Blautia producta displays potential probiotic properties against dextran sulfate sodium-induced colitis in mice
Bingyong Mao ,Weiling Guo ,Shumao Cui ,Qiuxiang Zhang ,Jianxin Zhao ,Xin Tang, ,Hao Zhang,c
a State Key Laboratory of Food Science and Resources, Jiangnan University, Wuxi 214122, China
b School of Food Science and Technology, Jiangnan University, Wuxi 214122, China
c National Engineering Research Center for Functional Food, Jiangnan University, Wuxi 214122, China
Keywords: Blautia producta D4 Colitis Intestinal mechanical barrier TLR4/NF-κB pathway Intestinal microbiot
ABSTRACT Blautia has attracted attention because of its potential eff icacy in ameliorating host energy metabolism and inflammation.This study aims to investigate the influences of Blautia producta D4 on colitis induced by dextran sulfate sodium (DSS) and to reveal the underlying mechanisms.Results showed that B.producta D4 intervention signif icantly relieved body weight loss,and suppressed the elevation of pro-inf lammatory cytokines (including interleukin-6 (IL-6),tumor necrosis factor-α (TNF-α),and interleukin-1β (IL-1β))and excessive oxidative stress (myeloperoxidease (MPO) activity,superoxide dismutase (SOD) activity,glutathione peroxidase (GSH-Px) activity,and malondialdehyde (MDA) level) in colitis mice.Moreover,the concentrations of tight junction proteins (occludin,claudin-1,and ZO-1) related to the intestinal barrier were obviously elevated,and colitis-related TLR4/NF-κB pathway activation was remarkably inhibited after B.producta D4 intervention.The intestinal microbial disorder was evidently ameliorated by increasing the relative abundance of Clostridium sensu stricto 1,Bifidobacterium,GCA-900066225,Enterorhabdus,and reducing the relative abundance of Lachnospiraceae NK4A136 group.In conclusion,oral administration of B.producta D4 could ameliorate DSS-induced colitis by suppressing inf lammatory responses,maintaining the intestinal barrier,inhibiting TLR4/NF-κB pathway,and regulating intestinal microbiota balance.These results are conducive to accelerate the development of B.producta D4 as a functional probiotic for colitis.
1.Introduction
Ulcerative colitis (UC) is a chronic inf lammatory disease of the gastrointestinal tract[1],and its incidence and prevalence have been gradually rising in the world and greatly affecting patient quality of life,especially affecting children and adolescents[2].It is generally believed that the occurrence and development of colitis are resulted from combining the effects of the intestinal barrier,intestinal microbiota,and mucosal immunity[3].The integrity of the intestinal barrier is the physical basis that provides a primal barrier against the invasion of antigens,toxins,and pathogenic microorganisms,and simultaneously maintains the proper development of epithelial barriers and the immune system[4].In addition,the accumulation of inflammatory cytokines and abnormal oxidative stress disturbs immunological responses,which are closely related to the occurrence and development of colitis.The previous study has shown that patients with colitis are characterized by high levels of interleukin-6 (IL-6),tumor necrosis factor-α (TNF-α),and interleukin-1β (IL-1β)[5].Therefore,the reduction of inf lammatory cytokines can be useful for the therapy of colitis.At present,drug therapy is thought to be the main treatment for colitis,while most therapeutic drugs still have a series of unpleasant side effects.How to prevent the development of colitis has become a major global issue in the medical community.
Probiotics are live microorganisms that can ameliorate host health when administered in adequate amounts[6].Previous studies exhibited that probiotics have promising potential in reducing the incidence and mortality of colitis[7-8].Blautiais widely distributed in the feces and intestines of mammals and has attracted growing interest due to its potential correlations with the alleviation of metabolic and inflammatory diseases,and inhibition of specific bacterial growth[9-11].Previous studies revealed that the abundance ofBlautiais positively associated with the improvement of lipid metabolism[12]and the protein and gene expressions of ZO-1 and occluding[13].Blautia productatreatment decreased the transcription of poly(I:C)-induced inflammatory genes in HT-29 cells,including DDX58,NFKBIA,IL-8,TNF,CXCL10 and CXCL11[14].These findings suggested thatBlautiamay play an anti-inflammatory role in the prevention and treatment of obesity and intestinal damage.
The gastrointestinal tract is a complex ecosystem consisting of gastrointestinal epithelium,mucus layer,immune cells,and intestinal microbiota,among which the disorders of intestinal microbiota may worsen colitis[6].A previous study has shown that oral administration of probiotics can ameliorate DSS-induced colitis by regulating the balance of intestinal microbiota[15].However,most studies mainly focused on the influence ofLactobacillusandBifidobacteriumon DSS-induced colitis[16-17].For instance,L.plantarumAR113 increased the abundance of Lachnospiraceae,Lactobacillus,Helicobacter,andRoseburia,which was associated with the remission of colitis[16].Bifidobacteriumtreatment decreased the abundance ofSutterella,Bacteroides,andOscillospirain colitis mice,which were positively correlated with the concentrations of tight junction proteins[17].Correlation analysis revealed thatBlautiamay be a potential probiotic in the regulation of intestinal microbiota and could be an effective therapeutic method for colitis prevention and treatment[18].However,whetherBlautiacan prevent/treat inflammation and the potential mechanism have not yet been elucidated.
We have successfully isolated one stain ofBlautia productafrom mice feces and intended to evaluate its effects against DSS-induced colitis.The underlying mechanisms ofB.productaD4 on colitis were first explored using high-throughput sequencing and reverse transcription-quantitative polymerase chain reaction.The association between the key intestinal microbial phylotypes and inflammatory cytokines was revealed by correlation analysis and visualization,aiming to provide a theoretical basis for developing new probiotics to improve colitis.
2.Materials and methods
2.1 Bacterial strains and culture conditions
B.productaD4 was isolated from mice feces,and cultured in Gifu Anaerobic Medium (GAM) at 37 °C under anaerobic conditions for 24 h.Subsequently,the cells were harvested by centrifugation(12 000 ×g,5 min at 4 °C) and washed three times using sterile normal saline,and re-suspended using 12% skim milk (5 × 109CFU/mL)and preserved at -80 °C.
