A food-grade and senescent cell-targeted f isetin delivery system based on whey protein isolate-galactooligosaccharides Maillard conjugate
Shuai Hou,Chutong Lai,Yukun Song,Haitao Wang,Jialu Ni,Mingqian Tan,
a Academy of Food Interdisciplinary Science, School of Food Science and Technology, Dalian Polytechnic University, Dalian 116034, China
b Institute of Cancer Stem Cell, Cancer Center, Dalian Medical University, Dalian 116044, China
c National Engineering Research Center of Seafood, Dalian Polytechnic University, Dalian 116034, China
d Collaborative Innovation Center of Seafood Deep Processing, Dalian Polytechnic University, Dalian 116034, China
Keywords: Fisetin Nanoparticle Cellular senescence Targeted delivery
ABSTRACT Cellular senescence is the results of aging and age-related diseases,and the development of anti-aging methods may improve health and extend longevity.The natural f lavonol f isetin has been shown to antagonize senescence in vitro and increases longevity in vivo,but has poor water solubility and limited bioavailability.In this study,a food-grade and senescent cell-targeted delivery system for f isetin was developed based on whey protein isolate-galactooligosaccharides (WPI-GOS) Maillard conjugate,which could recognize senescence associated β-galactosidase in senescent cells.The fisetin nanoparticles possessed a high encapsulation eff iciency,excellent dispersibility in water,good storage stability and well biocompatibility.Moreover,they could effectively accumulate and retain in senescent cells with excellent senescent cell-targeting eff icacy,and inhibit the oxidative stress-induced cellular senescence in vitro.Thus,this novel nanoparticle system based on WPI-GOS Maillard conjugate showed promise to deliver hydrophobic bioactive ingredients like f isetin to senescent cells to improve their bioavailability and anti-senescence effect.
1.Introduction
Cellular senescence,a state of growth arrest induced by endogenous or exogenous stress such as telomere shortening,oncogene activation,DNA damage,and oxidative stress,has been considered as a fundamental ageing mechanism that also contributes to multiple age-related diseases,including Parkinson disease,Alzheimer disease,atherosclerosis,osteoarthritis,and cancer[1-3].Senescent cells possess several key features including irreversible cell cycle arrest,increased activity of senescence associatedβ-galactosidase (SA-β-gal),high levels of p16INK4a,and a senescence-associated secretory phenotype (SASP)[1].Cellular senescence is def ined as an irreversible cell cycle arrest,typically at G1 phase due to accumulation of the cyclin dependent kinase inhibitors p21 and p16,and also sometimes at G2/M phase[4-5].SA-β-gal is the f irst and most widely used biomarker to detect senescent cell based on its increased lysosomal mass[1].It has been demonstrated that the increased activity of SA-β-gal during senescence was probably in part due to the increased expression of the lysosomalβ-galactosidase protein encoded byGLB1gene[6].Senolytic drugs have been explored to kill senescent cells to ameliorate healthy aging and age-related disease,otherwise known as senotherapy,which has been emerged as a therapeutic approach[7].The combination of the f irst generation senolytics (a new class of drugs that kill senescent cells selectively) dasatinib and quercetin has been proved an effective method to kill a broad range of senescent cell types and improve both health span and lifespan in aged mice[8-9].
In addition,bioactive compounds derived from natural plants have shown promising health benefits,particularly the prevention and control of pathophysiological events related to body disorder or disease[10-12].Some natural compounds like anthocyanins in vegetables and fruits showed improved anti-oxidative stress properties,thus extending the lifespan ofCaenorhabditis elegan[13].Moreover,fisetin(3,7,3’,4’-tetrahydroxyflavone) is a kind of bioactive flavonol that has been widely found in a variety of fruits,vegetables,teas,and anacardiaceae plants,such as strawberry (160 µg/g),onion (4.8 µg/g),and green tea (539 µg/g)[14].It is estimated that the average daily intake of fisetin is about 400 µg in individual[15].It has been demonstrated that fisetin exhibits multiple biological activities including antioxidant,anti-inflammatory,anticancer,anti-diabetic as well as neuroprotection[16].Recently,it was found that fisetin appeared to be a potential senolytics,and its effect is even better than other flavonoid polyphenols such as quercetin and curcumin[17-18].The potential mechanism is probably through inhibiting Bcl-2 family or PI3K/AKT pathway[19-20].Fisetin could reduce senescence in several cell typesin vitroand progeroid or aged wild-type micein vivo,and oral administration of fisetin in 60% Phosal 50 PG:30%PEG400:10% ethanol (not suitable for food application) to naturally aged mice extended heath span and lifespan[17-18].In spite of great application potential,the application of fisetin in food,health product and biomedical fields is unfortunately limited due to its poor water solubility and low oral bioavailability.The targeted delivery of fisetin to senescent cells is desirable which can enhance local concentration and curative effect[11].Therefore,there is an urgent need to develop delivery systems to improve both solubility and bioavailability of fisetin.At present,the delivery strategies of fisetin mainly include nanoparticles,emulsion,lipid vesicles,cocrystals,etc.[21].Among them,nanoparticles especially protein-based nanoparticles have obvious advantages in the delivery of hydrophobic bioactives due to their unique size and shape,good biocompatibility,high nutritional value,simple fabrication process,and excellent encapsulation performance[22-23].
