Lactobacillus plantarum CCFM1180 attenuates obesity induced by estrogen def iciency by activating estrogen receptor alpha in abdominal adipose tissue and regulating gut microbiota-derived metabolites
Qian Chen ,Chunxia Mei ,Min Guo,c,d ,Botao Wang,e ,Haiqin Chen,c,d ,Jianxin Zhao,c,d ,Gang Wang,c,d,,Wei Chen,c
a State Key Laboratory of Food Science and Resources, Jiangnan University, Wuxi 214122, China
b School of Food Science and Technology, Jiangnan University, Wuxi 214122, China
c National Engineering Research Center for Functional Food, Jiangnan University, Wuxi 214122, China
d (Yangzhou) Institute of Food Biotechnology, Jiangnan University, Yangzhou 225004, China
e Bloomage Biotechnology Co., Ltd., Jinan 250000, China
Keywords: Bile acid Gut microbiota Menopause Ovariectomy Short chain fatty acid
ABSTRACT Lipid metabolism disorders commonly occur during menopause.Estrogen deficiency has been shown to lead to excessive energy intake and abnormal lipid metabolism in ovariectomized rats,resulting in obesity.Probiotics exhibit anti-obesity properties,and their underlying mechanism has been widely reported.In this study,we demonstrated the metabolic benef its of Lactobacillus plantarum CCFM1180 in suppressing appetite,controlling body weight,correcting obesity-induced abnormalities,enhancing liver lipid metabolism,and protecting liver function in estrogen-def icient rats.The mechanisms associated with the anti-obesity and antidyslipidemia effects of CCFM1180 on estrogen-def icient rats were clarif ied.The results showed that CCFM1180 dramatically reduced food intake by activating the expression of estrogen receptor alpha (ERα) and increasing the level of leptin in abdominal adipose tissue.These changes,combined with the increased butyrate concentration and recovered bile acid structure,helped enhance lipid metabolism.Additionally,CCFM1180 treatment was found to be safer than exogenous estrogen supplementation.Thus,L.plantarum CCFM1180 could be considered a new therapeutic strategy for preventing and alleviating menopausal lipid abnormalities.
1.Introduction
Estrogens are associated with the regulation of glucose homeostasis and lipid metabolism[1].Changes in the hormonal environment and menopausal transition adversely inf luence metabolic health,leading to conditions such as blood glucose elevation,dyslipidemia,and abdominal fat accumulation[2].A study on Chinese women showed that the body mass index and total body fat percentage increased significantly during the menopausal transition,and postmenopausal women exhibited higher levels of glycosylated hemoglobin A 1c and blood lipid prof ile than premenopausal women[3].Notably,metabolic disorders during menopause promote the incidence and progression of diseases such as hypertension,nonalcoholic fatty liver disease,hyperlipidemia,and diabetes[4-7].Obesity-induced by menopause can be reversed by estrogen replacement therapy,and the prominent role of estrogen receptor alpha in fat accrual and liver metabolism has been recognized[8-10].ERα is important for the regulation of gluconeogenesis and lipid metabolism[11-12].
Probiotics are living microorganisms that confer health benef its to the host when administered in suff icient amounts[13].As a dietary strategy,the introduction of probiotics is gaining increasing attention for the health management of menopausal women as the cancer risks associated with hormone replacement therapy,a traditional treatment for menopause,can be alleviated.Probiotics have been noted to have several health benefits for menopausal women,for instance,in preventing postmenopausal osteoporosis and vaginal infections[14-15].Gut microbiota and host physiology have been noted to be strongly correlated[16].Furthermore,gut microbiota-derived compounds,such as short-chain fatty acids (SCFAs) and bile acids (BAs),have multiple biological functions[17].Probiotics that promote the metabolism of BAs,have been shown to lower cholesterol levels[18].BAs,as the main downstream products of cholesterol catabolism,serve to absorb dietary fats,promote lipid absorption,and signal systemic endocrine functions to regulate energy homeostasis[19].SCFAs,as another metabolite derived from gut microbiota,control body weight and insulin sensitivity by activating free fatty acid receptors and triggering the secretion of anorexia hormones[20].Probiotics,such as strains fromLactobacillusandBifidobacterium,can promote the production of SCFAs[21].
In this study,we examined the influence ofLactobacillus plantarumCCFM1180 on alleviating metabolic disorders induced by estrogen deficiency.To elucidate how CCFM1180 may affect obesity caused by estrogen deficiency,ERα expression in tissues was quantified;its influence on gut microbiota and gut microbiota-derived compounds was also assessed.
2.Materials and methods
2.1 L.plantarum strain
L.plantarumCCFM1180 was prepared as described in a previous study,in which this strain was namedL.plantarum30M5[22].Cell suspensions for animal oral administration were prepared by suspending cultured bacterial cells in sterile skim milk,at final cell densities of 1 × 109CFU/mL.The suspension solution (1 mL) was administered to the rats daily.
