 mice showed typical Th2-type responses to the model food allergen OVA through an AD-like skin lesion despite with different genetic backgrounds.Secondly,the C57BL/6 mice exhibited more severe disruption of the intestinal barrier integrity and dysbiosis of the gut microbiome than BALB/c mice after epicutaneous sensitization.Thirdly,the C57BL/6 mice were more suitable for establishing the epicutaneously sensitized mouse model of food allergy than the BALB/c mice.Collectively,these findings help to illustrate the role of genetic background in the establishment of mouse models of food allergy immunized by cutaneous exposure.
Previous reports showed that BALB/c mice epicutaneously sensitized to OVA exhibited higher anaphylactic scores,and elevated levels of OVA-specific IgE,and Th2-type cytokines,such as IL-4,IL-5,and IL-13,when compared to the unimmunized mice[9].Similarly,epicutaneously sensitized C57BL/6 mice showed significant increases in the serum levels of specific IgE and cytokines like IL-4,IL-5,and IL-13 in the skin-draining lymph node[32].Consistent with those reports,we demonstrated that the epicutaneously sensitized BALB/c and C57BL/6 mice both manifested remarkable symptoms of food allergy,including reduced rectal temperatures,increased anaphylactic scores,and elevated levels of OVA-specific antibodies,and Th2-type cytokines including L-4,IL-5,and IL-13 in the serum.In addition to symptoms of food allergy,the two strains of mice also displayed symptoms of AD,such as scratching and swelling of the ear skin.This suggested that both strains of mice can be used to establish mouse models of food allergy immunized by epicutaneous exposure.However,the effect of genetic background of mice on immune responses of different models of food allergy remains unknown.
It has been reported that the genetic background of mice constitutes an important factor to be considered when establishing mouse models of immune diseases,such as asthma[17],bronchial hyperresponsiveness[18],and immune-mediated blepharoconjunctivitis[12].Indeed,mice with genetic susceptibility are commonly used as models to mimic the features of human food-allergic diseases.As is well known that the BALB/c mice are a Th2-biased strain and are usually used to establish food allergic mouse models using different sensitization routes,such as oral gavage[11],intraperitoneal injection[11,33],and epicutaneous exposure[11,31].In keeping with the previous study[11],we found that epicutaneously sensitized BALB/c mice exhibited a reduced proportion of Th1 subsets and an increased proportion of Th2 subsets.In comparison,the C57BL/6 mouse is a Th1-prone strain and usually exhibited a relatively weak allergic response to allergens when compared to the BALB/c mice[3].Accordingly,we noticed both increased proportions of Th1 and Th2 subsets in epicutaneously sensitized C57BL/6 mice in the present study.A previous study on airway inflammation and bronchial hyperresponsiveness by Kodama and co-workers demonstrated that after epicutaneous sensitization,the BALB/c mice had a higher level of specific IgE than the C57BL/6 mice[18].Inconsistently,in this study,we found that the C57BL/6 mice had a higher level of OVA-specific IgE and greater increases in Th2-type cytokines like IL-5 and IL-13 when compared to the BABL/c mice upon cutaneous exposure.IL-5 as a central factor has been reported to play a vital role in mediating eosinophil expansion,priming,recruitment,and prolonged tissue survival in response to allergic stimuli[34-35].Moreover,IL-13 acts with a similar biological function to IL-4 and is involved in the isotype switch from IgM to IgE of B cells[34,36].Besides,it has been reported that alarmins,such as IL-25,IL-33,and thymic stromal lymphopoietin (TSLP),derived from damaged epithelial cells can induce ILC2s activation and secretion of IL-4,IL-5,and IL-13[29-30].The higher expression levels of IL-25 and IL-33 in the intestine of sensitized C57BL/6 mice in the present study may likely contribute to a Th2-biased microenvironment rich in IL-4,IL-13,and IL-5 to promote B cells to class switch to IgE[37].Therefore,in the present study,the higher increases in specific IgE and Th2-type cytokines in the C57BL/6 strain may be ascribed to the innate immune responses in the intestine.Altogether,these data demonstrated that the C57BL/6 mice showed a stronger Th2-skewed immune response than the BALB/c mice and may be more suitable for establishing the epicutaneously sensitized model of food allergy.