It is a challenge to find a unique marker for fisetin to attach onto senescent cells and enhance fisetin’s bio-accessibility,and specific phenotypic aspects might be used for identification of senescent cells[24].So far,there are few delivery strategies for senescent cell targeting,which are mainly focused on nanoparticles[25].Agostini et al.[26]reported the targeted delivery system with good targeting verified by cell model based on SA-β-gal and MCM-41-based mesoporous silica nanoparticles.The MCM-41 was coated with a layer of galactooligosaccharides (GOS) to block the release of the cargo from the carrier.When the carrier is taken up by the cell through endocytosis followed by endosomal fusion with lysosomal vesicles,normal cells cannot effectively degrade the outer layer of GOS due to lack of SA-β-gal,thereby re-secreting the carrier to the outside of the cell through exocytosis.While senescent cells can use SA-β-gal to degrade GOS to achieve responsive cargo release.This method was refined by Muñoz-Espín et al.[27]using linear homogeneous galacto-hexasaccharides instead of GOS,which was verified in senescent cellsin vitroand mice modelin vivo.In addition,molecularly imprinted nanoparticles or porous calcium carbonate nanoparticles were also designed to target senescent cells by targeting senescent cell surface marker of β2 microglobulin or CD9[28-29].Although the above strategies can target senescent cells,they all have the disadvantages of inedible raw materials and complex chemical preparation process,which limit their application in food.
Herein,a food-grade and senescent cell-targeted delivery system for fisetin was developed.Whey protein isolate (WPI),an important dairy protein,was selected as wall material due to many attractive properties,including desirable nutritional quality,good emulsifying properties,excellent encapsulation performance,and resistance to gastric digestion[30-31].Prebiotics GOS are nondigestible oligosaccharides which are produced by thetrans-galactosylation activity ofβ-galactosidase usually with various glycosidic bonds containing β(1→4),β(1→6),β(1→2) and β(1→3)[32].Maillard reaction is a series of complex reactions between the free amino groups of proteins and the reducing carbonyl groups of reducing sugars.It is a food-grade reaction which can occur naturally without introducing additional chemicals[33].Due to the unique characteristics including enhanced solubility,stability,emulsifying capacity and antioxidant properties,protein-reducing sugar conjugates via Maillard reaction have drawn great attention in food application especially for delivering nutraceuticals[34].GOS was first conjugated with WPI by Maillard reaction,and then fisetin loaded nanoparticles(WPI-GOS-FIS) were prepared through an emulsification-evaporation technique (Fig.1).The major novelty of this work is using WPI-GOS system to target senescent cells through a one-step Maillard reaction.No extra organic solvents were needed during the preparation of delivery system.The wall materials of the delivery system are all food-grade with good biocompatibility.By using this system,we hypothesize that cellular uptake of WPI-GOS-FIS occurs via endocytosis,and,after fusion with lysosome,the nanoparticles are released by exocytosis in normal cells or achieve the release of fisetin under the action of SA-β-gal in the lysosome from senescent cells(Fig.1)[27,35].In this study,the physical and chemical properties,senescent cell-targeting efficacy,in vitrocytotoxicity,and effect on cellular senescence of the fisetin loaded nanoparticles were evaluated.
2.Materials and methods
2.1 Materials
WPI (HilmarTM9410) was obtained from Hilmar Ingredients (CA,USA).GOS were purchased from Carbosynth China Ltd.(Suzhou,China).Fisetin was bought from MedChemExpress (Shanghai,China).Potassium bromide was acquired from J&K Chemical Ltd.(Shanghai,China).Fluorescent dye sulfo-cyanine5 (CY5) was obtained from Shanghai Macklin Biochemical Co.,Ltd.(Shanghai,China).Cell counting kit-8 (CCK8) was purchased from Seven Biotech (Beijing,China).Propidium iodide (PI) was bought from Sigma Aldrich (Shanghai) Trading Co.,Ltd.(Shanghai,China).The human fetal lung fibroblast cell line MRC5 was obtained from Haixin Lei’s lab in Dalian Medical University,China.Fetal bovine serum(FBS) and EMEM medium were bought from Biological Industries(Kibbutz Beit-Haemek,Israel).All other chemicals were of analytical grade reagents in this research.
2.2 Synthesis and characterization of WPI-GOS
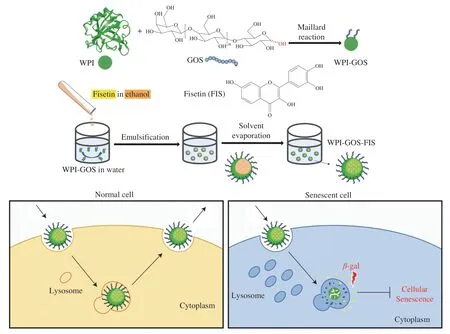
Fig.1 Schematic representation of the design and synthesis strategy of senescent cell-targeted nanoparticles loaded with fisetin,and illustration of the targeting and working mechanism[27].