2.2 Animal experiments
Twenty-four male C57BL/6 mice (7 weeks old) were obtained from Charles River (Beijing,China).All mice were raised in controlled laboratory conditions without pathogens (temperature,(23 ± 1) °C;humidity,(55 ± 5)%;light,08:00-20:00).All mice were given free access to water and food.
After the adaptation period (1 week),all mice were randomly assigned to 3 groups (n=8).The Control and Model groups were fed with 0.2 mL of skim milk (12%,m/V),and the D4 group was fed with 0.2 mL (1 × 109CFU) ofB.productaD4.Mice in the Control group were given free access to water during the entire experiment.Mice in the Model and D4 groups were given free access to water for 7 days,and then were provided drinking water consisting of 3.0% (m/V)dextran sodium sulfate (DSS,MW 36-50 kDa) from day 8 to day 14.The body weight was measured every day.The animal experiments were approved by the Experimental Animal Ethics Committee of Jiangnan University (JN.No20210430c1500608[097]).
2.3 Measurement of disease activity index (DAI)
During the experiment,the DAI was measured according to a previous report[19].DAI scores consist of weight loss score,stool consistency score,and stool bleeding score.The score information is briefly shown in Table 1.DAI was calculated according to the following formula:
DAI=weight loss score+stool consistency score+stool bleeding score

Table 1 Calculated DAI score.
2.4 Measurement of anti-inflammatory biomarkers and antioxidant system in the colon
Colon tissue from all mice was harvested,and homogenized.The homogenate was centrifuged,and the supernatant was collected.The concentration of protein was measured using commercial kits (Beyotime,Shanghai,China) according to the manufacturer’s protocols.Colonic IL-6,TNF-α,IL-1β,IL-10,and mucin2 (Muc2)levels were analyzed using the commercial ELISA kits (R&D Systems Co.,Ltd,USA) and relativized to total protein.The myeloperoxidease(MPO) activity,superoxide dismutase (SOD) activity,glutathione peroxidase (GSH-Px) activity,and malondialdehyde (MDA) level were performed using commercial kits (Jiancheng Biotechnology Co.,Ltd.,Nanjing,China).
2.5 Histological analysis
Fresh colons were removed and fixed in formalin at 4 °C for 12 h and embedded in paraffin after gradient alcohol dehydration.Hematoxylin-eosin staining,alcian blue staining,and PAS staining were performed as previously described[19].Three randomly selected areas were observed per slide.The scores of colitis were obtained in line with the criteria illustrated previously (Table S1)[19].
2.6 Immunohistochemical analysis
Immunohistochemical analysis was performed as previously described[20].Briefly,the sections of the colon were incubated with a primary antibody to occludin,claudin-1,and ZO-1 for 60 min,and then incubated with secondary antibody and streptavidinbiotin peroxidase for 120 min.Ultimately,the brown color was measured under 3,3’-diaminobenzidine tetrahydrochloride using a light microscope.Sections were observed using a panoramic scanner(Pannoramic MIDI,3D HISTECH,Hungary).
2.7 Measurement of short-chain fatty acid (SCFA)
Cecal SCFA concentrations were measured as previously described[21].Briefly,the dry cecal contents (50 mg) were dissolved in 0.5 mL of saturated NaCl solution.The solutions were stood still for 30 min and homogenized.Then,sulfuric acid (10%,V/V) was added to the solution to adjust the pH to 2-3.SCFAs were extracted using 0.8 mL of C2H5OC2H5and then homogenized (60 Hz,30 s),and centrifugated (14 000 ×g,15 min).The supernatant was transferred to a new centrifuge tube (containing 0.25 g of anhydrous Na2SO4),and the solution was centrifugated (14 000 ×g,15 min).The supernatant was collected and applied to determine SCFAs concentrations using gas chromatography-mass spectrometry (GC-MS).
2.8 RNA extraction and quantitative real-time PCR
Total RNA was extracted from the colon using a commercial kit(Takara,Dalian,China) following the manufacturer’s protocols.The levels of RNA were measured by NanoDrop 2000 spectrophotometer(Thermo,USA).Then,colonic RNA was reverse-transcribed into cDNA using a commercial kit (Vazyme Biotechnology Co.,Ltd.,Nanjing,China).The SYBR Green PCR Master Mix kit (Vazyme Biotechnology Co.,Ltd.,Nanjing,China) was applied to perform qRT-PCR.The mRNA transcription levels were measured using an RT-qPCR system (Bio-Rad,CA,USA).The expression of genes related to colitis was counted based on the GAPDH expression.All primers of these genes are shown in Table S2.
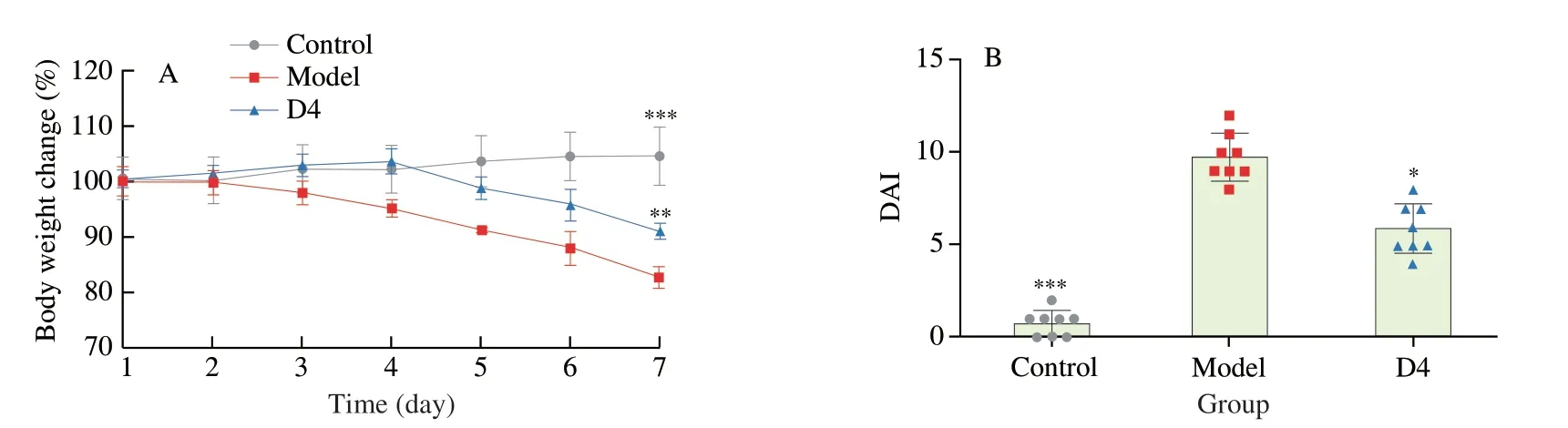
Fig.1 Influence of B.producta D4 intervention on the DSS-induced colitis.Body weight (A),DAI (B),colon length (C) and macroscopic pictures of colons (D).Values are showed as the mean ± SD (n=8).Significance set at *,**and ***means P < 0.05,P < 0.01 and P < 0.001 when compared with the DSS group,respectively.
