Lactobacillus plantarum AR495 improves stress-induced irritable bowel syndrome in rats by targeting gut microbiota and Mast cell-PAR2-TRPV1 signaling pathway
Hongyun Zhng,Gungqing Wng,Zhiqing Xiong,Zhun Lio,Yngyn Qin,Xin Song,Li Sui,Linzhong Ai,Yongjun Xi,
a Shanghai Engineering Research Center of Food Microbiology, School of Health Science and Engineering, University of Shanghai for Science and Technology, Shanghai 200093, China
b Department of Gastroenterol, Digestive Endoscopy Center, Shanghai Hospital, Shanghai 200093, China
Keywords: Lactobacillus plantarum Irritable bowel syndrome Visceral sensitivity Dorsal root ganglia Tryptase Microbiota
ABSTRACT Probiotics have great potential in regulating intestinal pain.In this study,the effects of Lactobacillus plantarum AR495 on the visceral sensitivity and gut microbiota of irritable bowel syndrome (IBS) rats were studied.The results showed that tryptase released after mast cell activation and degranulation plays a key role in visceral pain,and L.plantarum AR495 reduced the stimulation of colonic mast cells and the expression of protease-activated receptor 2 (PAR2) and TRPV1 in dorsal root ganglia.Research further showed that supplementation with L.plantarum AR495 increased the level of short-chain fatty acids (SCFAs)and enhanced the barrier function of the colo n.In addition,the microbiota analysis of the colon indicated that L.plantarum AR495 promoted the proliferation of Bifidobacterium and inhibited the proliferation of Lachnospiraceae,which alleviated the imbalance of the intestinal microbiota caused by IBS to a certain extent.In total,L.plantarum AR495 might reduce visceral sensitivity through the Mast cell-PAR2-TRPV1 signaling pathway by maintaining the homeostasis of the intestinal barrier.
1.Introduction
Probiotics are non-pathogenic microorganisms,which have been reported to be beneficial in regulating various enteric diseases ranging from diarrhea,inflammatory bowel disease,and lactose intolerance by maintaining or improving the intestinal microbiota[1].Probiotics have strong viability and superior probiotic characteristics,especiallyLactobacillusandBifidobacterium[2].As an important part of the gut microbiota,they play an important role in maintaining the intestinal barrier function,regulating gut immune homeostasis,defense against pathogens,and producing some beneficial bacterial-derived substances[3-4].According to research reports,Lactobacillushad the effects of reducing the permeability of colon cells and inhibiting the contraction of the cytoskeleton of colonic epithelial cells[5].Lactobacillus plantarumDM5 could produceγ-aminobutyric acid (GABA),a major inhibitory neurotransmitter in the mammalian brain[6].In addition,studies have reported that someLactobacilluseven can relieve intestinal pain.
Irritable bowel syndrome (IBS) is a type of functional gastrointestinal disease caused by multiple pathogeneses,which widely affects young and middle-aged people[7].Up to 94% of IBS patients show evidence of visceral hypersensitivity,this may also lead to behavioral symptoms such as anxiety,fear,and depression,which significantly reduces the patient’s quality of life[8].Further studies on gut-brain interaction would be useful to reveal the pathogenesis of IBS.Several studies have shown that the composition of the fecal or mucosal microbiota of patients with IBS has changed compared with healthy patients[9-10].Therefore,regulating the homeostasis of the intestinal environment by supplementingLactobacilluscould lead to promising outcomes[11].
However,howLactobacillusreduces visceral hypersensitivity and whether it is related to the barrier function of the colon has not been well studied.To explain these disparate observations,we evaluated the ability ofL.plantarumAR495 to reduce visceral hypersensitivity in IBS rats.Then the influence of theLactobacilluson the intestinal barrier function and the changes in intestinal flora were studied.
2.Materials and methods
2.1 Bacterial cultures and growth conditions
L.plantarumAR495,which is kept at the China General Microbiological Culture Collection Center (the preservation number,CGMCC No.14004) was obtained from the Shanghai Engineering Research Center of Food Microbiology,the University of Shanghai for Science and Technology (Shanghai,China).The bacteria were streaked on separate de Man,Rogosa,and Sharpe (MRS) agar plates in an anaerobic workstation at 37 °C.After culture,a single colon was separately activated for two generations in MRS liquid medium under incubation at 37 °C for 16 h and harvested by centrifugation(2 000 r/min,10 min at 4 °C).The bacterial suspension was prepared daily to administer orally 109CFU/mL in PBS after being washed twice with sterile phosphate-buffered saline (PBS,pH 7.4).
2.2 Animals and experimental protocols
Female Wistar rats weighing 180-210 g (5-6 weeks old) were purchased from the Shanghai BK Laboratory Animal Co.,Ltd.(Shanghai,China).Rats were maintained on a 12-h light/dark cycle according to the standards of the holding facility with food and water provided.After purchase,rats were left untreated under experimental conditions,and 4 rats per cage were acclimated to the environment for 1 week.All protocols of animals were approved and performed following the guidelines of the Institutional Animal Care and Use Committee of Shanghai Jiao Tong University by the animal protocol(201801130).
The experimental protocol was shown in Fig.1A.The rats were weighed and randomly divided into 3 groups (n=8) as follows:(a) Control group,(b) IBS model group (IBS),and (c) treatment group(IBS+AR495).The IBS model was induced as a literature report[12-13].First,the rats in the IBS model group and AR495 treated group were lightly anesthetized and infused with 1 mL of 40 mL/L acetic acids at 8 cm proximal to the anus for the 30 s.Second,after flushing the colon with 1 mL of 0.01 mol/L PBS,it was returned to the cage to move and eat freely.From the second day onwards,wrap the upper extremities and chest shaft with tape every day.All stress sessions were performed at the same time of day (between 10 a.m.and 12 a.m.) to minimize the influence of circadian and motility rhythms.Rats were then placed in their home cage[14].Stress was given in the same vicinity of the animal facility.One week later,the stress and probiotic treatment were applied in parallel.The rat in the IBS group orally received 1 mL PBS daily and the rat in AR495 treated group was givenL.plantarumAR495 once a day by oral gavage.Two weeks later,the rats were fasted overnight before being sacrificed after the colon expansion test.After the rat was euthanized,segments of the colon were removed for H&E and toluidine blue staining,and dorsal root ganglia (DRG) (L6-S1) were isolated and stored at -80 °C for further analyses.