Given that growing evidence has demonstrated that dysfunctions in the intestinal epithelial barrier are involved in the development of food allergy[15,38-39],we further investigated and compared the gut barrier integrity of the two strains of mice epicutaneously sensitized with OVA.As is well known that intestinal barrier destruction is mainly manifested by the loss of TJ proteins[40].TJ proteins as a critical part of the intestinal epithelial barrier can effectively prevent the pathogenic antigen to translocate from the lumen into the mucosal lamina propria and further into the circulation by regulating the selective permeability of the intestinal epithelial barrier[41].Occludin as a major member of the transmembrane protein can not only regulate the permeability of TJ but also maintain cell polarity[40].ZOs are members of the peripheral membrane proteins family and mainly keep the integrity of the TJ complex by linking claudins,occludin,and cytoskeletal proteins[42].Previous studies have reported that mice with allergies exhibited a significant down-regulated expression of TJ proteins,including ZO-1,occludin,and claudin-2,not only at the mRNA level but also at the protein level[22].Similar results were also observed in the present study.After epicutaneous sensitization,the two strains of mice both showed disruptions in the gut barrier integrity,accompanied by down-regulated mRNA expression levels of ZO-1 and claudin-2 in the intestine.Of note,the epicutaneously sensitized C57BL/6 mice,not the BALB/c mice,showed a statistically lower expression level of occludin in the tissue,demonstrating severer destruction of the gut barrier in the strain.
Imbalance of the gut flora is closely associated with the etiology of food allergy[33].Individuals with food allergies commonly exhibited reduced richness and diversity of the gut microbiota,and various changes in the composition and structure of the community[32,43].Consistently,in this study,we observed that the cutaneously sensitized C57BL/6 mice,not the immunized BALB/c mice,had a significant reduction in the richness and diversity of the gut microbiota community.For taxonomic compositions,Liu and co-workers reported that the relative abundance of Bacteroidetes was increased,while that of the Firmicutes was reduced in intraperitoneally sensitized BALB/c mice[15].In another similar mouse model,it was noted that the relative abundance of both of the two phyla was depleted upon intraperitoneal sensitization[33].Previous studies have documented increases in the abundance of Bacteroidaceae as a marked feature of infants with cow’s milk allergy[44].In particular,a high abundance of Bacteroides was observed in 3-6-month subjects with persistent milk allergy[45].Interestingly,alterations of Bacteroidaceae in the gut flora were rarely reported in orally or intraperitoneally sensitized murine models of food allergy.Of note,in the present study,we demonstrated that the abundance of Bacteroidaceae (includingBacteroides) and Tannerellaceae (includingParabacteroides) was significantly increased in both strains of mice after epicutaneous sensitization,especially in C57BL/6 mice.These outcomes indicated that the C57BL/6 strain displayed severer disruptions in gut microbial homeostasis and may help to mimic dysbiosis in microbiota composition associated with the pathogenesis of food allergy in humans[46-47].
Some unique microbial features were also observed in C57BL/6 or BALB/c mice after epicutaneous sensitization.The abundance of Lachnospiraceae was significantly reduced in immunized BALB/c mice when compared to their control counterparts,in line with the previous report of Liu et al.[33]who found that Lachnospiraceae spp.,as a family that included some genera of short-chain fatty acids (SCFAs)-producing bacteria,have been reported to be inversely associated with the progression of intestinal inflammation and anaphylactic diseases[33,48].Furthermore,in a previous study,Lachnospiraceae was negatively correlated to the proportions of Th2 in the peripheral immune organ[49].In comparison,the abundance of Muribaculaceae was significantly depleted in sensitized C57BL/6 mice when compared to the controls.It has been reported that Muribaculaceae spp.as the dominating gut microbiota identified in healthy individuals that can degrade some types of polysaccharides to generate SCFAs,including succinate,acetate,and propionate[50].Reductions in the abundance of those SCFAs-producing bacteria would promote colonic epithelium and intestinal permeability and further affect the barrier integrity and function[40].It was also pointed out that antibiotic-induced dysbiosis in gut microbiota promoted the development of food allergy with a signature of decreases in the abundance of Muribaculaceae[22].Xu and co-workers[51]proved that Muribaculaceae was associated with OVA sensitization and negatively correlated with OVA-specific IgE and IgG,which was in agreement with our present study and could be another reason for the higher level of OVA-specific IgE in epicutaneously sensitized C57BL/6.Furthermore,Lactobacillaceae (includingLactobacillus) as a type of classic probiotic is noted to be reduced in intraperitoneally sensitized mouse models of food allergy[32],while surprisingly,was significantly increased in epicutaneously sensitized C57BL/6 mice in the present study.Similar to our findings,Ling and co-workers[52]found that the abundance ofLactobacillusandBifidobacteriumwas enriched in food-allergic infants,again suggesting the advantages of the C57BL/6 mouse model in mimicking features of disordered gut homeostasis in human allergic diseases.Altogether,these findings demonstrated that both strains of mice,especially the C57BL/6 mice,suffered dysbiosis in gut flora mimicking real pathological alterations after being epicutaneously sensitized with OVA.According to the results of immune responses and changes in the integrity of gut barrier and gut flora,the C57BL/6 mouse,rather than the BALB/c mouse,is more suitable for establishing an epicutaneously sensitized model of food allergy.