Fig.1 (Continued)
2.9 Bioinformatics analysis of 16S rRNA gene sequences
Genomic DNA of frozen feces was extracted using a DNA isolation kit (MoBio,USA) according to the manufacturer’s protocols,and the 16S rRNA genes (V3-V4 region) were PCR amplified using the forward primer (338F) and reverse primer (806R).The PCR products were purified using Ampure magnetic purification beads (Agencourt Brea,CA,USA) and then pooled into equal concentrations.Qubit 2.0 Fluorometer (Thermo Fisher Scienticfic,USA) was applied to assess the quantity of sequencing libraries and sequenced on the Illumina MiSeq platform.
The raw data from high-throughput sequencing were demultiplexed and quality filtered using the QIIME2 platform.The results were assigned to operational taxonomic units (OTUs) by UCLUST with a threshold of 97%.Principal component analysis(PCA) plots were implemented using R software (v 4.1.2).STAMP(v 2.1.3) software was applied to identify gut microbial phylotypes with obvious differences among all groups.Pearson correlation coefficients and network between specific intestinal microbiota and the concentrations of inflammatory cytokines,antioxidant enzymes,and SCFAs were obtained using “ggplot2” package of R software and Cytoscape software (v 3.6.1),respectively.
2.10 Statistical analysis
The experimental results are expressed as the mean ± SEM using Prism 7.0 (GraphPad Software,CA,US).Statistical analysis was carried out using one-way analysis of variance (ANOVA),followed by Tukey test using SPSS 20.0 (IBM,Chicago,USA).Statistically significant differences are indicated as*P< 0.05,**P< 0.01,and***P< 0.001.
3.Results
3.1 B.producta D4 intervention relieves the colitis symptoms
As shown in Fig.1A,the body weight loss in the Model group after 7 days of DSS treatment exhibited a significant decreasing trend.However,oral administration ofB.productaD4 could significantly delay and attenuate the reduction of body weight induced by DSS(P< 0.01).Furthermore,DAI scores were widely applied to assess the influences ofB.productaD4 on colitis mice.The DSS treatment evidently elevated the DAI scores of mice in the model group by comparison with the Control group (P< 0.01) (Fig.1B).Interestingly,B.productaD4 intervention significantly reversed these trends caused by DSS (P< 0.05).The colon length of mice in the Model group was obviously shortened compared with the Control group (P< 0.01)(Figs.1C and 1D).However,the oral administration ofB.productaD4 ameliorated the shortening of colon length in colitis mice(P< 0.05).Thus,B.productaD4 intervention effectively alleviated the symptoms of colitis,including the reduction of body weight,DAI scores,and colon shortening.
3.2 B.producta D4 intervention relieves colonic inflammatory cytokines in colitis mice
The anti-inflammatory role ofB.productaD4 on DSS-treated mice was explored by determining the concentrations of pro-inflammatory cytokines (TNF-α,IL-6,and IL-1β),and anti-inflammatory cytokine(IL-10).As shown in Fig.2,the concentrations of colonic TNF-α,IL-6,and IL-1β were remarkably increased in the Model group compared with the Control group (P< 0.001),while the concentration of colonic IL-10 was remarkably reduced (P< 0.001).B.productaD4 intervention dramatically reduced the colonic TNF-α,IL-6,and IL-1β concentrations (P< 0.01),and dramatically increased the colonic IL-10 concentration (P< 0.001).These results showed thatB.productaD4 may promote the secretion of anti-inflammatory cytokines and inhibit the secretion of pro-inflammatory cytokines,thus ameliorating the colitis induced by DSS.

Fig.2 Influence of B.producta D4 intervention on the colonic inflammatory cytokines.Values are showed as the mean ± SD (n=8).(A-D) TNF-α,IL-6,IL-1β,IL-10.Significance set at **and ***means P < 0.01 and P < 0.001 when compared with the DSS group,respectively.
3.3 B.producta D4 intervention relieves colonic oxidative stress in colitis mice
After the mice were treated with DSS,the colonic MPO activity and MDA level were dramatically increased,which indicated an increasing exacerbation of oxidative stress (P< 0.01) (Fig.3A).However,B.productaD4 intervention dramatically decreased the colonic MPO activity and MDA level compared with the Model group(P< 0.05).In addition,the colonic GSH-Px and SOD activities in the Model group were obviously lower than that in the Control group(P< 0.001).WhereasB.productaD4 intervention resulted in significant increases of GSH-Px and SOD activities in colitis induced by DSS (P< 0.01).
Detailed histological analysis of colonic lesions displayed that DSS treatment resulted in an extensive distortion of the crypts,inflammatory cell infiltration,loss of epithelial and goblet cells compared with the Control group.However,B.productaD4 intervention dramatically prevented pathological changes induced by DSS,including reducing inflammatory cell infiltration,inhibiting foci of crypt loss,and decreasing the damage of goblet cells (Fig.3B and Fig.S1).Thus,B.productaD4 effectively mitigated the damage to the colon induced by DSS.