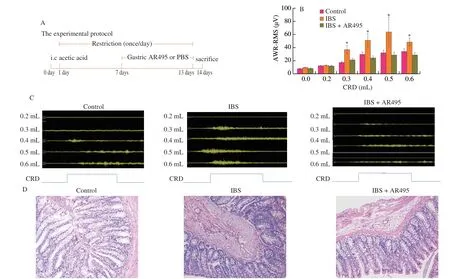
Fig.1 L.plantarum AR495 alleviated the visceral hypersensitivity of IBS in rats.(A) Protocol diagram of the animal experiment.(B) The visceromotor responses of the abdominal musculature induced by colorectal distension (n=8).(C) Changes in visceromotor responses in response to rectal distensions (n=8).(D) Representative histological examination micrographs of colonic slides (n=8),200× magnification.Results were expressed as the mean ± SEM for each experimental group.The significance of differences between the data was assessed using One-way ANOVA by Dunnett’s tests,with the level of significance set at *P < 0.05.
2.3 Colorectal distension and electromyography recording
The visceromotor responses (VMR) were recorded by quantifying reflex contractions of the abdominal musculature induced by colorectal distension (CRD)[15].An 8F disposable silicon balloonurethral catheter for pediatrics was used.After anesthetizing by intraperitoneal injection of 10% chloral hydrate (0.3 mL/100 g/rat),the balloon was carefully inserted into the rectum until 7 cm distal from the end of the balloon on the catheter to the anus and then the catheter was taped to the base of the tail to prevent displacement.After this procedure,the rats were placed in a transparent cubicle and allowed to recover and acclimate for 1.5-2 h before testing.The catheter was connected to a 1 mL syringe filled with water through a three-way connector.
Portable electromyography evoked potential meter (NDI-093,China) was used to continuously monitor a pressure-response curve under a series of rectal distensions.The rats were equipped with two sets of hypodermic needle electrodes implanted into the external oblique muscle 2 cm higher than the inguinal ligament.First,scanned the baseline signal without expansion,and recorded the myoelectric activity under different degrees of colon balloon inflation after the baseline was stable.The CRD protocol consisted of 5 phasic distensions between 0 mL and 0.6 mL,and each expansion lasted for 30 s,with a 5 min interval between consecutive distensions.Rats were accustomed to placing them in a transparent cubicle for a few days before CRD to minimize recording artifacts.
2.4 H&E and toluidine blue staining
After sacrifice,the colon tissue of 5 mm was cut off and fixed in neutral buffered formalin or Carnoy’s fixative for 18 h,dehydrated,and embedded in paraffin wax for haematoxylin and eosin (H&E)staining or toluidine blue[16].The stained slices of colon tissue were scanned with a Pannoramic MIDI digital section scanner and photographed.For histological examination experiments,n=6 biologically independent samples for each group.H&E tissue sections were analyzed by experienced gastrointestinal pathologists to rule out inflammation of the colonic mucosa.For the toluidine blue section,5 random fields of view (200× magnification) were observed using a microscope,and the average mast cell count was determined by 3 independent and unsuspecting researchers.The results were expressed as the average number.
2.5 Assessment of tryptase content
The colonic mast cells tryptase in each group was determined using a rat trypsin ELISA assay kit (Shanghai Tongwei Biotechnology Co.,Ltd.) according to the manufacturer’s instructions.The tryptase level represents the degree of activation and degranulation of mast cells.The colon samples were prepared as follows: after removing fat and feces from the sample,60 mg of colonic tissue was accurately weighed and then homogenized in 0.6 mL PBS.A tissue homogenate was then prepared in a tissue dispersing machine and centrifugation,the supernatant was taken to detect tryptase content by enzyme-linked immunosorbent assay.
2.6 Transmission electron microscopic (TEM) of colonic mast cells
The colon tissue was fixed in glutaraldehyde and sliced.After staining with uranyl acetate and lead acetate,the activation and degranulation of colonic mast cells were observed with TEM (Tecnai G2 Spirit,FEI).
2.7 RNA extraction and real-time quantitative polymerase chain reaction (RT-qPCR) analysis
The gene expression of colonic barrier proteinsoccludin,ZO-1,claudin1,andclaudin4were analyzed by real-time PCR.Total RNA was isolated from the colon according to the manufacturer’s instructions using Trizol reagent (Invitrogen,US),and the concentration and purity of the extracted RNA were measured using a NanoDrop ND2000C spectrophotometer (Thermo Scientific,DE) at anA260nm/A280nmratio.RNA integrity was determined by agarose gel electrophoresis using a Gel Doc XR+system (Bio-Rad,Hercules,CA).cDNA was then prepared by reverse transcription using a Prime-Script RT reagent kit (TaKaRa Bio) and amplified by RT-qPCR using SYBR Premix ExTaqII (TaKaRa Bio).RT-PCR was performed on a LightCycler® Nano thermal cycler (Roche Applied Science,Penzberg,Germany),using the SYBR Green FastStart kit (Roche,Basel,Switzerland) and the appropriate primers (Table 1).PCR cycles were as follows: 1 cycle of 95 °C for 30 s,40 cycles of 95 °C for 5 s,60 °C for 30 s,and 72 °C for 30 s,followed by 1 cycle of 95 °C for 10 s,65 °C for 1 min and 97 °C for 1 s.PCR primer sequences for the genes were presented in Table 1,which were synthesized by Sangon Biological Engineering,Shanghai,China.Relative expression was calculated using the 2-ΔΔCtmethod.