2.2 Animal models and sample collection
Twelve-week-old specific pathogen free (SPF)-grade female Sprague Dawley rats (n=24),purchased from SPF Biotechnology Co.,Ltd.(Beijing,China),were randomly divided into four groups(n=6 per group).Rats were housed under standard laboratory conditions and allowedad libitumaccess to standard food and water.One group of rats was subjected to Sham operation (named the Sham-operated group) and treated orally with skim milk (1 mL)every day for 4 weeks from the second week after the surgery.The other groups of rats were subjected to bilateral ovariectomy.After a week of rest after the surgery,the ovariectomized rats were administered the following treatments daily: intraperitoneal injections ofβ-oestradiol (OVX+E2group),oral treatment with skim milk (OVX group),or oral treatment withL.plantarumCCFM1180 (OVX +CCFM1180 group) for 4 weeks.Details of the treatments are shown in Fig.1.All procedures involving rats were performed according to the Experimental Animal Management and Animal Welfare Ethics Committee of Jiangnan University (Protocol number: JN.No 20210330S0720611[045]).The food intake and body weight of the rats were recorded weekly.Fresh faeces were collected,and then the rats were subjected to overnight fasting before being anaesthetised with isoflurane and sacrificed.The serum,abdominal adipose,brain,liver,and contents of the distal ileal and colon were collected and stored at -80 °C.

Fig.1 Animal experiment procedure.β-Oestradiol was dissolved in olive oil and administered according to the body weight of the rats.
2.3 Serum and liver biochemical analysis
The level of serum total cholesterol (TC),triglyceride (TG),highdensity lipoprotein cholesterol (HDL-C),low-density lipoprotein cholesterol (LDL-C),alanine aminotransferase (ALT),aspartate aminotransferase (AST),alkaline phosphatase (ALP),and lactate dehydrogenase (LDH) were measured using an automatic biochemistry analyser (BS-480 Automatic Biochemical Analyzer,Shenzhen Myriad Biomedical Electronic Co.,Ltd.China).The levels of serum circulating peptide YY (PYY),leptin,and interleukin-6 (IL-6)and that of liver CYP7A1 were determined using enzyme-linked immunosorbent assay kits (Nanjing SenBeiJia Biological Technology Co.,Ltd.,Nanjing,China).The concentrations of superoxide dismutase (SOD),malondialdehyde (MDA),and TC in the liver were measured using commercial kits from the Nanjing Jian Cheng Bioengineering Institute.Protein in tissue were measured using kits from Beyotime Biotechnology Co.,Ltd.(Shanghai,China) to quantify the concentrations of other molecules in tissues.
2.4 Expression of ERα and concentration of leptin in abdominal adipose tissue
RNAs in the hypothalamus and abdominal adipose tissue were extracted as previously described[22].The following primers were used for the reverse transcription polymerase chain reaction tests: ERα_F: 5’-CAAACCAATGCACCATCGATAA-3’,ERα_R: 5’-TTTTCGTATCCCGCCTTTCA-3’,Gapdh_F: 5’-CAACGGGAAACCCATCACCA-3’,and Gapdh_R:5’-ACGCCAGTAGACTCCACGACAT-3’.The quantitative expression results were analysed using the 2-ΔΔCtmethod.The levels of hepatic ERα were determined using enzyme-linked immunosorbent assay kits (Jiangsu Meimian Biotechnology Co.,Ltd.,Nanjing,China).Leptin levels in abdominal adipose tissue was measured using commercial kits(Nanjing SenBeiJia Biological Technology Co.,Ltd.).
2.5 BA determination and analysis
BAs were extracted from the terminal ileum contents using the method described by John et al.[23].Specifically,the freezedried contents of the terminal ileum (approximately 50 mg) were weighed and then crushed and dissolved in methanol (1 mL).The supernatant was obtained by centrifugation (15 000 ×g,15 min) and analysed using a liquid chromatography system (Dionex UltiMate 3000,Thermo Fisher,USA) coupled to a mass spectrometry (MS)system (Q Exactive Orbitrap,Thermo Fisher,USA).Samples were separated using ACQUITY UPLC® HSS T3 columns (2.1 × 100 mm,1.8 µm;Waters,Ireland) at 35 °C.Water with 1 mmol/L ammonium acetate was selected as mobile phase A,and methanol with 1 mmol/L ammonium acetate was selected as mobile phase B.The elution conditions were as follows: 1) Phase B: 20% to 60% for 6 min;2) Phase B: 60% to 100% for 19 min;maintained for 100% for 1 min;3) Phase B: 100% to 50% for 2 min;4) Phase B: 50% to 80% for 2 min.The flow rate was 0.3 mL/min.MS detection was performed in the negative mode,and the mass to charge ratio (m/z) ranged fromm/z50 tom/z750 in the full MS scan mode.The BAs were identified through a comparison with the retention times andm/zvalues of standard products.
2.6 SCFA determination and analysis
SCFAs,including acetate,propionate,butyrate,and valeric acid,in the freeze-dried colon contents were detected and analysed according to the previously described methods[24].