Fig.3 Influence of B.producta D4 intervention on the activity of antioxidant-related parameters and the histological injury in colon.The activities of MPO,MDA,GSH-Px,and SOD (A);Histological analysis of colon tissue (scale bar=20 μm) (B).The crypts are marked using blue rectangle and the goblet cells are marked using black arrows.Values are showed as the mean ± SD (n=8).Significance set at *,**and ***means P < 0.05, P < 0.01 and P < 0.001 when compared with the DSS group,respectively.
3.4 B.producta D4 intervention attenuates intestinal barrier integrity in colitis mice
The barrier integrity of the colon in colitis mice may be damaged,thus stimulating the inflammatory responses and promoting the process of colitis.Alcian blue and PAS staining were applied to explore the influence ofB.productaD4 on the colon barrier in colitis mice (Fig.S2A and S2B).Alcian blue and PAS staining of colon sections exhibited that the number of goblet cells in the Model group was dramatically decreased compared with the Control group.Nevertheless,B.productaD4 intervention obviously reduced mucus layer damage.Moreover,B.productaD4 intervention dramatically elevated the colonic Muc2 levels compared with the Model group (Fig.S3).
In addition,Fig.4 showed the immunohistochemical analysis of occludin,claudin-1,and ZO-1,respectively.The concentrations of colonic occludin,claudin-1,and ZO-1 proteins were dramatically reduced in the Model group compared with the Control group(P< 0.001).Interestingly,B.productaD4 intervention obviously increased the concentrations of colonic occludin,claudin-1,and ZO-1 proteins compared with the Model group (P< 0.01).These results suggested thatB.productaD4 intervention effectively protected against the reduction of the tight junction proteins induced by DSS.
3.5 Effects of B.producta D4 on the production of SCFAs in colitis mice

Fig.4 Influence of B.producta D4 intervention on the tight junction proteins (A,occludin,B,claudin-1,C,ZO-1) (scale bar=20 μm).Significance set at **and ***means P < 0.01 and P < 0.001.

Fig.4 (Continued)
SCFAs are produced by the fermentation of intestinal microbiota,and their function is to regulate the host’s metabolism and immune system.The concentrations of cecal SCFAs (including acetic acid,propionic acid,isobutyric acid,butyric acid,valeric acid,and isovaleric acid) were measured using GC-MS.As illustrated in Fig.5,the concentrations of acetic acid,propionic acid,isobutyric acid,and butyric acid were significantly decreased in the Model group compared with the Control group (P< 0.05),but there were no significant differences in cecal valeric acid and isovaleric acid between the Control and Model groups (P> 0.05).Intriguingly,B.productaD4 intervention significantly elevated the concentrations of cecal acetic acid and butyric acid in colitis mice (P< 0.05).The concentrations of propionic,isobutyric,valeric,and isovaleric acids in D4 group were slightly higher than that of the Model group(P> 0.05).This finding suggested thatB.productaD4 intervention could regulate the cecal SCFAs concentrations,resulting in the potential to ameliorate colitis.
3.6 B.producta D4 intervention regulates the signaling pathway-related colitis
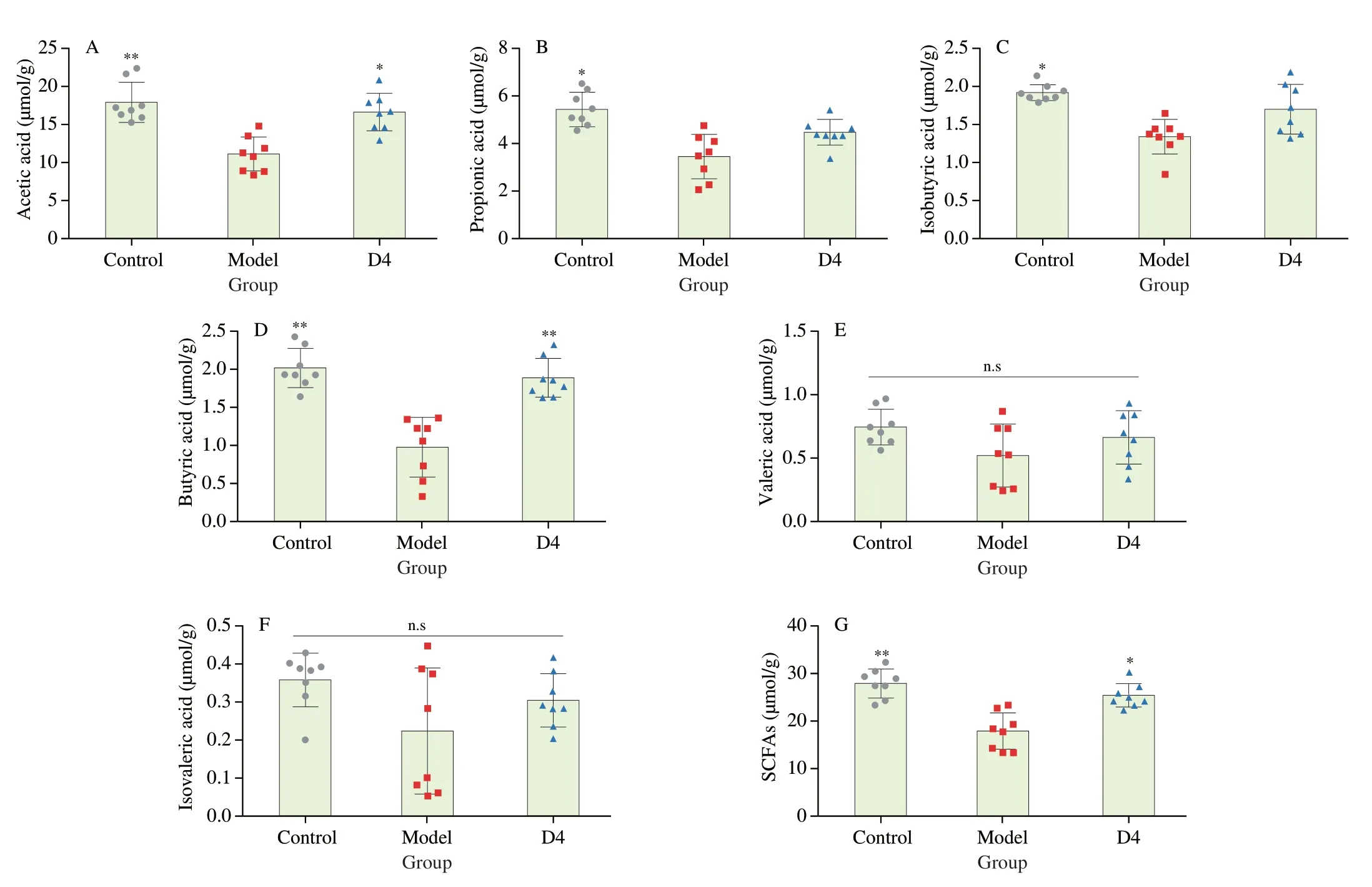
Fig.5 Influence of B.producta D4 intervention on colonic SCFAs.(A-G) acetic acid,propionic acid,isobutyric acid,butyric acid,valeric acid,isovaleric acid,SCFAs.Values are showed as the mean ± SD (n=8).Significance set at *,**and n.s means P < 0.05, P < 0.01 and not significant when compared with the DSS group,respectively.