2.8 Western blot in colon and DRG
Colon and DRGs (L6-S1) were weighed and homogenized with a homogenizer in RIPA lysis buffer (Beyotime Biotechnology,Shanghai,China).Upon centrifugation at 12 000 ×gfor 30 min at 4 °C,the supernatant was collected,and the total protein content was measured using a BCA Protein Assay Reagent Kit (Biyotime,china).Equivalent protein concentrations were loaded onto 12.5%SDS-polyacrylamide gels.Electrophoretically separated samples were transferred to polyvinylidene fluoride (PVDF) membranes.Membranes were blocked with Western sealing fluid (Beyotime,P0252) and incubated with rabbit anti-occludin (abcam,ab612327),rabbit anti-claudin1 (Abcam,ab615098),rabbit anti-ZO-1 (Thermo,61-7300),rabbit anti-claudin4 (Affinity),rabbit anti-PAR2 (Abcam,ab180953),rabbit anti-TRPV1 (Abcam,ab6166) or mouse anti-β-actin primary antibody at 4 °C overnight.Then the proteins were visualized by the ECL chemiluminescence substrate kit (Beyotime,P0018) using a horseradish peroxidase-conjugated anti-rabbit secondary antibody.Images were acquired using a ChemiDoc MP imaging system(Bio-Rad,Hercules,CA) and densitometry was performed for each detected protein.
2.9 Immunohistochemistry in DRG
The fresh DRGs (L6-S1) tissue was fixed in 4% paraformaldehyde and embedded in paraffin.After deparaffinization and antigen retrieval,the sections were blocked in 10% BSA (Beyotime,ST025)phosphate buffer for 1 h,and combined with rabbit anti-PAR2(1:20 000,abcam ab180953) or rabbit anti-TRPV1 (1:2 000,abcam ab6166) at 4 °C overnight.On the second day,the sections were incubated with goat anti-rabbit IgG secondary antibody (Beijing Zhongshan Jinqiao Biotechnology Co.,Ltd.,PV-9000) for 2 h at 37 °C.After washing 3 times in PBS,it was developed with a DAB Substrate kit (Beijing Soleibao Technology Co.,Ltd.,DA1010).The positive expressing areas and density of PAR2 and TRPV1 were counted by Image-Pro plus 6.0 software.
2.10 Fecal short-chain fatty acids (SCFAs) extraction and gas chromatography-mass spectrometry (GC-MS) analysis
Fresh fecal pellets were collected from each mouse individually after sacrifice.The extraction procedures of SCFAs were referred to in relevant literature[17].Briefly,60 mg fecal samples were mixed with 700 μL deionized water,then homogenized and incubated at 4 °C with shaking for 30 min,followed by centrifugation for 30 min at 12 000 ×g.The acidified fecal supernatant by 10 uL of 5 M HCl was extracted three times in a turn by adding 100 uL of anhydrous diethyl ether (1:1,V/V).The diethyl ether layer (containing SCFAs) was pooled and further derived by adding 5 µL of BSTFA (N,O-bis(trimethylsilyl)trifluoroacetamide,Thermo).After being incubated at 70 °C for 20 min,and 37 °C for 2 h,the derivatized samples were loaded to GC-MS.
G C-M Sanalys is was performed by a 7890 Bg as chromatograph/5977 mass selective detector (Agilent Technologies,Santa Clara,CA,USA) with an HB-5 ms capillary column(30 m × 0.25 mm × 0.25 µm film thickness) (Agilent Technologies).The injector,ion source,quadrupole,and the GC-MS interface temperature were 260,230,150,and 280 °C,respectively.The flow rate of helium carrier gas was kept at 1 mL/min.1 µL of the derivatized sample was injected with a 3 min solvent delay time and split ratio of 10:1.The initial column temperature was 40 °C and held for 2 min,ramped to 200 °C at the rate of 20 °C/min and held for 2 min,and then finally increased to 300 °C at the rate of 30 °C/min and kept at this temperature for 10 min.The ionization was carried out in the electron impact (EI) mode at 70 eV.The MS data were acquired in full scan mode fromm/z40-400 with an acquisition frequency of 12.8 scans/s.The identification of compounds was confirmed by injection of pure standards and a comparison of the retention time and corresponding MS spectra.The analytes were quantified in the selected ion monitoring (SIM) mode using the target ion and confirmed by confirmative ions.The target ion of acetic,propionic,and butyric arem/z117,131,and 145,respectively.Addingm/z75 to all of them andm/z117 to acids with butyric acts as a confirmation ion.Data were analyzed using Mass Hunter programs.The contents of SCFAs were calculated with external standard (Volatile Free Acid Mix,Sigma) methods.
2.11 Fecal microbiota analysis
All fecal samples before the rats were sacrificed were collected separately and stored at -80 °C for microbiota analysis.Fecal DNA was extracted using the E.Z.N.A.® soil DNA Kit (Omega Bio-Tek,Norcross,GA,U.S.A.) according to the manufacturer’s instructions.The DNA extract was checked on 1% agarose gel,and DNA concentration and purity were determined with NanoDrop 2000 UV-vis spectrophotometer (Thermo Scientific,Wilmington,USA).The hypervariable region V3-V4 regions of the bacterial 16S rRNA genes were amplified using universal primers 338F 5’-ACTCCTACGGGAGGCAGCA-3’ and 806R 5’-GGACTACHVGGGTWTCTAAT-3’[18].PCR amplicons products were extracted from agarose gels and purified using a DNA gel extraction kit (Axygen Biosciences,China).The quality of PCR products was quantified using the QuantiFluor™-ST system(Promega,USA) according to the standard protocols.Then,purified PCR products were sequenced on an Illumina MiSeq platform(Illumina,USA) at Majorbio Bio-Pharm Technology Co.,Ltd.,Shanghai,China.