2.7 Faecal microbiota analysis
The faecal bacterial DNA was extracted as described in our previous work[25].The alpha diversity was considered to characterise the bacterial communities in each sample,based on the faith_pd,observed_otus,and shannon indices.The beta diversity was examined using the principal co-ordinate analysis (PCoA) plots to represent the faecal microbiota diversity.
2.8 Statistical analysis
The GraphPad Prism version 9.0 software (GraphPad Software Co.,Ltd.,San Diego,CA,USA) was used to perform the statistical analyses and obtain plots.The statistical analyses involved an ordinary one-way analysis of variance with Dunnett’s multiple comparisons test to compare the different experimental groups.The results are presented as means ± standard deviations.Pvalues less than 0.05 corresponded to statistically significant differences.The PCoA and correlation analysis of the fecal microbiota and BAs or SCFAs were performed in R (version 3.6.2).
3.Results
3.1 CCFM1180 controls the body weight, appetite, and hyperlipidemia of ovariectomized rats
Ovariectomy resulted in abnormal energy metabolism.As shown in Fig.2A,exogenous estrogen supplementation in ovariectomized rats significantly decreased the weight gain compared with OVX group.Notably,the weight gain of ovariectomized rats treated with CCFM1180 was similar to that of the Sham-operated group but significantly lower than that of the OVX group.The food intake of all rats was monitored for 4 weeks (Fig.2B).Rats in the OVX group consumed more food than those with adequate sources of oestradiol(Sham-operated and OVX+E2groups).In addition,the food intake of rats in the OVX+CCFM1180 group was significantly lower than that in the OVX group,and their food intake gradually returned to the normal level with the extension of the CCFM1180 intervention.Althoughβ-oestradiol and CCFM1180 treatment slightly increased PYY levels in ovariectomized rats,no significant difference existed between different groups (Fig.2C).As shown in Fig.2D,CCFM1180 effectively increased the serum leptin levels in ovariectomized rats.The concentrations of serum HDL-C,LDL-C,and TC did not exhibit significant differences between the groups (Figs.2E-G).However,CCFM1180 effectively alleviated dyslipidemia in ovariectomized rats by decreasing the TG levels (Fig.2H).As an inflammatory factor associated with metabolic disorders,the levels of serum IL-6 were investigated (Fig.2I).Rats without bilateral ovaries exhibited high concentrations of serum IL-6.Treatment withβ-oestradiol and CCFM1180 significantly decreased the IL-6 concentrations in ovariectomized rats.
When Sabatella, for that was the name of the peasant s wife, saw the little beast, she sighed deeply and said, Even the snakes have their brood; I alone am unfortunate and have no children

Fig.2 Anti-obesity effects of CCFM1180 in ovariectomized rats.(A) Body weight changes during treatment.(B) Daily food intake was reduced with treatment of CCFM1180 for 28 days.(C) The concentration of PYY in serum.(D) The concentration of leptin in serum increased by CCFM1180 treatment.(E-H) Lipid metabolites in serum (HDL-C,LDL-C,TC and TG separately).(I) The concentration of IL-6 in serum significantly decreased by CCFM1180 treatment.Compared with OVX group,*P < 0.05,**P < 0.01,***P < 0.001,****P < 0.000 1.
3.2 CCFM1180 reduces hepatic lipid levels and improves hepatic function in ovariectomized rats
Ovariectomy significantly increased the hepatic lipid levels(Fig.3A).Similar toβ-oestradiol treatment,CCFM1180 treatment decreased the hepatic lipid levels in ovariectomized rats,as indicated by lower levels of liver TC in the CCFM1180 group than those in OVX group.The biochemical markers of liver function,including the AST,ALT,ALP,and LDH levels,were tested.As shown in Figs.3B-E,ovariectomy significantly increased serum ALT,AST,ALP,and LDH concentrations in rats.CCFM1180 treatment considerably decreased the levels of ALT,AST,and LDH in ovariectomized rats.E2-treated ovariectomized rats exhibited significantly decreased ALP and LDH levels.The SOD and MDA levels in the liver were measured to determine the degree of liver oxidative damage.The OVX rats exhibited significantly high levels of SOD and MDA in the liver (Figs.3F and G).In contrast,the SOD and MDA levels of ovariectomized rats treated with CCFM1180 were similar to those of Sham-operated rats.Ovariectomized rats treated withβ-oestradiol exhibited a significantly lower level of MDA in the liver than those not subjected to any treatment.Furthermore,the concentration of hepatic CYP7A1 was completely restored afterβ-oestradiol and CCFM1180 treatment (Fig.3H).