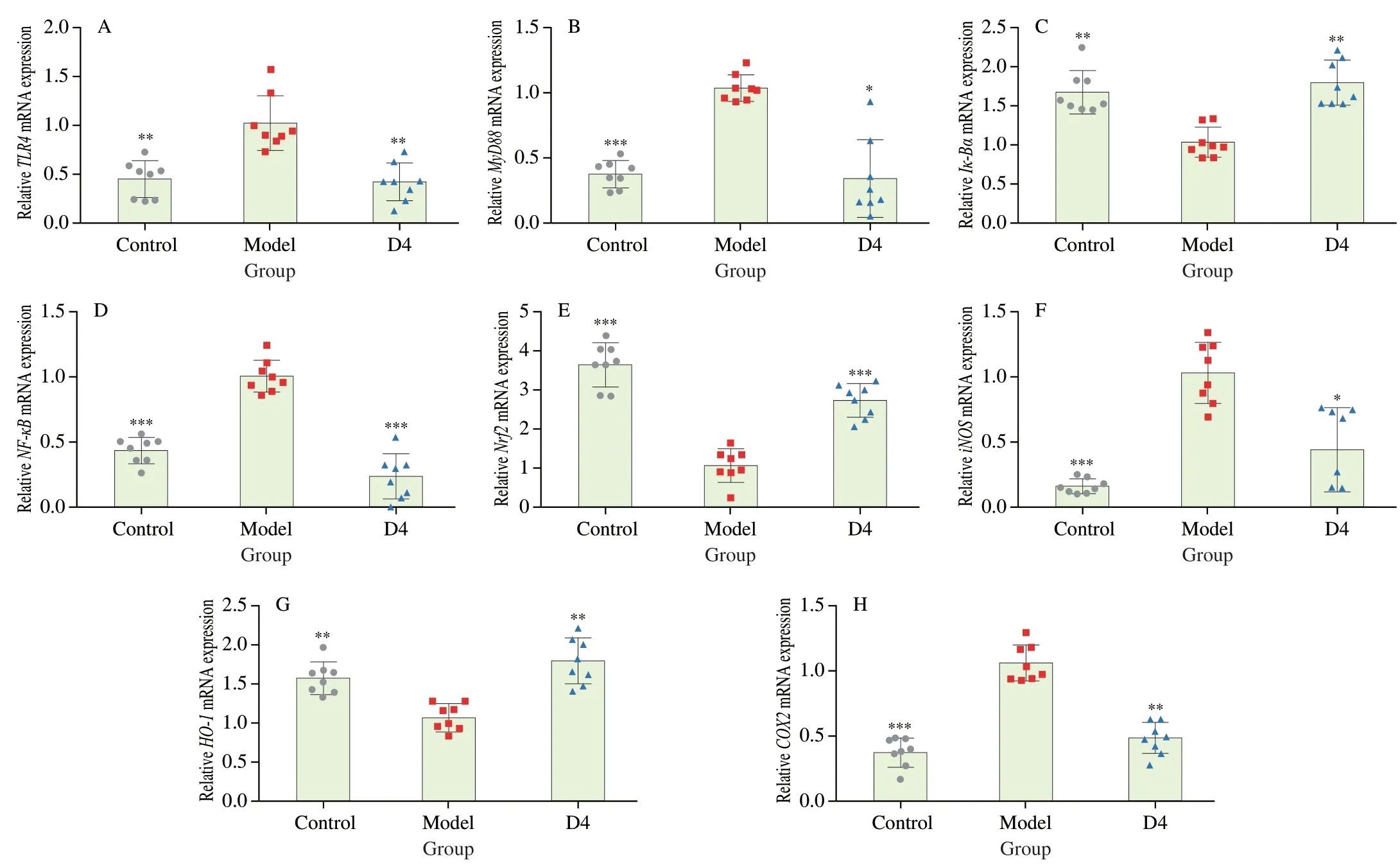
Fig.6 Influence of B.producta D4 intervention on the mRNA expression of genes-related NF-κB signaling pathway.(A-H) TLR4,MyD88,Ik-Bα, NF-κB,Nrf2,iNOS,HO-1,COX2.Values are showed as the mean ± SD (n =8).Significance set at *,**and ***means P < 0.05,P < 0.01 and P < 0.001 when compared with the DSS group,respectively.