All raw reads were demultiplexed and quality-filtered using QIIME (version 1.9.1) with the following criteria: 1) The 300 bp reads were truncated at any site receiving an average quality score < 20 over a 10 bp sliding window abandoned the truncated reads that were < 50 bp;2) Sequences that reads containing ambiguous characters,or two nucleotide mismatch in primer matching were removed.Operational taxonomic units (OTUs) were set as at least 97% identified sequences.The invalid sequences were cut-off using UPARSE (version 7.0.1090,http://drive5.com/uparse/).RDP Classifier (version 2.11,http://rdp.cme.msu.edu/) was used to analyze the taxonomy of each 16S rRNA gene sequence,and against the SILVA (version 132) 16S rRNA database using a confidence threshold of 70%[19-20].
2.12 Statistical analysis
Data were expressed as the mean ± SD normally.Significant differences among groups (AWR score) at each distension pressure were statistically analyzed using ANOVA and expressed as the mean ± SEM.Immunohistochemistry and Western blot data were analyzed by one-way ANOVA.A Dunnett’s test was used after ANOVA analysis where appropriate.Differences withP< 0.05 were considered significant.Multiple comparisons between groups were corrected by SPSS 25 statistics software (SPSS Inc.,Chicago,IL,USA).The Wilcoxon rank-sum test was used to examine differences in bacterial composition between the two groups.P< 0.05 was considered to be statistically significant.
3.Results
3.1 L.plantarum AR495 alleviates visceral hypersensitivity with IBS
To assess the visceral sensitivity of IBS patients under stress conditions,we conducted acetic acid enema and stress restraint modeling in rats,and explored theL.plantarumAR495 to alleviate the response level of electromyogram under colorectal distention (Fig.1).The schematic diagram of the animal experiment was shown in Fig.1A.Rectal balloon inflation at lower volumes produces painful sensations in IBS patients,The abdominal withdrawal reflex (AWR)score was used to assess the visceral sensitivity[21-22].In this experiment,the AWR score to colorectal distention at distension volume of 0.3,0.4,0.5,or 0.6 mL in the IBS model group (36.07 ± 7.25,50.34 ± 10.96,63.38 ± 22.87,47.83 ± 6.53) was significantly higher than that in the Control group (16.89 ± 2.04,28.93 ± 4.42,31.19 ± 4.83,33.26 ± 5.00).The pain threshold of the model group was reduced,and the visceral sensitivity was significantly higher than that of the Control group,showing visceral hypersensitivity.The administration ofL.plantarumAR495 (20.98 ± 2.43,23.76 ± 3.59,28.09 ± 4.73,27.94 ± 4.06),decreased the visceral sensitivity of IBS at the above expansion volume (Fig.1B,P< 0.05).Probiotic intervention could alleviate the sensitivity and increase the pain threshold of colon dilation.As shown in Fig.1C,L.plantarumAR495 significantly attenuated visceral motor responses induced by rectal distension.The integrity of colon tissue and the degree of mucosal inflammation was evaluated by H&E staining.In each group,the colonic membrane structure was intact,without epithelial sloughing,rich in goblet cells,and tightly arranged villi without inflammatory cells appearing in the mucosa (Fig.1D).
3.2 L.plantarum AR495 inhibits activation and degranulation of mast cells
It has been reported that patients with IBS were often accompanied by an increase in the number of mast cells in the gut and that the activation of mast cells in the distal colon was associated with the symptoms and severity of IBS[23-24].To confirm the role of mast cells in visceral hypersensitivity,toluidine blue staining was used to identify the mast cell population (Figs.2A-C).The colon tissue was stained red,and the mast cells were round or spindle-shaped blue particles.Through statistical techniques,we observed the mast cells in colonic segments 8 cm from the anus,the number of mast cells in the IBS group (14.17 ± 5.91) was significantly higher than that in the Control group (3.50 ± 1.52) (Fig.2G,P <0.05).The mast cell number in AR495 treated group (7.89 ± 1.50) was lower than that in the IBS group (P <0.05).
To further study the changes of mast cells in visceral hypersensitivity,we adopted TEM to observe the degranulation of mast cells.As shown in Figs.2D-F,most mast cells were mainly distributed in the lamina propria of the intestinal mucosa and around the muscular plexus of the intestine,which contained many particles with higher electron density.The mast cells in the Control group were in a quiescent state,with high electron density,intact cell membrane,and no obvious degranulation.The mast cell was in a degranulation state with the intracellular dense particles reduced in the model group.Among them,degranulation led to rupturing of the cell membrane,granules gushing out,and cells appearing as irregular shapes.In AR495 treated group,there was fewer mast cell in the colon muscular layer,and the activation and degranulation of mast cell were significantly alleviated.We proved that not only the excessive proliferation of mast cells but also the abnormal activation and degranulation of mast cells existed in the colon of IBS rats.L.plantarumAR495 may reduce visceral sensitivity by inhibiting mast cells due to the count and the degranulation of cells in the colon muscular layer of rats.
There were a large number of inflammatory mediators,such as tryptase,serotonin (5-HT),and histamine (HIS) in mast cells.The release of cytokines could further activate mast cells and promote their continuous degranulation[25-26].In addition,they were also involved in the functional activities of the nervous system and other functions,which in turn produce cross-effects between the neuro-endocrineimmune network system[27].Thus,the tryptase activity released in mast cells of the intestinal tract was detected.The tryptase content was significantly increased in the IBS group ((58.96 ± 11.46) ng/g)than in the Control group ((28.44 ± 8.47) ng/g),(Fig.2F,P <0.05).Treatment withL.plantarumAR495 alleviated the release of tryptase to (16.20 ± 4.61) ng/g.