3.3 CCFM1180 increases the expression of ERα and levels of leptin in abdominal adipose tissue
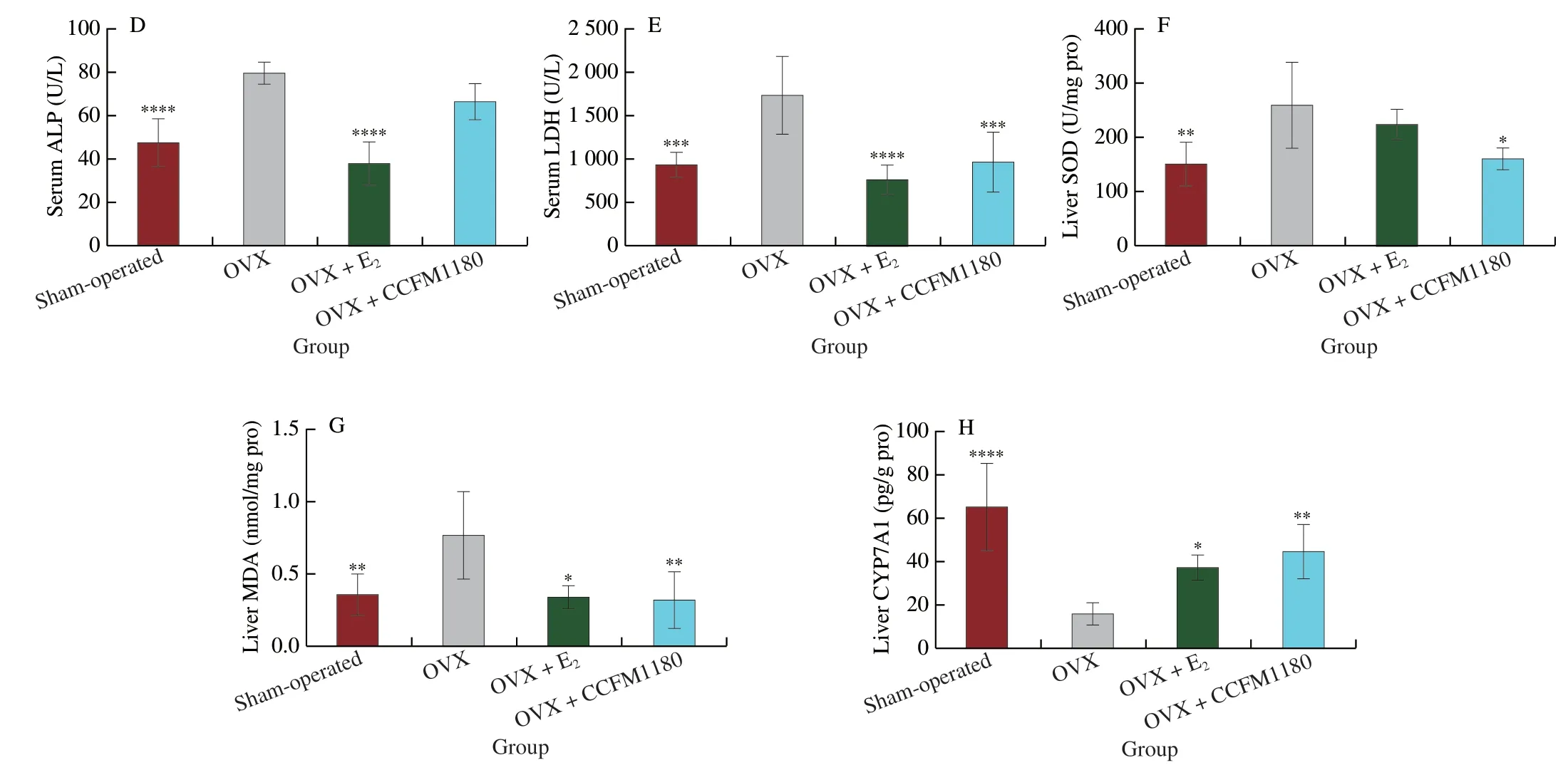
Fig.3 (Continued)
Even in the absence of ovarian function,the expression of ERα in the abdominal adipose tissue of ovariectomized rats was significantly increased by CCFM1180 (Fig.4A).To determine the effects of CCFM1180 on estrogen receptors in other tissues,we evaluated the levels of ERα in the liver and hypothalamus (Figs.4B and C).In contrast to that in the case of abdominal adipose tissue,the ERα levels in the liver and hypothalamus of ovariectomized rats were considerably affected only by the oestradiol treatment and not by the CCFM1180 treatment.The leptin levels in the abdominal adipose of ovariectomized rats were lower than those in Sham-operated rats(Fig.4D).In addition,the leptin levels in abdominal adipose tissue were significantly increased by CCFM1180 treatment.Unlike CCFM1180,β-oestradiol treatment did not considerably influence the leptin levels in abdominal adipose tissue of ovariectomized rats.
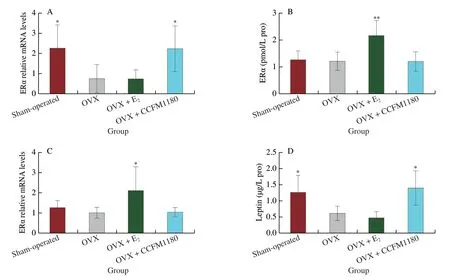
Fig.4 Effect of CCFM1180 on abdominal fat.(A-C) Expression of estrogen receptors in abdominal adipose,liver and hypothalamus,respectively.(D) Leptin in abdominal adipose.Compared with OVX group,*P < 0.05,**P < 0.01.