To further investigate the underlying mechanism of action by whichB.productaD4 intervention protected against colitis induced by DSS,transcription levels of the genes related to colitis were further measured using RT-qPCR (Fig.6).Compared with the Control group,the transcription of toll-like receptor 4 (TLR4),myeloid differentiation primary response gene 8 (MyD88),nuclear factor-kappa B (NF-κB),inducible nitric oxide synthase (iNOS),and cyclooxygenase 2 (COX2) were dramatically up-regulated in the Model group (P< 0.01),whereas the transcription of inhibitor of NF-κB (Ik-Bα),nuclear factor erythroid 2-related factor 2 (Nrf2),and heme oxygenase-1 (HO-1) was dramatically down-regulated(P< 0.01).However,B.productaD4 intervention dramatically suppressed the transcription of colonicTLR4,MyD88,NF-κB,iNOS,andCOX2in colitis mice,and dramatically elevated the transcription of colonicIk-Bα,Nrf2,andHO-1(P< 0.01).
3.7 B.producta D4 intervention alters the composition of intestinal microbiota in colitis mice
In order to reveal the influence ofB.productaD4 on the intestinal microbiota structure of colitis mice,the V3-V4 region of the 16S rRNA was sequenced using Illumina MiSeq.As shown in Fig.7A,there was a remarkable distinct clustering in intestinal microbiota between the Control and Model groups.However,there was a statistical separation between the Model and D4 groups,suggesting thatB.productaD4 intervention had a substantial influence on the intestinal microbial structure of DSS-treated mice.To further explore the influence ofB.productaD4 on the structures of intestinal microbial in DSS-treated mice,extended error bar plot was applied to identify the specific altered bacterial phenotypes.The colitis mice had a higher proportion of Lachnospiraceae NK4A136 group,[Eubacterium]fissicatenagroup,Negativibacillus,Ruminiclostridium9 and a lower proportion ofLactobacillus,Alloprevotella,Enterococcus,[Eubacterium]xylanophilumgroup,Adlercreutzia,Muribaculum,PrevotellaceaeUCG-001,Catabacter,unculturedBacteroidales bacterium,[Eubacterium]ventriosumgroup (Fig.7B).However,B.productaD4 supplementation increased the proportion ofClostridium sensu stricto1,Bifidobacterium,GCA-900066225,Enterorhabdus,and reduced the proportion of Lachnospiraceae NK4A136 group (Fig.7C).These data suggested thatB.productaD4 intervention obviously changed the structure of intestinal microbiota in DSS-treated mice.
3.8 Correlation between colitis-related parameters and specific intestinal microbiota altered by B.producta D4
Spearman’s analysis was used to reveal the possible correlation between the colitis-related parameters and the specific intestinal microbiota (Figs.8A and B).The relative abundance ofAdlercreutzia,Prevotellaceae UCG-001,Catabacter,[Eubacterium]ventriosumgroup,[Eubacterium]xylanophilumgroup,Lactobacillus,Muribaculum,unculturedBacteroidalesbacterium,Alloprevotella,andEnterococcuswas negatively associated with the colonic TNF-α,IL-6,IL-6,IL-1β,MPO,and MDA levels.However,the relative abundance of Lachnospiraceae NK4A136 group,[Eubacterium]fissicatenagroup,Ruminiclostridium9,GCA-900066225,Clostridium sensu stricto1,andEnterorhabduswas positively associated with the cecal SCFAs levels,colonic Muc2 level,SOD activity,and GSH-Px activity.The relative abundance ofBifidobacteriumandNegativibacilluswas negatively associated with the cecal SCFAs levels,colonic Muc2 level,SOD activity,GSH-Px activity,IL-10 level,and body weight.
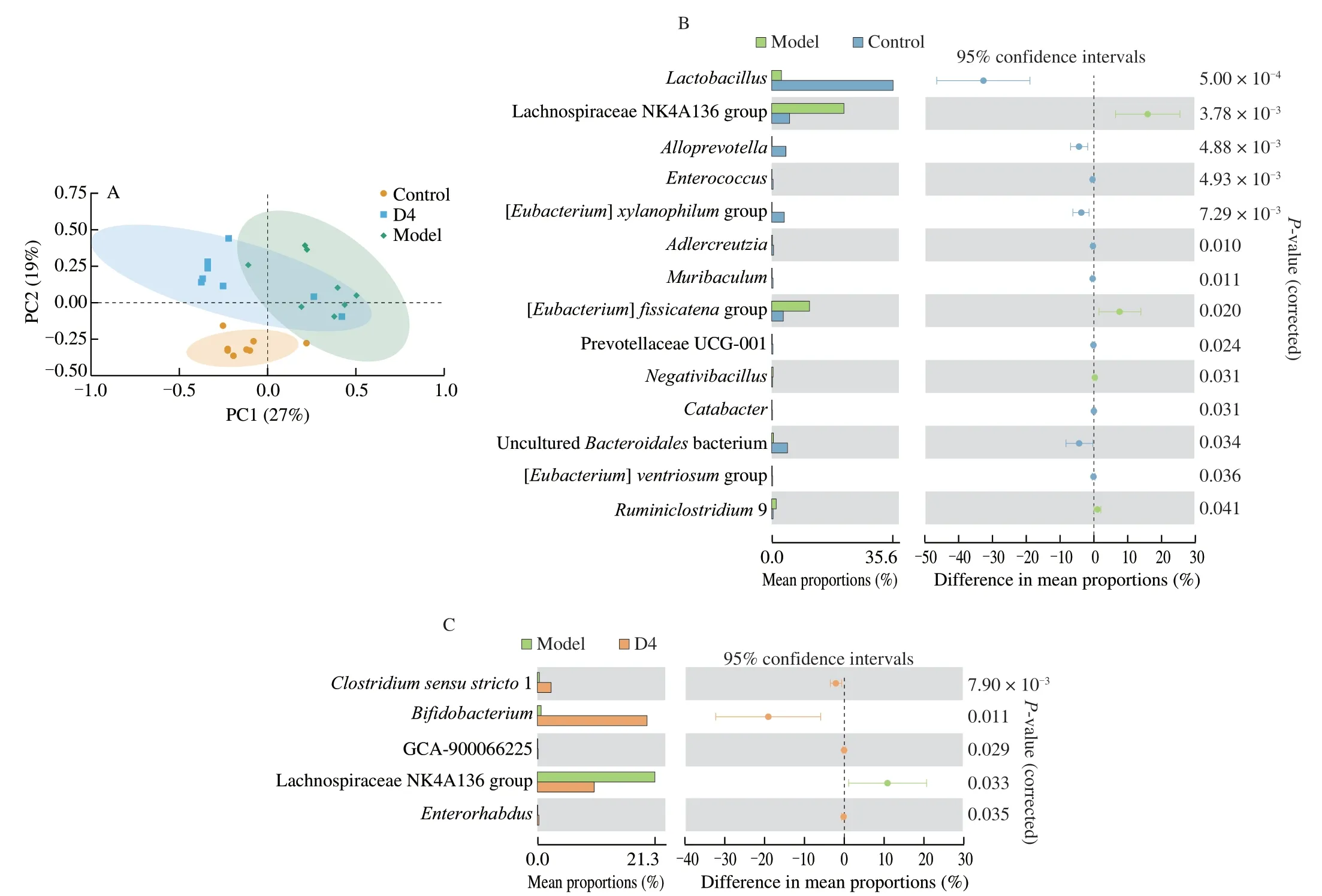
Fig.7 Influence of B.producta D4 intervention on the intestinal microbiota at the genus levels.PCA analysis (A);The remarkable differences of intestinal microbiota between the Control group and the Model group (B),the Model group and the D4 group (C).