3.3 L.plantarum AR495 relieves nerve hypersensitivity
Previous studies have reported that tryptase can activate sensory neurons in the DRG in a PAR2-dependent manner.In addition,PAR2 was expressed in capsaicin-sensitive primary sensory neurons and sensitizes TRPV1 in the gastrointestinal tract[28].Therefore,we further investigated whetherL.plantarumAR495 alleviated visceral hypersensitivity through the PAR2-TRPV1 pathway.
Immunohistochemical studies showed that the percentage and staining intensity of PAR2 and TRPV1 immuno-positive neurons in all DRG neurons in the IBS group increased significantly (Figs.3A-D).Western blot indicated that the PAR2 protein level in the IBS group(1.54 ± 0.36) in DRGs was higher than that in the Control group(1.02 ± 0.18) and in AR495 treated group (0.96 ± 0.28) (Fig.3E,P< 0.05).The TRPV1 protein level in DRGs in the IBS group(1.82 ± 0.42) was higher than that in the Control group (0.91 ± 0.21)and in AR495 treated group (1.33 ± 0.38) (P< 0.05).Therefore,L.plantarumAR495 could reduce the visceral hypersensitivity caused by overexpression of PAR2 and TRPV1 in DRG ganglia by inhibiting tryptase production.
3.4 L.plantarum AR495 alters the gut microbiota composition
Notably,many diseases could result in microbiota dysbiosis,including IBS,inflammatory bowel diseases,obesity,and diabetes[29-30].It is well known that dysbiosis in the gut microbiota can exacerbate the development of many intestinal diseases.The results of PCA showed that the gut microbiota structure was different between groups (Fig.4B),suggesting that the IBS model interferes with normal gut microbiota.After AR495 intervention,novel microbial communities formed in the rat gut.
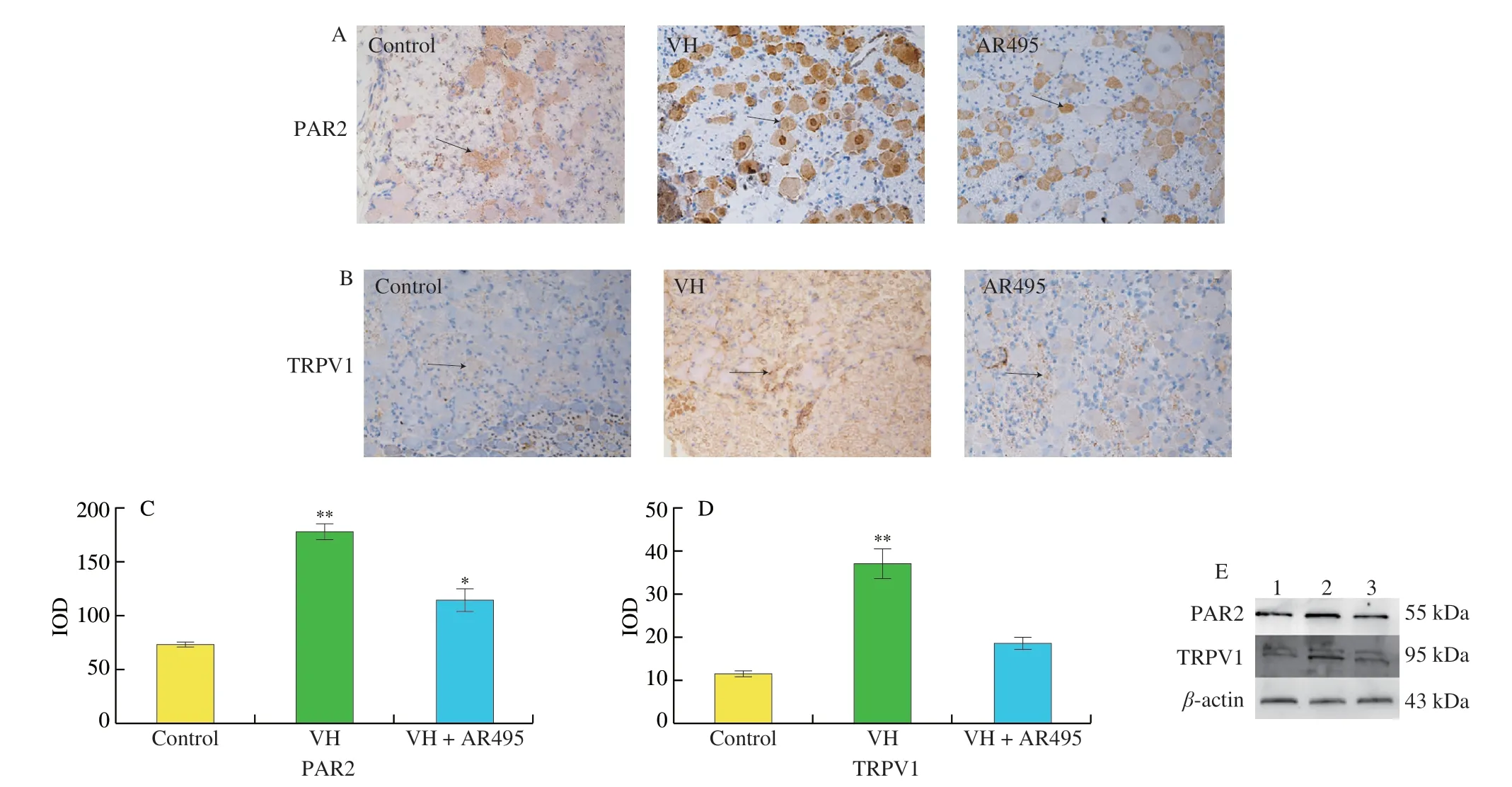
Fig.3 Effects of L.plantarum AR495 on PAR2 and TRPV1 proteins in L6-S1 DRGs.Immunohistochemistry staining of (A) PAR2 and (B) TRPV1 expression in L6-S1 DRGs (Image magnification ×400).(C-D) Immunohistochemical integral optical density (IOD) analysis.(C) PAR2,(D) TRPV1.(E) Protein expression of PAR2 and TRPV1.Results were expressed as the mean ± SD for each experimental group (n=8).The significance of differences between the data was assessed using One-way ANOVA by Dunnett’s tests,with the level of significance set at *P < 0.05,**P < 0.01.