WPI-GOS was prepared by dry-heating method of Maillard reaction.Briefly,WPI and GOS with a mass ratio of 1:1 were dissolved in 0.1 mol/L PBS (pH 7.0),mixed well with complete hydration,and then lyophilized.Subsequently,the powder mixtures were incubated in a desiccator at a relative humidity of 45% using saturated Mg(NO3)2solution at 60 °C for 0-48 h.The reaction was stopped by keeping the powder on ice for 10 min.Then,the reaction product was washed with 75% ethanol for 3 times to remove the free unreacted GOS,salt,pigment and other impurities.Finally,the precipitate was re-dissolved with water,lyophilized,and then stored at 4 °C.
The UV absorbance of different reaction time conjugates at a concentration of 2.5 mg/mL at 294 and 420 nm was measured with a UV-vis spectrophotometer (UV-5200,Shanghai Yuanxi Instrument Co.,Ltd.,China).In addition,the fluorescence of the samples(2.5 mg/mL) was determined at an excitation wavelength of 280 nm and emission wavelength of 300-450 nm using a fluorescence spectrophotometer (F-2700,Hitachi,Japan).
The content of free amino groups was determined following the ortho-phthaldialdehyde (OPA) method described by Mao et al.with minor modifications[36].The OPA reagent was prepared freshly by mixing 40 mg of OPA dissolved in 1 mL methanol,25 mL of 0.1 mol/L sodium borate buffer (pH 9.85),5 mL of 10% SDS water solution,100 μL ofβ-mercaptoethanol,and then fixing the volume to 50 mL with deionized water.Then,100 μL of sample solution(10 mg/mL) and 2 mL of OPA reagent were mixed and incubated at 35 °C for 2 min.The absorbance at 340 nm was immediately measured by UV-vis spectrophotometer (UV-5200,Shanghai Yuanxi Instrument Co.,Ltd.,China).Free amino group contents were calculated using a calibration curve (y=0.427 1x+0.001 9,R2=0.999 6) based onL-lysine (0.125-2 mmol/L) as a standard.
2.3 Preparation of fisetin nanoparticles
To prepare fisetin nanoparticles,0.5% WPI or WPI-GOS solutions were prepared in ultrapure water,and stirred for 3 h at room temperature firstly.Then,0.05% fisetin was prepared in absolute ethanol,added dropwise to the aqueous phase at a volume ratio of 1:9,and then stirred overnight at room temperature.Ethanol was subsequently removed by rotary evaporation at 40 °C to obtain fisetin-loaded nanoparticles.The samples were finally freeze-dried and stored at -20 °C until use.
2.4 Encapsulation efficiency (EE) and loading capacity (LC)analysis
Fisetin was first extracted from nanoparticles using methanol and dichloromethane following the methods by Shen et al.[30]with some modifications.Briefly,0.5 mL sample was mixed with 2 mL methanol/dichloromethane (1:1,V/V),vortexed and then centrifuged.The upper layer containing fisetin was collected and brought up to 2 mL.The concentration of fisetin was analyzed by UV-vis spectrophotometer at 364 nm,and calculated according to the calibration curve.EE and LC were calculated as follows:
2.5 Characterization of fisetin nanoparticles.
The average particle size and polydispersity index (PDI) of nanoparticles were determined based on the Cumulant fit by dynamic light scattering using 3D LS (LS Instruments,Switzerland) at a fixed scattering angle (90°).
The surface morphology of nanoparticles was observed by cryo-scanning electron microscopy (Cryo-SEM) (SU8010,Hitachi,Japan).Samples (approximately 10 µg/mL) were placed on a round aluminum table and frozen rapidly with liquid nitrogen sludge.After sublimation,samples were sprayed with gold and scanned at an accelerating voltage of 10 kV.
Fourier transform infrared spectroscopy (FT-IR) spectra in the range of 4 000-400 cm-1were obtained using FT-IR spectrometer(PerkinElmer,USA) based on KBr method.Circular dichroism (CD)spectra in the range of 190-280 nm were applied using J-1500 CD spectrometer (JASCO,Japan) and the secondary structures were analyzed based on Yang’s reference[37].
2.6 Senescent cell-targeting efficacy of WPI-GOS-FIS
MRC5 cells were cultured in EMEM medium supplemented with 20% FBS and 1% penicillin/streptomycin.Cells were seeded in 12-well plate,treated with 400 µmol/L H2O2for 2 h,washed twice with PBS,and maintained in fresh medium for 4 days to induce cell senescence.In order to visualize the intracellular distribution of nanoparticles,fluorescent dye hydrophobic CY5 was used to represent fisetin.After 2 h incubation of WPI-GOS-CY5(0.25 mg/mL),one group of cells were washed with PBS and fixed immediately (0 h group) followed by SA-β-gal staining to see the cellular uptake efficiency of nanoparticles.Another group of cells were washed with PBS and incubated in fresh medium for another 4 h (4 h group) and then fixed for staining to see the cellular efflux efficiency of nanoparticles.Images were recorded by fluorescence microscope (Revolve RVL-100,ECHO,USA).