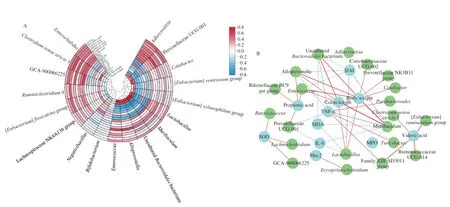
Fig.8 Heatmap of association between the key intestinal microbiota and colitis-related indicators (A);Visualization of the correlation network based on the correlation (B).
4.Discussion
Restoration of colitis demands many factors,such as repair of the intestinal barrier,inhibition of pro-inflammatory cytokines,and epithelial cell proliferation and differentiation.Drug treatment is thought to be the primary treatment for colitis,while some drugs have adverse toxicity and side effects.Previous studies have shown thatBlautiamay be related to a variety of biological activities[11,22-24],including treatment or prevention of diarrhea[22],inhibition of bacterial infections of the gastrointestinal tract[23],and remission of inflammation[24].However,these studies are mainly based on correlation analysis,and no verification test was performed with theBlautiastrains.In this study,we explored the protective effects ofB.productaD4 on intestinal barrier damage and inflammation in DSS-induced colitis.Our results suggested thatB.productaD4 was a potential probiotic that can ameliorate the development of colitis by preventing the damage of the intestinal barrier,inhibiting the secretion of pro-inflammatory cytokines,controlling the TLR4/NF-κB pathway activation,and regulating the structure of intestinal microbiota (Fig.9).As far as we know,this may be the firstin-vivoexperiment to prove that a specificB.productastrain has the effect of improving intestinal inflammation,which may encourage more research on the physiological activities ofBlautiastrains in this field.
The obvious characteristics of DSS-induced colitis are loss of body weight and increases in DAI score[25].Therefore,inhibition of body weight loss and the DAI score increase is thought to relieve the development of colitis.In addition,colitis is closely associated with high levels of pro-inflammatory cytokines,such as TNF-α,IL-1β,and IL-6[26].Secretion of TNF-α is monitored by IL-1β,which is a central monitor in the initiation of the inflammatory response[27].IL-6 is another common inflammatory cytokine,and is frequently found in metabolic inflammation and glycolipid metabolism disorders[28].While IL-10 is a vital immunoregulatory cytokine that can ameliorate inflammatory responses by inhibiting the production of CD4 and CD8 T cells[29].B.productaD4 intervention effectively suppressed the loss of body weight,the increases in DAI score,and levels of colonic TNF-α,IL-6,and IL-1β,simultaneously increased the levels of colonic IL-10 in colitis mice,indicating thatB.productaD4 could relieve the development of colitis.
Abnormal status of oxidative stress can accelerate the development of colitis,and even induce rectal cancer[30],which can be reflected by MDA level,MPO,SOD,and GSH-Px activities.MDA served as the end product of polyunsaturated fatty acid peroxidation[31],and could damage the integrity of cell by the crosslinking and polymerization of proteins[32].Elevation of MPO activity destroyed intestinal epithelium and promoted the development of colitis[33-34].SOD is an antioxidant enzyme and helps to eliminate reactive oxygen species to accelerate the formation of hydrogen peroxidein vivo,which was further metabolized to oxygen and water by activating GSH-Px[35].B.productaD4 intervention suppressed the MDA levels and MPO activity,while the SOD and GSH-Px activities were elevated,indicating thatB.productaD4 protects against colitis likely through altering the activities of anti-oxidative enzymes.However,this is only a phenotypic phenomenon,and the underlying mechanisms needs to be further explored.
DSS can elicit the injury of the intestinal barrier,characterized by the decreases in mucus layer thickness and tight junction proteins,and the disorder of mucosal and crypt structure[36].Therefore,restoring altered barrier functions is an essential direction in colitis treatment.The mucus layer can reflect the integrity of the intestinal barrier and protect epithelia from harmful substances[37].The decrease in the concentration of Muc2 and tight junction protein (ZO-1,occludin,and claudin-1) is an obvious symptom of colitis.ZO-1 is the first epithelial tight junction protein and is critical to epithelial repair,and could control the Wnt-β-catenin signaling and mitotic spindle orientation[26,38].Notably,dysregulation ofβ-catenin is closely related to colorectal cancer.Thus,we speculate thatB.productaprotects against the occurrences of colorectal cancer by elevating the expression of ZO-1 (Fig.4).Besides ZO-1,B.productaD4 intervention also increased the expression of occludin and claudin-1,indicating thatB.productaD4 could repair the intestinal barrier and maintain the functions.

Fig.9 Exposure of B.producta D4 attenuates intestinal barrier damage via regulating the TLR4/NF-κB signaling pathway.