3.4 CCFM1180 modulates the gut microbiota composition in ovariectomized rats
The faith_pd,shannon,and observed_otu indices indicated that the alpha diversity of the faecal microbiota in ovariectomized rats decreased (Fig.5A).CCFM1180 orβ-oestradiol treatment increased the alpha diversity.According to the results of the UniFrac-weighted PCoA,the gut microbiota communities did not exhibit any distinct cluster between different groups (Fig.5B).CCFM1180 altered the community structure of the fecal microbiota in ovariectomized rats.At the phylum level,CCFM1180 slightly decreased the abundance of Firmicutes and significantly enriched the abundance of Bacteroidetes(Fig.5C).Furthermore,the ratio of Firmicutes to Bacteroidetes (F/B)was significantly enhanced by ovariectomy but evidently decreased byβ-oestradiol and CCFM1180 treatments (Fig.5D).The linear discriminant analysis effective size (LEfSe) was used to identify the taxa that could be used as biomarkers for the dominant microbiome of each group (Fig.5E).At the family level,the relative abundances of Bacteroidaceae,Staphylococcaceae,and Tannerellaceae were comparable between the OVX+CCFM1180 rats and sham-operated rats (Fig.5F).In addition,the CCFM1180-treated group exhibited higher relative abundances of Akkermansiaceae and Streptococcaceae and lower abundances of Erysipelotrichaceae and Desufovibrionaceae than the other groups.At the genus level,the gut microbiota in ovariectomized rats lackedRuminiclostridium6 (Fig.5G).However,this genus reappeared after CCFM1180 andβ-oestradiol treatments.Compared with the other groups,the CCFM1180-treated group exhibited a considerable increase in the abundance ofBacteroides(Fig.5H).Adlercreutziawas the genus which only shown in the CCFM1180-treated groups (Fig.5I).

Fig.5 (Continued)
3.5 CCFM1180 promotes the production of SCFAs in the colon
The SCFA (acetate,propionate,butyrate,and valeric acid) levels in the colonic contents of sham-operated and ovariectomized rats were similar (Fig.6).Compared with rats in OVX group,CCFM1180 andβ-oestradiol treatments promoted the production of the SCFAs.Theβ-oestradiol treatment significantly enhanced the contents of acetate,propionate,butyrate,and valeric acid (Figs.6A-D),while the CCFM1180 treatment considerably increased the butyrate and valeric acid contents (Figs.6C and D).

Fig.6 SCFAs levels in colonic contents.(A) Acetate,(B) propionate,(C) butyrate,(D) valeric acid.Compared with OVX group,*P < 0.05.

Fig.6 (Continued)
3.6 CCFM1180 alters BA metabolism in the distal ileum of ovariectomized rats
Before and after ovariectomy,the secretion of the bile exhibited an obvious increase in the distal ileum of ovariectomized rats(Fig.7).The concentrations of ileal BAs,including unconjugated and conjugated BAs,in the ileum of the rats were elevated postovariectomy (Fig.7A).The proportion of unconjugated BAs in the ileum of ovariectomized rats decreased from 98.17% to 87.8%.After treatment with CCFM1180,the contents of conjugated BAs and total BAs significantly decreased,and the proportion of unconjugated BAs was maintained at 95.99%.In contrast,unconjugated BAs in the OVX+E2group accounted for only 28.72% of the total BA,considerably lower than that in the other groups.In addition,the structure of the BA pools in each group was examined using partial least squares discriminant analysis (PLS-DA) (Fig.7B).The results indicated that the structure of the BA pools in the E2-treated group was significantly different from that in the other groups.In contrast,the structure in the CCFM1180-treated group was similar to that in the sham-operated group.The BA composition of the groups is shown in Fig.7C.The dominant BA in the ileum of sham-operated rats was cholic acid (CA),followed by beta-muricholic acid (β-MCA).Similar to the PLS-DA results,the compositions of BA pools in the OVX and OVX+CCFM1180 groups were similar to those in the shamoperated group.However,a significant difference was observed between the OVX+E2and sham-operated groups.For example,the proportions of CA andβ-MCA were significantly lower and those of chenodeoxycholic acid (CDCA) was considerably higher in OVX+E2group.The specific concentrations of the BAs detected in the ileum are shown in Fig.7D.Compared with Sham-operated group,the concentrations of taurocholic acid (TCA),glycocholic acid (GCA),taurine-beta-muricholic acid (T-β-MCA) and taurohyodeoxycholic acid (THDCA) were significantly increased in ovariectomized rats,which returned to normal levels after CCFM1180 treatment.