The histograms in Fig.4A illustrate the relative abundances of major gut microbes at the genus level.It can be seen that the abundance ofLactobacillusis the highest in the model group and AR495 group.Notably,although the model group was not supplemented withLactobacillus,the abundance in the model group increased.A multi-omics study of IBS reports that severe IBS-D has a higher relative abundance of more than 20Lactobacillusgenera compared with mild to moderate IBS-D[31].This study was consistent with the phenomenon presented here,explaining the association of IBS withLactobacillus.Further comparison of the relative abundance of gut microbes between the Control and IBS groups at the genus level revealed that the abundance ofBacteroidesandOscillospiraceae(P< 0.05) was significantly decreased in the IBS group compared with the Control group (Fig.4C).Bacteroidesfragiliscould improve the micro-ecological environment in the intestinal tract and regulate the body’s immune system[32].One for the relationship of the microbiome with extraintestinal pain and psychological distress symptoms in persons with IBS found that Oscillospiraceae abundance was negatively correlated with intestinal pain[30].This phenomenon further indicates that there was a close connection between IBS and the gut microbiota,and the imbalance of the gut microbiota could lead to the imbalance of the body’s homeostasis and induce the phenomenon of hyperalgesia.As shown in Fig.4D,L.plantarumAR495 could mitigate these stressed-induced changes in bacterial abundance to a certain extent.For example,at the genus level,L.plantarumAR495 increased the relative abundance ofBifidobacterium(P< 0.05) by at least 2.38 times,and the abundance of Lachnospiraceae reduced by 3.94 times compared with the corresponding abundances in the IBS group.This study found that AR495 could improve the flora imbalance caused by IBS.Although there was a difference in the proportion of flora in the original steady state,AR495 could restore the flora imbalance in the model group to a certain extent and improve the allodynia in IBS rats.Although no strains directly related to IBS have been found,the results were instructive for the study of the correlation between gut microbiota and IBS.
3.5 L.plantarum AR495 maintains the integrity of the intestinal barrier
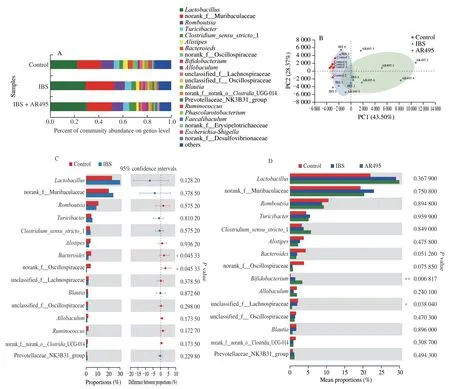
Fig.4 Effects of L.plantarum AR495 on gut microbiota in rats.(A) Community bar-plot analysis of the relative abundance of gut microbiota at the genus level.(B) Principal coordinate analysis plots in the fecal microbiota of experiment groups.(C) Phylotypes significantly different between the Control group and the IBS group.The Wilcoxon rank-sum test was used to examine differences in bacterial composition between the two groups.(D) Phylotypes significantly different between each group.The Kruskal-Wallis H test was used to examine differences in bacterial composition between the three groups.Results were expressed as the mean ± SEM for each experimental group (n=6),*P < 0.05,**P < 0.01.
As the key physical barrier of the intestine,tight junction (LJ) was mainly composed of the claudin family,occludin,and intracellular tight junction protein (ZO) family inter-connected[33].The complex connected adjacent intestinal epithelial cells to form a stable epithelial cell layer,thereby maintaining the normal intestinal epithelial barrier function and permeability.Probiotic has an excellent ability to repair the colonic barrier,and we speculated that probiotics could reduce mast cell activation by reducing and repairing intestinal tissue damage.Therefore,we evaluated the expression of intestinal epithelial cells by Q-PCR and western blot to further verify the alleviation mechanism ofL.plantarumAR495 to visceral sensitivity.
As shown in Figs.5A-D,the intestinal epithelial cell junction proteins were severely damaged.Compared with the Control group,the gene expression of claudin1 and claudin4 protein were significantly reduced by 4.83 times and 1.37 times,respectively.L.plantarumAR495 treatments effectively restored the gene expression levels of claudin1 and claudin4.As a key component that drives the formation of the characteristic fibrillar structure in the LJ,different claudin proteins participate in the formation of a hybrid band that closes the epithelial cell gap[34].Disruption of this protein results in damage to the highly selective intestinal permeability barrier,resulting in mast cell stimulation and subsequent pain responses.
Western blot analysis further showed similar results to gene levels.As shown in Fig.5E,The IBS group significantly reduced occludin and claudin1 protein expression compared to the Control group.Western blot indicated that the occludin protein level in the IBS group (0.66 ± 0.16) in the colon was lower than that in the Control group (1.05 ± 0.20) (Fig.5F,P< 0.05),and the expression of claudin1 (0.70 ± 0.14) in the IBS group was also reduced compared to the Control group (1.14 ± 0.32) (P> 0.05).L.plantarumAR495 treatments effectively restored the destruction of occludin and claudin1 protein levels.Among LJ proteins,occludin and claudins were considered to be the main components of epithelial ion and size-selective barrier,which play an important role in maintaining and regulating paracellular permeability[35].Studies have shown thatL.plantarumAR495 significantly reduced intestinal tissue damage and repaired intestinal epithelial structure.