Based on the results of body weight loss,DAI score,inflammatory responses,oxidative status,and intestinal barrier,we confirmed thatB.productaD4 could protect against DSS-induced colitis.However,the underlying protective mechanisms remain unanswered.SCFAs are the end metabolites of gut microbes,and have been recognized as a vital mediator between the commensal microbiota and the immune system.A previous study exhibited that microbiotaderived SCFAs promoted Th1 cell IL-10 production to maintain intestinal homeostasis[39].In this study,we found thatB.productaD4 intervention significantly increased the cecal SCFAs concentrations,and the colonic IL-10 concentration.There may be a connection between these two matters,but we failed to provide direct evidence.We speculated that SCFAs may be an important factor forB.productaD4 to ameliorate colitis,but needs to be further revealed.Blautia,a new genus with probiotic potential,is poorly studied and its metabolic properties in the gut are unclear[40].It is possible to discover new and different metabolites that improve colitis after further and detailed metabolomic analysis.
To explore the potential mechanism wherebyB.productaD4 treatment relieves colitis induced by DSS,the mRNA levels of genes related to colitis were measured by RT-qPCR,including TLR4,MyD88,Iκ-Bα,NF-κB,Nfr2,iNOS,HO-1,andCOX2.TLR4has been involved to promote the cellular release of cytokines and chemokines[41].Activated TLR4 promotes the recruitment ofMyD88and then elevates nuclear translocation ofNF-κB,which triggers activation of the downstream NF-κB pathway and secretion of proinflammatory mediators that contribute to intestinal inflammation.The high expression ofNF-κBis related to interaction withIκ-Bα.Through phosphorylation,NF-κBp65 can be released from the p65-p50-Iκ-Bα complex,which is followed by nuclear translocation and resultant production of cytokines such as IL-1β,TNF-α,and IL-6[42].In addition,NF-κBmodulates the expression ofiNOSandCOX2.Overexpression ofiNOSdestroys the structure of morphology,leading to the formation of a large number of nitric oxides[43],which further elevates the formation ofCOX2.COX2is an inducible cyclooxygenase that is responsible for the synthesis of several vital regulators related to the initiation and resolution of inflammation[44].B.productaD4 intervention dramatically suppressed the colonicTLR4,NF-κB,iNOS,andCOX2expression in colitis mice,which may be the reason for the decreases in the levels of colonic TNF-α,IL-6,and IL-1β.Meanwhile,the expression of colonicNfr2andHO-1was dramatically elevated.Emerging evidences exhibited that Nfr2 takes a crucial part in the regulation of genes related to detoxifying and antioxidant enzymes[45-46].Nrf2could also regulate the expression ofHO-1,which possesses a broad scope of antioxidant properties and anti-inflammatory functions.
Dysbiosis of intestinal microbiota occurs frequently in patients with colitis and is regarded to contribute to the disease.Previous studies suggested that the relative abundance of Lachnospiraceae NK4A136 group,[Eubacterium]fissicatenagroup andNegativibacilluswas significantly increased in colitis mice[47-50].The relative abundance of [Eubacterium]fissicatenagroup was negatively related to the level of tight junctional proteins(including occluding and ZO-1)[49-50],and the relative abundance ofNegativibacilluswas negatively related to the level of IL-10[51].In this study,B.productaD4 significantly decreased the relative abundance of Lachnospiraceae NK4A136 group,while the relative abundance ofEnterorhabduswas significantly increased afterB.productaD4 intervention.These results suggested thatB.productaD4 intervention could change the intestinal microbiota,which may be related to the relief of colitis.However,the relationship between microbiota and colitis is only based on correlation analysis,lacking definite evidence.Moreover,the relative abundance of [Eubacterium]fissicatenagroup andNegativibacilluswas not significantly changed afterB.productaD4 intervention,though the levels of tight junctional proteins and IL-10 were significantly increased,indicating that there are no direct causal relationships between them.This is inconsistent with previously reported inferences[49-51].Therefore,more research is required to reveal the material basis for functional activities of gut bacteria,rather than still on the correlation analysis.One of the great difficulties faced is the isolation and culture of specific gut bacteria.We believe thatB.productais a potential bacterial species,and we will continue to carry out in-depth research in the future in order to reveal the material basis of its functions.
4.Conclusion
Our results exhibited thatB.productaD4 could relieve colitis by inhibiting the secretion of pro-inflammatory cytokines,reducing oxidative stress,preventing the damage of the intestinal barrier,regulating the TLR4/NF-κB signaling pathway,and reshaping intestinal microbiota.We have discussed the potential probiotics properties ofBlautiabefore and this study provided the first direct evidence ofB.productain protecting against DSS-induced colitis,which may inspire more researchers to investigate the probiotic properties ofBlautia.Nevertheless,other signaling pathways need to be investigated to determine the specific mechanism by whichB.productaD4 ameliorates colitis symptoms.Future studies are also required to further elucidate the metabolite in the gut that could prevent the occurrence and development of inflammation using metabolomics and metagenomics analysis.Moreover,other potential physiological functions ofBlautianeed to be further explored as we reported before.More studies on the isolation of differentBlautiastrains and functional evaluations are required,which may be helpful to accelerate the development ofBlautiainto probiotics.
Conflict of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This work was supported by National Natural Science Foundation of China (31972086,32172173,32072197) and Collaborative Innovation Center of Food Safety and Quality Control in Jiangsu Province.
Appendix A.Supplementary data
Supplementary data associated with this article can be found,in the online version,at http://doi.org/10.26599/FSHW.2022.9250060.
- 食品科学与人类健康(英文)的其它文章
- Protective effects of oleic acid and polyphenols in extra virgin olive oil on cardiovascular diseases
- Diet and physical activity inf luence the composition of gut microbiota,benef it on Alzheimer’s disease
- Inf luence of nitrogen status on fermentation performances ofnon-Saccharomyces yeasts: a review
- Ganoderma lucidum: a comprehensive review of phytochemistry,eff icacy,safety and clinical study
- Resveratrol combats chronic diseases through enhancing mitochondrial quality
- Demonstration of safety characteristics and effects on gut microbiota of Lactobacillus gasseri HMV18