2.7 Cell viability assay
The CCK8 assay was performed according to the manufacturer’s protocol to assess cell viability.Briefly,MRC5 cells were plated in 96-well plate,and treated with free fisetin or fisetin nanoparticles(with an equivalent fisetin concentration ranging from 25 µmol/L to 100 µmol/L and the corresponding additives).After 24 h incubation,cells were washed twice with PBS,and then replaced by fresh medium containing 10% (V/V) CCK8 for 2-4 h.The absorbance at 450 nm was determined using a micro-plate reader(Infinite M200 NanoQuant,Tecan,Switzerland).The cells cultured only in the medium were used as blank control.Cell viability was calculated as follows:
2.8 Cell treatment with H2O2 and fisetin nanoparticles
MRC5 cells were seeded in 12-well plate (for SA-β-gal staining)or 60 mm dishes (for cell cycle analysis),and pre-treated overnight with 25 µmol/L free fisetin or fisetin nanoparticles with the same loading.Then,cells were treated with 400 µmol/L H2O2for 2 h,washed twice with PBS,and maintained in fresh medium containing free fisetin or fisetin-loaded nanoparticles for 4 days (for SA-β-gal staining) or 3 days (for cell cycle analysis).
2.9 SA-β-gal staining
SA-β-gal detection assay was performed based on SA-β-gal Staining Kit (Beyotime,China) according to the manufacturer’s protocol.Briefly,MRC5 cells were cultured in 12-well plate,before staining,cells were washed once with PBS,fixed in fixative solution for 15 min at room temperature.Subsequently,fixed cells were washed with PBS for 3 times,and stained with freshly prepared staining solution at 37 °C overnight.Images were then captured using Ti-S microscope (Nikon,Japan) and the percentage of positive cells was calculated.
2.10 Cell cycle analysis
Cells were harvested and fixed with ice-cold 75% ethanol at -20 °C overnight.After wash with PBS for two times,cells were then incubated with PI solution containing RNase at 37 °C for 30 min.Subsequently,the cell cycle was detected by FACSVerse flow cytometer(BD,USA),and the data were analyzed with FlowJo7.6 software.
2.11 Statistical analysis
Data were presented as mean ± SD (standard deviation).Student’st-test was used for statistical analyses with statistical significance defined as*P< 0.05,**P< 0.01 and***P< 0.001.
3.Results and discussion
3.1 Formation and characterization of WPI-GOS
First,WPI-GOS conjugates were prepared through the foodgrade Maillard reaction between the free amino groups of WPI and the reducing carbonyl groups of GOS.Dry-heating method was used,as shown in Fig.2A,with the increase of reaction time,the color of dry powder gradually changed from white to orange,and the dissolved solution also changed from colorless and transparent to orange.The longer the time was,the darker the color became.The Maillard reaction process mainly includes three stages,namely the early stage,the intermediate stage and the advanced (or final) stage[34].In general,it is necessary to coordinate the relationship between crosslinking and degradation using Maillard reaction to crosslink molecules,and it is better to control the reaction in the early stage of crosslinking without degradation and harmful effects[33-34].It has been reported that UV-absorbance at 294 nm could be used to monitor the amount of intermediate products in Maillard reaction,while UV-absorbance at 420 nm reflected the amount of advanced products[38].As shown in Fig.2B,both of them showed a gradually increasing trend as a function of time,indicating that Maillard reaction continued,and intermediate products and final products gradually accumulated.In addition,the protein has endogenous fluorescence,which mainly comes from its tryptophan and tyrosine residues.The fluorescence spectra showed that WPI had an obvious fluorescence emission peak at 340 nm when excited at 280 nm.As the reaction proceeded,the emission peak underwent a significant red shift,and the fluorescence intensity gradually decreased,which might be due to the conformation change and the shielding effect after glycation[39-40](Fig.2C).The OPA detection of free amino groups showed that the degree of substitution of amino groups reached(63.8 ± 3.0)% after 24 h reaction,and there was no obvious change when the reaction continued (Fig.2D).These results indicated that WPI-GOS conjugates could be obtained by only one-step Maillard reaction,and WPI-GOS prepared by 24 h glycation was used as the wall material for fisetin encapsulation in the subsequent experiments.
3.2 Preparation and characterization of WPI-GOS-FIS nanoparticles
Next,an emulsification-evaporation technique was used to prepare fisetin loaded WPI-GOS nanoparticles (WPI-GOS-FIS).As shown in Fig.3A,the aqueous solution with clear and transparent appearance and light-yellow color was obtained.The encapsulation efficiency of fisetin was greater than 90% for both samples with no significant difference between each other (Fig.3B).The loading capacity of fisetin in WPI-FIS and WPI-GOS-FIS nanoparticles was (1.23 ± 0.10)%and (1.18 ± 0.06)% respectively.DLS analysis showed that the average particle size of WPI-FIS nanoparticles was 236 nm with PDI of 0.39,while the average particle size of WPI-GOS-FIS nanoparticles was 171 nm and PDI was 0.45 (Figs.3C and D).Cryo-SEM further revealed that both nanoparticles exhibited spherical structure with an average particle size of 120 and 87 nm,respectively (Fig.3E).The storage stability experiment showed that the nanoparticle solutions showed good stability and no obvious aggregation and color change were observed for over 28 days under 4 °C dark condition (Fig.3F).Together,these data suggested that WPI-GOS could form water-soluble and stable nanoparticles with fisetin.