3.6 L.plantarum AR495 regulates the SCFAs in intestinal homeostasis
It is known that the (SCFAs in the intestine are an important energy source for maintaining the barrier function of the intestine,which could be synthesized by fermenting indigestible cellulose and other polysaccharides such as anaerobic Bacteroides,Bifidobacterium,Eubacteria,Streptococcus,andLactobacillus[36-37].As shown in Fig.6A,the contents of propionic acid and butyric acid in the cecum((17.13 ± 5.98),(3.06 ± 0.56) µmol/g) of rats in the IBS group were significantly lower than those in the Control group ((21.80 ± 1.65),(4.72 ± 1.38) µmol/g) (P< 0.05).AfterL.plantarumAR495 intervention,the content of propionic acid and butyric acid ((19.59 ± 2.00),(5.67 ± 1.51) µmol/g) increased in the intestinal tract,and the butyric acid was significantly different in the IBS group (P< 0.05).
The content of SCFAs in the colon content was different from that in the cecum.In addition to the increase in acetic acid content in the colon,the consumption of propionic acid and butyric acid were reduced in the Control and IBS group.As shown in Fig.6B,compared with the other two groups,L.plantarumAR495 intervention significantly increased the SCFAs content in the colon,where the content of propionic acid ((23.77 ± 7.11) µmol/g) was 1.2 times and butyric acid ((3.08 ± 0.88) µmol/g) was 3.8 times compared to the IBS group.

Fig.5 Effects of L.plantarum AR495 on junction proteins in colonic tissue.mRNA expression of (A) occludin,(B) ZO-1,(C) claudin1,(D) claudin4;(E) Protein expression of junction proteins;(F) Optical density analysis of protein expression.Results were expressed as the mean ± SD for each experimental group(n=8).The significance of differences between the data was assessed using One-way ANOVA by Dunnett’s tests,with the level of significance set at P < 0.05,and different letters represent significant differences.
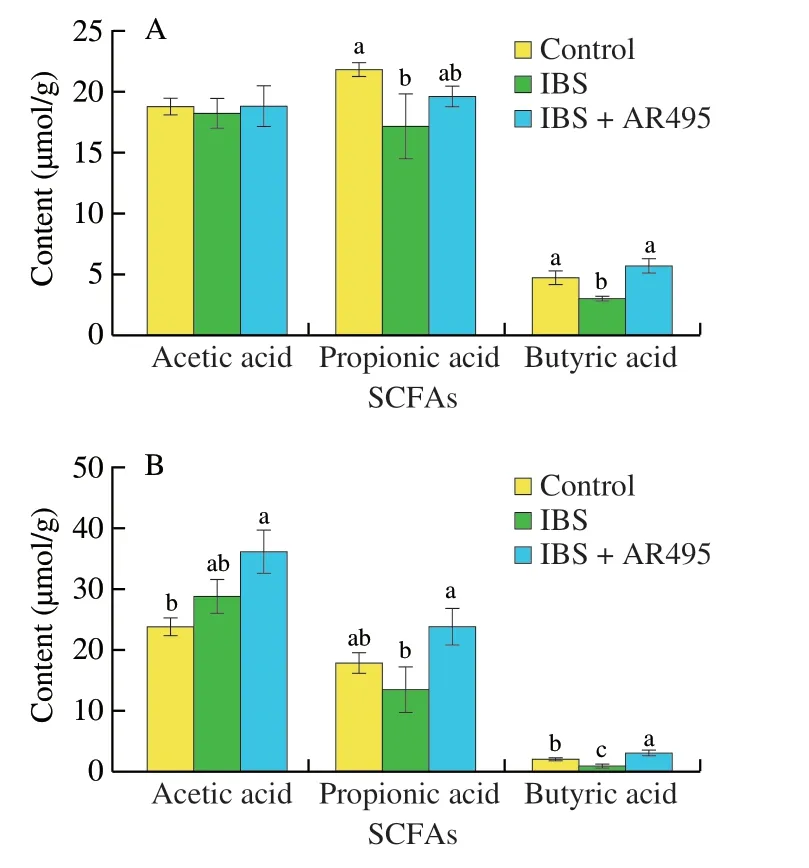
Fig.6 The variation trend of SCFAs in the intestinal environment in(A) cecum and (B) colon.Results were expressed as the mean ± SD for each experimental group (n=8).The significance of differences between the data was assessed using One-way ANOVA by Dunnett’s tests,with the level of significance set at *P < 0.05,and different letters represent significant differences.
4.Discussion
The pathophysiology of IBS involves a wide range,including abnormal stool,gastrointestinal motility changes,intestinal mucosal barrier abnormalities,gut microbiota imbalance,gut-brain axis dysfunction,and psychological disorders[38],which are the most prominent feature of the disease visceral pain sensation.In this study,the IBS model was constructed by acetic acid enema and restraint stress method,and an increase in the number of mast cells was found in the colon of IBS rats,accompanied by visceral hyperalgesia.Tryptase plays an important role in the pathogenesis of visceral hypersensitivity in IBS[23].The experiment found that the tryptase content in the colon supernatant of IBS rats was significantly increased and closely related to a highly activated mast cell in intestinal epithelial cells.Animal models and clinical studies support that tryptase increases the excitability of visceral and mesenteric afferent nerves,leading to deleterious processes associated with visceral pain.In addition,the study also foundL.plantarumAR495 significantly inhibited the proliferation and activation of mast cells,and reduced the tryptase content in the colon of the IBS mode.Some studies have also shown thatBifidobacteriumstrains inhibited IgE-mediated mast cell degranulation and subsequent late-stage response through the TLR2-dependent mechanism of FceRIa downregulation[39].In addition,treatment withLactobacillus rhamnosusJB-1 significantly inhibited the release of mast cell mediators in response to a series of stimuli by IgE-mediated activation[40].In conclusion,L.plantarumAR495 could effectively alleviate visceral hypersensitivity in IBS rats by inhibiting mast cell degranulation.