The present study has some limitations.Firstly,we used two strains of mice,i.e.,the BALB/c and C57BL/6 mice,to establish epicutaneously sensitized mouse models of food allergy.There is evidence that other strains of mice,such as the C3H/HeJ and AKR/J mice,can also be used to develop models for peanut allergy[3].Thus,more strains of mice should be considered in studying the influence of genetic backgrounds on AD-like skin lesions associated with food allergies.Secondly,some results,such as flow cytometry and cytokines,of the cells from the mesenteric lymph nodes (MLN)should be provided to enforce the stringency of the present study.Thirdly,in this study,we did not provide an in-depth exploration into the mechanisms by which different genetic backgrounds affect the immune response to food allergens and regulate the relationship between the skin barrier and gut homeostasis.Despite these,the current data provide researchers with clues to choosing an appropriate strain for establishing mouse models of food allergy via epicutaneous sensitization.The involved mechanisms that explain variations between the two genetic strains regarding sensitization pathways and microflora disruptions warrant investigations in the future.
5.Conclusion
In conclusion,this study,for the first time,compared differences in two genetic backgrounds of mouse (BALB/c and C57BL/6 mice)immunized by epicutaneous sensitization in the context of foodallergic immune responses as well as changes in gut barrier function and homeostasis.Both strains of mice can be used to establish foodallergic mouse models due to the strong IgE-mediated immune responses to epicutaneously sensitized OVA.Comparison analysis clarified that,after cutaneous exposure,the C57BL/6 mice displayed stronger Th2-biased responses and severer disruptions in the barrier function and gut flora than the BALB/c mice,which were fitter for studying the features of human food allergies associated with ADlike skin lesions.This study highlights the role of genetic variations of mice on the pathogenesis of food allergy and helps to choose an appropriate strain of mice to study human food-allergic disease in association with cutaneous exposure.
Conflict of interest
Yanbo Wang and Linglin Fu are editorial board members forFood Science and Human Wellnessand were not involved in the editorial review or the decision to publish this article.The authors have no conflict of interest to declare.
Acknowledgments
We gratefully acknowledge the financial support received from the Natural Science Foundation of China (32202202 and 31871735),the Zhejiang Provincial Natural Science Foundation of China(LGN22C200027),and the Open Fund of the Key Laboratory of Biosafety Detection for Zhejiang Market Regulation (2022BS004).
Appendix A.Supplementary
Supplementary data associated with this article can be found,in the online version,at http://doi.org/10.26599/FSHW.2022.9250056.
- 食品科学与人类健康(英文)的其它文章
- Protective effects of oleic acid and polyphenols in extra virgin olive oil on cardiovascular diseases
- Diet and physical activity inf luence the composition of gut microbiota,benef it on Alzheimer’s disease
- Inf luence of nitrogen status on fermentation performances ofnon-Saccharomyces yeasts: a review
- Ganoderma lucidum: a comprehensive review of phytochemistry,eff icacy,safety and clinical study
- Resveratrol combats chronic diseases through enhancing mitochondrial quality
- Demonstration of safety characteristics and effects on gut microbiota of Lactobacillus gasseri HMV18