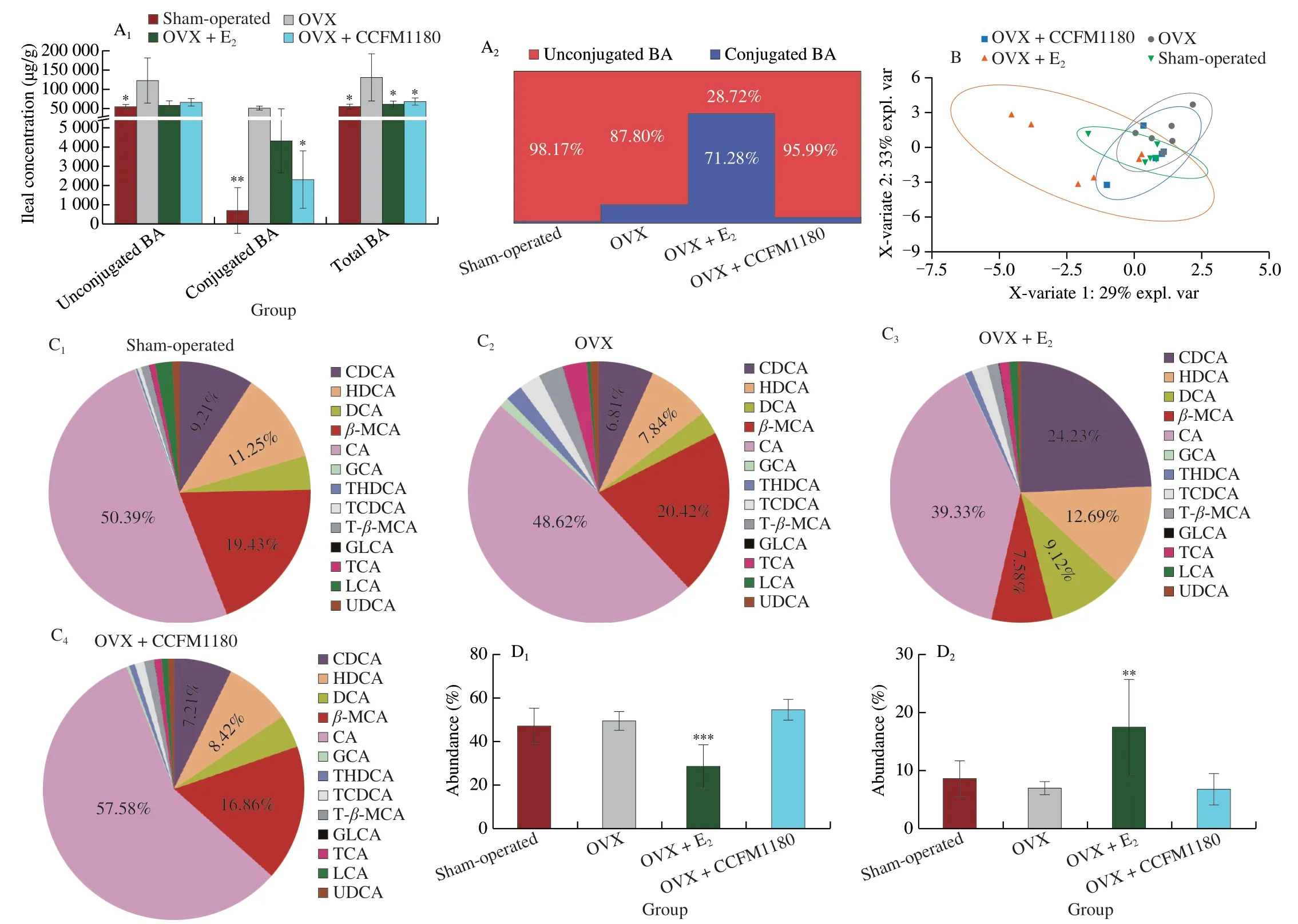
Fig.7 Effects of CCFM1180 on ileal BAs metabolism of ovariectomized rats.(A) BAs concentration in ileal content.(B) PLS_DA analysis.(C) The relative composition of bile acids in different groups.(D1-D8) The abundance of typical bile acids of CA,CDCA,β-MCA,TCA,TCDCA,GCA,DCA,HDCA,T-β-MCA,THDCA.Hyodeoxycholic acid: HDCA,taurochenodeoxycholic acid: TCDCA,deoxycholic acid: DCA,glycolithocholic acid: GLCA,lithocholic acid: LCA.Compared with OVX group,*P < 0.05,**P < 0.01,***P < 0.001.
3.7 Correlation analysis
Fig.8A shows the correlation between the expression of ERα in abdominal adipose tissue and leptin levels (in serum or abdominal adipose tissue).The leptin levels in both serum and abdominal adipose tissue were positively correlated with the levels ofERαmRNA.The leptin levels in abdominal adipose tissue exerted a significantly positive phase effect on serum leptin.A multivariate correlation analysis was performed to describe the directional relationships between the gut microbiota-related metabolites and clinical results (Fig.8B).The correlation heatmap indicated that butyrate was negatively and positively correlated with TG and HDL-C,respectively,while TC in liver was positively correlated with BAs (GCA,TCDCA,T-β-MCA,and TCA).CDCA and acetate were negatively correlated with liver abnormality.