The complete intestinal epithelial barrier was composed of intestinal epithelial cells,tight junction complexes between cells,and intestinal secretions[41].When the gut barrier was damaged,mast cells could rapidly respond to a variety of factors,such as bacteria in the lumen,food,toxins,and endogenous peptides.After activation and degranulation,it could move to the vicinity of intestinal nerve fibers,and send sensitizing signals to sensory neurons,causing the release of peptides in sensory nerve endings to induce visceral hypersensitivity[24].The results of H&E staining showed that the colonic epithelium of IBS rats had a complete structure,no inflammatory pathological changes,and abundant goblet cells.However,the expression of claudin1 and occludin protein in the intestinal epithelial cells of model rats decreased,suggesting that the colonic tight junction complex has certain destruction.When mast cells degranulate and lead to the release of tryptase,which further acts on the colonic tissue,it leads to the degradation of tight junction proteins and enhances the permeability of the colon.Treatment withL.plantarumAR495 up-regulated the transcription levels of tight junction-related genesoccludin,claduin1,claduin4,and the expression of occludin and claduin1 proteins,indicating that they protected the stability of the colonic epithelial structure and effectively prevented the invasion of pathogens and antigens.These results indicated thatL.plantarumAR495 could maintain intestinal epithelial integrity and reduce mast cell activation.
Alteration in the gut microbiome and microbial metabolites underlie IBS and symptom flares,which was validation confirmed in 73% of IBS patients[42].Gastrointestinal motility,intestinal secretion,visceral hypersensitivity,and intestinal permeability,all IBS pathogenesis changes could be modified by the gut microbiome[43].Lactic acid bacteria could pass with epithelial cells competitive adhesion,competition with nutrients,and reduction of intestinal oxidative stress to change the balance of intestinal microbes.In our study,the intra-individual variability of the stool-related flora of the IBS model varies greatly and has a certain degree of dispersion.In addition,the lower similarity of the microbial flora after the intervention ofL.plantarumAR495 reflected that the degree of differentiation of the microbial flora between individuals varies over time,and a certain degree of variability appears.This was consistent with the results reported in the literature.Hypoxanthine could serve as an energy source for intestinal epithelial and promote the development and recovery of the intestinal cell barrier after injury or hypoxia.Research showed that Lachnospiraceaecan consume hypoxanthine.Compared toBifidobacterium longumcolonized mice,it had significantly lower hypoxanthine levels in cecal contents of mice colonized with Lachnospiraceae.This experiment found thatL.plantarumAR495 could significantly reduce the content of Lachnospiraceae in the model group and promote the growth ofBifidobacterium,which was consistent with the study that the oral administration ofL.plantarumCCFM8610significantly restored the diversity of the gut microbiota and increased the abundance ofBifidobacterium[44].
In addition,Lactobacilluscould also regulate the intestinal microflora and inhibit the growth and proliferation of harmful bacteria by lowering the pH of the intestinal tract and synthesizing SCFAs and defensins[45].Now a large amount of evidence showed SCFAs were the main energy source of colonic epithelial cells,and oral supplementation of SCFA after intestinal injury could inhibit the increase of colonic epithelial permeability[46].Previously reported the propionate,butyrate,and acetate to be significantly lower in the stool samples of patients with IBS,which drives changes in gastrointestinal physiology[31].The present study found that the content of propionic and butyric acid in the gut decreased after restraint stress,but returned to normal levels after supplementation withL.plantarumAR495.Notably,the propionic acid content was higher in the three groups of rats,which may be attributed to the homeostatic regulation of the intestinal barrier in rats after colonic dilation.SCFAs inhibited the permeability of isolated colonic mucosa and Caco-2 cell monolayers in a dose-dependent manner in the short term[47].Among them,the physiological concentration of propionic acid immediately enhanced the barrier function of colonic epithelium in a short timein vitro.Moreover,SCFA also could modulate the serotonergic pathway in host gastrointestinal tissue[48].This study found thatL.plantarumAR495 enhanced the concentration of SCFAs in the colon,especially propionic acid and butyric acid.Studies claimed that oral administration of 200 mmol/L propionic acid can increase the expression levels of claudin and occludin,which was consistent with the results of this experiment[49].Therefore,this study found thatL.plantarumAR495 could repair the intestinal mucosal barrier by improving gut microbiota imbalance,and relieving visceral sensitivity of IBS rats through the Mast cell-PAR2-TRPV1 pathway.
5.Conclusions
In summary,the alleviating effect ofL.plantarumAR495 on visceral sensitivity of IBS was investigated in Wistar rats.L.plantarumAR495 could effectively reverse the abnormal activation of mast cells and tryptase content in the IBS model,thereby reducing the continuous sensitization by blocking up the over-expression of PAR2 and TRPV1 in the DRG.
The results also showed that the gut microbiota was dysregulated and gut homeostasis was disrupted in IBS rats.L.plantarumAR495 promoted the production of SCFAs to maintain a healthy gut barrier,especially propionate and butyrate.By regulating the imbalance of gut microbiota and promoting the proliferation of beneficial bacteria such asBifidobacterium,the visceral sensation of IBS rats could be improved through the gut-brain axis.This report lays the foundation for mechanistic studies of probiotics for intervention in IBS.
Conflict of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This work was supported by the shanghai agriculture applied technology development program (2019-02-08-00-07-F01152);the national science fund for distinguished young scholars (32025029);the shanghai engineering research center of food microbiology program (19DZ2281100);and the national key R&D program of china (2018YFC1604305).
- 食品科学与人类健康(英文)的其它文章
- Protective effects of oleic acid and polyphenols in extra virgin olive oil on cardiovascular diseases
- Diet and physical activity inf luence the composition of gut microbiota,benef it on Alzheimer’s disease
- Inf luence of nitrogen status on fermentation performances ofnon-Saccharomyces yeasts: a review
- Ganoderma lucidum: a comprehensive review of phytochemistry,eff icacy,safety and clinical study
- Resveratrol combats chronic diseases through enhancing mitochondrial quality
- Demonstration of safety characteristics and effects on gut microbiota of Lactobacillus gasseri HMV18