Fig.2 Changes in total color (A),absorbance at 294 and 420 nm (B),intrinsic fluorescence spectra (C),and degree of graft (D) of the WPI-GOS conjugate obtained by Maillard reaction at different heating time.

Fig.3 Appearances (A),encapsulation efficiency (B),particle size (C),PDI (D),representative cryo-SEM images (E),and photographs during 28 days storage under 4 °C dark condition (F) of the WPI-FIS and WPI-GOS-FIS nanoparticles.n.s.: no significance.
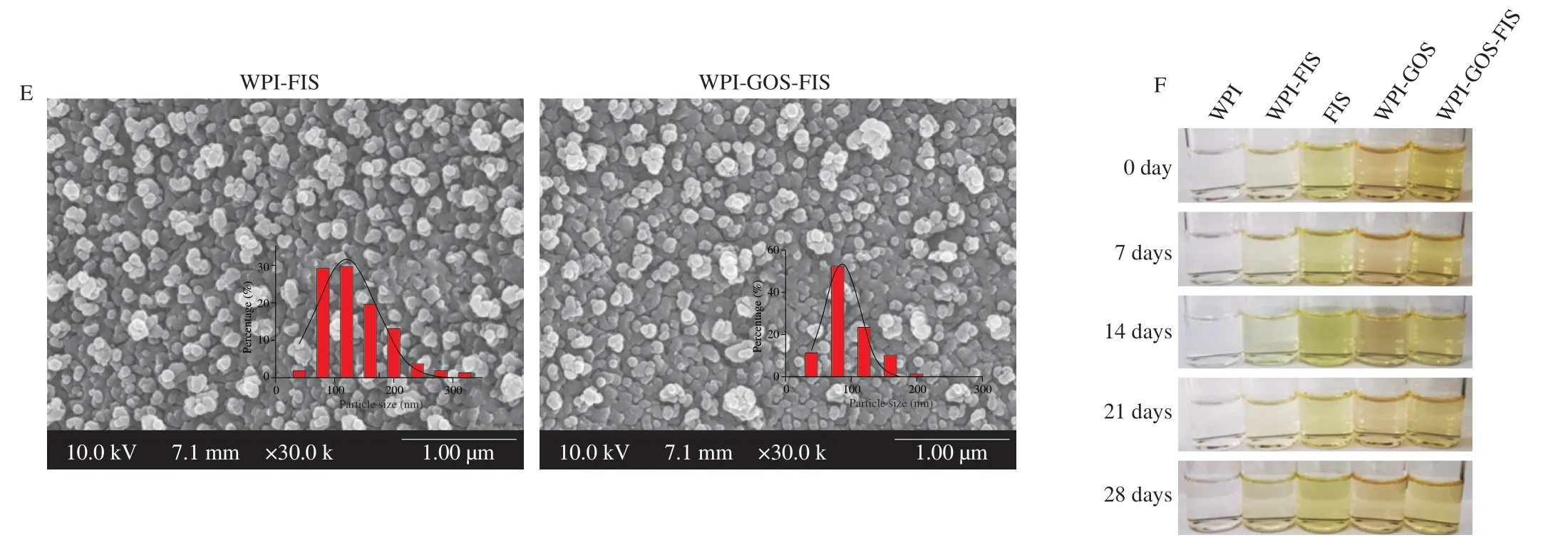
Fig.3 (Continued)
3.3 FT-IR and CD analysis
FT-IR spectroscopy in the range of 4 000-400 cm-1was adopted for the analysis of WPI-GOS conjugate and its interaction with fisetin(Fig.4A).The WPI spectrum showed a broad absorption band at 3 292 cm-1,attributed to O-H/N-H stretching vibration.The bands observed at 1 650 and 1 533 cm-1were assigned to amide I (C=O stretching vibration) and amide II (C-N stretch coupled with N-H bending mode),respectively.For GOS,the bands at 3 337,2 894,1 376,1 147,and 1 072/1 039 cm-1,were ascribed to O-H stretching vibration,C-H stretching vibration,deformation of -CH2,anti-symmetric stretching of C-O-C bridge,and C-O stretching vibration,respectively.After conjugation with GOS through Maillard reaction,the amide I band and C-O-C anti-symmetric stretching band of WPI-GOS moved to 1 662 and 1 161 cm-1,indicating the formation of Schiff base and glycosylamine (early stage products) produced during Maillard reaction.