4.Discussion
Obesity is a chronic disease that has attracted widespread concern.Menopause is a notable period in a woman’s life that marks a decline in ovarian function and estrogen production.With the onset of menopause,women tend to experience weight gain and fat accumulation,especially abdominal fat accumulation,owing to metabolic disturbances.Dyslipidaemia is the most common metabolic disorder that occurs during menopause.The results in this study demonstrated that the anti-menopausal obesity and dyslipidemia effects of CCFM1180 are associated with the upregulation of ERα mRNA,increased leptin production in abdominal adipose tissue,and regulation of the microbiota-derived active metabolites (SCFAs and BAs).

Fig.8 Correlation analysis.(A) Correlation between ERα in abdominal adipose and leptin.Pearson correlation coefficient test was used for statistical analysis.(B) Multivariate analysis of bacteria-derived metabolites (bile acids and SCFAs) and clinical indicators.*P < 0.05,**P < 0.01.
In a previous study,we reported that ovariectomy-induced significant weight gain[25].Unlike ovariectomized mice,the weight gain in ovariectomized rats was noted to be associated with increased food intake.Rats and mice exhibit species-specific responses to ovariectomy.Ovariectomy-induced weight gain is mediated by hyperphagia and hypoactivity in rats,whereas it is mainly mediated by hypoactivity and decreased metabolic rate in mice[26].Nevertheless,ovariectomy-induced hyperphagia can be corrected by oestradiol replacement.Oestradiol reduces food intake through upregulated ERα transcriptional activity in the hypothalamus under ad libitum feeding[27].Similarly,our results showed that estrogen supplementation significantly increased the expression of estrogen receptors in the hypothalamus.In addition,CCFM1180 appeared to control the body weight of ovariectomized rats by reducing their food intake.The appetite of ovariectomized rats returned to normal after the CCFM1180 treatment in a time-dependent manner.Unlike the estrogen treatment,CCFM1180 treatment did not inhibit the appetite in ovariectomized rats by stimulating estrogen receptors in the hypothalamus.In general,probiotics can stimulate L cells and increase the secretion of gut hormones such as glucagon-like peptide 1 and PYY[28-29].As the largest secretory organ in the body,the intestine secretes intestinal hormones,which play a significant role in controlling appetite[30].The experimental results showed that CCFM1180 upregulated the level of the ‘anorexia hormone’PYY;however,the degree of its regulation was not significant,and thus,the regulatory effect of PYY was not the main cause of reduced food intake.In our previous study,lactic acid bacteria were noted to promote ERα expression in the abdominal adipose tissue of ovariectomized mice[25].Thus,we measured the ERα expression in the adipose tissue of ovariectomized rats.Expectedly,the ERα expression in the abdominal adipose tissue of ovariectomized rats was also stimulated by CCFM1180.Leptin is a peptide hormone secreted by adipose tissue,and the circulating leptin level reflects energy stores and food intake[31].Yi et al.[32]reported that ERα induced leptin expression.Considering this aspect,we measured the leptin levels both in serum and abdominal adipose tissue.CCFM1180 significantly enhanced the leptin levels in both circulation and adipose tissue.According to the correlation analysis results,the leptin levels in adipocytes were significantly positively correlated with theERαmRNA levels.Moreover,theERαmRNA level was significantly positively correlated with the serum leptin level.Thus,CCFM1180 suppressed the appetite in ovariectomized rats by increasing the secretion of leptin by stimulating the expression of ERα in the abdominal fat.In contrast,β-oestradiol supplementation did not influence the ERα expression in abdominal fat.Estrogen exposure has been noted to upregulate ERα expression in subcutaneous adipocytes in women,although it cannot alter theERαmRNA expression in adipocytes from intra-abdominal origins[33].Another study showed that no effect of 17β-oestradiol was seen on ERα in the visceral adipose tissue of early and late postmenopausal women,whereas 17β-oestradiol treatment decreased the ratio of ERα:ERβ by 20% in adipose tissue from the late postmenopausal women group[34].Therefore,the regulation of estrogen receptors by estrogen supplementation is not only tissue-specific,but may also be related to the type of estrogen receptors.Overall,our results indicate that CCFM1180 inhibits appetite in a different manner than estrogen supplementation.