For fisetin,a series of bands at 3 520 cm-1(aromatic C-H stretching vibration),3 252 cm-1(O-H stretching vibration),1 603 cm-1(amide I,C=O stretching vibration),1 570/1 508 cm-1(the skeletal vibrations of aromatic rings),1 168 cm-1(anti-symmetric stretching of C-O-C bridge),and 1 117/1 099/1 017 cm-1(C-O stretching vibration) were observed.However,these characteristic bands almost disappeared in the spectrum of WPI-GOS-FIS and no new bands were observed,indicating that fisetin might be successfully encapsulated in the nanoparticles through non-covalent interaction.Compared with WPI-GOS,the O-H/N-H stretching vibration band of WPI-GOS-FIS shifted from 3 292 cm-1to 3 298 cm-1,and showed an increased intensity,suggesting the hydrogen-bonding interaction between WPI-GOS and fisetin.Furthermore,as fisetin is hydrophobic and glycated WPI also has many hydrophobic regions,the hydrophobic interaction was thought to be another driving force responsible for the self-assembly of nanoparticles.In addition,the amide I band of WPI-GOS-FIS shifted from 1 662 cm-1to 1 649 cm-1,implying that the secondary structure of WPI-GOS might be changed due to the interaction of fisetin with WPI-GOS.
The conformational changes before and after conjugation with GOS and encapsulation of fisetin were further measured by CD.The CD spectra in the range of 190-280 nm were shown in Fig.4B and the contents of secondary structure derived from Yang’s equation[37]were shown in Fig.4C.WPI contained 19.6%α-helix,27.4%β-sheet,17.7%β-turn and 35.3% random coil.After glycation with GOS,theα-helix (14.7%,P< 0.001) andβ-turn (14.1%,P< 0.05) contents were decreased while theβ-sheet (34.0%,P< 0.05) content was increased,indicating that glycation led to conformational changes in WPI molecules.For WPI-FIS,negligible difference was detected compared with WPI.However,in comparison with WPI-GOS,the content ofα-helix,β-sheet,β-turn and random coil in WPI-GOS-FIS were 11.7%,41.5%,10.4% and 36.4%,respectively,with a decrease inα-helix (P< 0.01) andβ-turn (P< 0.05) and an increase inβ-sheet(P< 0.01),further suggesting that the encapsulation of fisetin could influence the secondary structure of WPI-GOS.
Together,these data further supported that WPI could conjugate with GOS through Maillard reaction and then successfully interact with fisetin to form encapsulation system.

Fig.4 FT-IR spectra (A),CD spectra (B),and relative contents of secondary structure (C) of the fisetin-loaded nanoparticles and their raw materials.
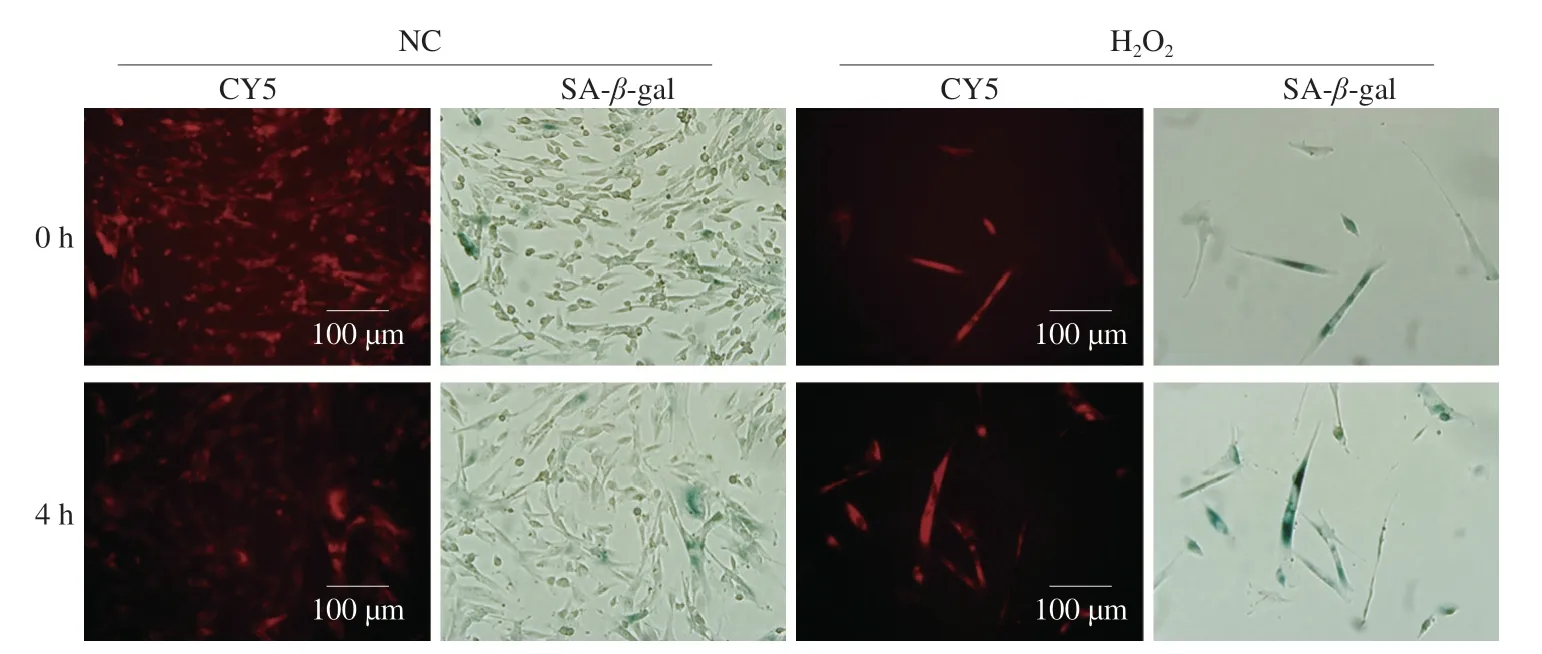
Fig.5 Representative fluorescence and SA-β-gal staining images of H2O2-untreated and H2O2-treated MRC5 cells at 0 or 4 h after WPI-GOS-CY5 incubation.
3.4 WPI-GOS-FIS targets senescent cells
A hallmark of senescent cells is the high activity of lysosomalβ-galactosidase,which can catalyze the hydrolysis ofβ-galactoside.Previous studies have shown that mesoporous silica nanoparticles coated with GOS could target senescent cells and realize the controlled release of the cargo[26-27].Next,the targeting efficacy of WPI-GOS-FIS was evaluated using H2O2-induced senescent MRC5 cells.As shown in Fig.5,H2O2treatment significantly induced cellular senescence with more enlarged and positive SA-β-gal staining cells.
At 0 h after WPI-GOS-CY5 incubation,nearly all the H2O2-untreated MRC5 cells showed weak red fluorescence intensity,and H2O2-treated MRC5 cells also showed weak red fluorescence intensity in SA-β-gal staining negative cells but much stronger fluorescence intensity in SA-β-gal staining positive cells (Fig.5),indicating that senescent cells can uptake nanoparticles more efficiently compared with normal cells.At 4 h after WPI-GOS-CY5 incubation,the fluorescence intensity decreased or disappeared in H2O2-untreated normal cells,but almost unchanged in H2O2-treated senescent cells (Fig.5),implying that senescent cells can inhibit the efflux of nanoparticles.Taken together,these data suggested that WPI-GOS system can effectively target senescent cells by promoting the uptake and inhibiting the efflux.
3.5 Cell viability assay
Cytotoxicity is a valuable indicator to evaluate the biosafety of fisetin-loaded nanoparticlesin vitro.Therefore,the effect of WPI-GOS-FIS on normal cell viability was next checked in MRC5 cells using CCK8 assay.The results of the cell viability assay after treatment of the cells with different concentrations of free fisetin or fisetin-loaded nanoparticles for 24 h were shown in Fig.6.Because WPI and GOS are both natural organic dietary substances with good biocompatibility,unsurprisingly,the cell viability of WPI and WPI-GOS treated cells showed no cytotoxicity or even promoting effect at concentration from 0.6 mg/mL to 2.4 mg/mL,indicating that WPI-GOS conjugate could be served as a safe nanocarrier for fisetin delivery.As for fisetin,both free ((115.2 ± 3.5)%) and encapsulated fisetin((132.8 ± 3.1)% for WPI-FIS and (122.9 ± 1.9)% for WPI-GOS-FIS)could promote cell proliferation at a low concentration of 25 µmol/L.With the increasing concentration of fisetin,the cell viability exhibited a slight drop and decreased to about (84.4 ± 3.4)% (50 µmol/L)and (71.0 ± 1.5)% (100 µmol/L) for free fisetin,(84.5 ± 2.6)%(50 µmol/L) and (74.8 ± 2.7)% (100 µmol/L) for WPI-FIS,(87.7 ± 1.1)% (50 µmol/L) and (86.1 ± 1.5)% (100 µmol/L) for WPI-GOS-FIS,respectively.It demonstrated that fisetin encapsulated by WPI-GOS might have less cytotoxicity than free fisetin (P< 0.001)and fisetin encapsulated by WPI (P< 0.01) under high concentration of 100 µmol/L,probably due to its better encapsulation and targeted release effect.Thus,the concentration of 25 µmol/L fisetin was used in the follow-up experiments.In summary,WPI-GOS-FIS did not induce cytotoxicity at a low concentration of 25 µmol/L fisetin and could reduce cytotoxicity at a high concentration compared with free fisetin,which was reflected in its good safety and biocompatibility.

Fig.6 Cell viability of MRC5 cells treated with free fisetin,WPI,WPI-GOS,and fisetin-loaded nanoparticles at different fisetin concentrations.**P < 0.01,***P < 0.001.
3.6 WPI-GOS-FIS inhibition of H2O2-induced cellular senescence
Recently,fisetin was reported to have potent senotherapeutic activity in several cell typesin vitroby suppressing cell senescence[17-18].As mentioned before,fisetin could also be effectively delivered to senescent cells by WPI-GOS-FIS.Consequently,the effect of WPI-GOS-FIS on cellular senescence was next examined based on H2O2-induced senescent MRC5 cell model.The high expression of SA-β-gal is one of the most important hallmarks of senescent cell,therefore,to investigate whether WPI-GOS-FIS could inhibit H2O2-induced senescence in MRC5 cells,the activity of SA-β-gal was first investigated (Fig.7A).SA-β-gal staining showed that H2O2treatment significantly induced cellular senescence((47.6 ± 2.1)% in H2O2treatment compared to (3.2 ± 1.1)% in control,P< 0.001).Treatment of H2O2-induced senescent cells with free fisetin resulted in striking decrease of SA-β-gal positive cells((29.5 ± 0.8)%,P< 0.001),with only WPI ((43.6 ± 2.7)%,P> 0.05)or WPI-GOS ((42.9 ± 3.3)%,P> 0.05) did not alleviate the percentage of SA-β-gal positive cells.However,the WPI-FIS and WPI-GOS-FIS treatment both significantly reduced the number of SA-β-gal positive cells,(26.4 ± 1.2)% in the case of WPI-FIS-treated cells,and(19.0 ± 0.6)% for WPI-GOS-FIS-treated cells (Fig.7A).Compared with free fisetin (P< 0.001) or WPI-FIS (P< 0.01) treatment,the effect of WPI-GOS-FIS was much stronger,indicating that the targeted carrier could increase the anti-senescence effect of fisetin.
Another hallmark of cellular senescence is cell cycle arrest,thus,the influence of WPI-GOS-FIS on cell cycle distribution was next determined.As shown in Fig.7B,the cell cycle distribution of H2O2-treated MRC5 cells was (46.9 ± 3.5)% in the G0/G1 phase and (34.9 ± 1.3)% in the G2/M phase,compared to (57.9 ± 0.5)%in the G0/G1 phase and (20.2 ± 1.5)% in the G2/M phase in the case of untreated cells,suggesting that H2O2treatment significantly resulted in cell cycle arrest at G2/M phase in MRC5 cells (P< 0.001),which was similar to some previous reports[41-42].When the H2O2-treated MRC5 cells were treated with free fisetin,WPI-FIS or WPI-GOS-FIS,the proportions of cells in the G2/M phase were reduced to (26.3 ± 0.7)% (P< 0.001),(27.4 ± 1.5)% (P< 0.01),or(25.0 ± 0.7)% (P< 0.001),respectively,indicating that the cell cycle arrest at G2/M phase induced by H2O2was dramatically attenuated by free or encapsulated fisetin.

Fig.7 Analysis of SA-β-gal activity (A) and cell cycle distribution (B) in normal and H2O2-induced senescent MRC5 cells treated with free fisetin (25 µmol/L) or fisetin nanoparticles with the same loading.B1-B8.-H2O2 NC,+H2O2 NC,+H2O2 WPI,+H2O2 WPI-GOS,+H2O2 FIS,+H2O2 WPI-FIS,+H2O2 WPI-GOS-FIS,cell cycle distribution.N is the number of chromosomes.**P < 0.01,***P < 0.001.
Together,these observations suggested that WPI-GOS-FIS could be absorbed by senescent cells and then inhibit the oxidative stress-induced cellular senescence.
4.Conclusion
To sum up,food-grade WPI-GOS-FIS nanoparticles for senescent cell-targeted fisetin delivery were successfully constructed based on the facile Maillard reaction and self-assembly approach.The fisetin nanoparticles exhibited spherical shape with mean particle size around 171 nm,high encapsulation efficiency (> 90%),high biocompatibility,and good dispersibility and stability in aqueous solutions during four weeks of storage.The fluorescence and SA-β-gal staining colocalization imaging proved an accumulated and retained effect of nanoparticles in senescent cells with excellent senescent cell-targeting efficacy owing to GOS targeting with SA-β-gal.SA-β-gal staining and cell cycle analysis indicated that WPI-GOS-FIS could effectively alleviate the oxidative stress-induced cellular senescence.All these results indicated that the strategy of targeting delivery of fisetin using GOS-modified protein-based nanoparticles was beneficial for senescence intervention.
Declaration of competing interest
Mingqian Tan is an editorial board member forFood Science and Human Wellnessand was not involved in the editorial review or the decision to publish this article.The authors declare no conflict of interest.
Acknowledgements
This work was supported by Dalian Youth Science and Technology Star Project (2020RQ121),the National Science Fund for Distinguished Young Scholars of China (31925031),Doctoral Scientific Research Foundation of Liaoning Province (2020-BS-211),and Liaoning Province Education Administration (J2020101).
- 食品科学与人类健康(英文)的其它文章
- Protective effects of oleic acid and polyphenols in extra virgin olive oil on cardiovascular diseases
- Diet and physical activity inf luence the composition of gut microbiota,benef it on Alzheimer’s disease
- Inf luence of nitrogen status on fermentation performances ofnon-Saccharomyces yeasts: a review
- Ganoderma lucidum: a comprehensive review of phytochemistry,eff icacy,safety and clinical study
- Resveratrol combats chronic diseases through enhancing mitochondrial quality
- Demonstration of safety characteristics and effects on gut microbiota of Lactobacillus gasseri HMV18