The metabolic benefits of CCFM1180 in periods with estrogen deficiencies were also reflected in the significant improvement of blood lipid and liver lipid metabolism disorders in ovariectomized rats and inhibition of liver injury.The liver is a notable entity that coordinates the metabolism of fatty acids,triglycerides,and cholesterol to ensure normal physiological metabolism,and this coordination may not be effective in obese individuals due to liver functional damage[35].Estrogen regulates the hepatic glucose homeostasis and hepatic cholesterol output through ERα activity[36].Notably,the ERα expression levels in the ovariectomized rat livers were comparable to those in rats with intact ovaries and increased significantly only afterβ-oestradiol treatment.These results were consistent with those of Mohamed et al.[37].It is worth emphasizing that while CCFM1180 showed a positive effect on ERα in adipose tissue,no similar effect on the ERα in liver were found.In ovariectomized rats,the site of estrogen production shifts from the ovary to many extragonadal sites.Adipose tissue is an important site for extragonadal estrogen synthesis.Estrogen synthesized in adipose tissue is biologically active in a paracrine or secretory manner[38].Therefore,we speculated that CCFM1180 treatment stimulated the synthesis of estrogen in adipose tissue.However,due to the limited total amount of estrogen synthesized by adipose tissue,it can only exert biological effects locally.Thus,unlike estrogen treatment,the metabolic regulation exerted by CCFM1180 in ovariectomized rats was independent of hepatic estrogen receptors.
Lactic acid bacteria can regulate lipid metabolism by modulating the structure and diversity of the gut microbiota[39].The microbiota compositions of obese and lean individuals are different,and reduced microbial richness is often observed in obese individuals[40].CCFM1180 increased the richness of the gut microbiota in ovariectomized rats,which manifested as increased alpha diversity.Increased F/B ratios during the postmenopausal period lead to excess energy storage[41].The F/B ratios in ovariectomized obese rats were regulated by CCFM1180.Although an increase in the F/B ratio is not recommended as an indicator of obesity,the relative abundances of the two major bacterial phyla constituting the majority of the intestinal microbiota differ between lean and obese mice[42-43].Firmicutes have more carbohydrate-metabolizing enzymes than Bacteroides,and thus,a higher abundance of Firmicutes is associated with greater energy harvesting in obese individuals[44].In contrast,a higher abundance ofBacteroidesis positively linked with a loss of body fat[45].Analyses in both Chinese and Korean contexts have demonstrated that the abundance ofg_Bacteroides,belonging to Bacteroidetes,is negatively correlated with the serum TG level,which suggests the benefits of Bacteroidetes to metabolic homeostasis[46-47].The significant increase in the abundance of Bacteroidetes,e.g.,g_Bacteroidesand f_Tannerellaceae,induced by CCFM1180 led to decreased F/B ratios.
In addition to changes in the structure of the gut,changes in the metabolites derived from the gut microbiota,such as the SCFAs and BAs,considerably influence the energy homeostasis of the host.SCFAs,including acetate,propionate,and butyrate,are produced by commensal bacteria fermenting dietary fibers or resistant starches in the colon[48].SCFAs can modulate the host’s energy metabolism during the development of diet-induced obesity[49].Although the SCFAs in the sham-operated and OVX group were similar in our study,the SCFA content drastically increased by estrogen supplementation.This increase likely occurred owing to the more significant intestinal metabolic effects required to increase the metabolism of estrogen[50].CCFM1180 significantly increased the butyrate content in our study,attributable to the change in the gut microbiota induced by CCFM1180.In particular,the SCFAs are enhanced by microorganisms such asRuminiclostridiumthat can degrade polysaccharides to produce butyrate[51-52].The results of the correlation analysis demonstrated a positive association between butyrate and HDL-C levels and a negative association between butyrate and TG levels.Butyrate has been noted to prevent and treat dietary-induced obesity and insulin resistance in mice by increasing adenosine triphosphate consumption and stimulating mitochondrial function[53].Thus,increased butyrate promotes the suppression of lipid disorders caused by estrogen deficiency.BAs are another group of metabolites that influence the microbial-host co-metabolism in the gut[54].BAs originate from the catabolism of cholesterol in the liver and facilitate the absorption and digestion of dietary fats in the gut[55].BAs are normally held in the gallbladder until food intake stimulates their release into the small intestine.Subsequently,most of the BAs are absorbed in the gut[56].In this study,we observed that a large amount of BAs appeared in the ileum of ovariectomized rats owing to overfeeding,likely because high BA concentrations are required to adequately absorb cholesterol and fat from the intestine and facilitate the excretion of certain insoluble substances[57-58].However,the expression of CYP7A1,the only rate-limiting enzyme in BA synthesis,decreased after ovariectomy.This decrease likely occurred because of the BA feedback repression mechanism,through which the liver can efficiently increase or decrease BA synthesis,mediated by the BA-activated nuclear receptor farnesoid X receptor in response to changes in BA levels to maintain a constant pool of BAs[59].Changes in BA homeostasis are expected to affect the liver metabolic homeostasis and lead to liver inflammation and pathogenesis of metabolic diseases[55].Severe liver oxidative stress (MDA and SOD) and abnormal liver function in ovariectomized rats,as well as elevated cholesterol levels in the liver,may be associated with unusual BA synthesis.For example,the OVX group exhibited higher levels of GCA,TCDCA,T-β-MCA,and TCA,which were positively correlated with the liver TC level.The CCFM1180 treatment recovered these changes in the BA composition in ovariectomized rats.The composition of the BA pool in the CCFM1180-treated group was similar to that in the sham-operated group.Consistent with the CCFM1180 treatment,estrogen supplementation achieved appetite suppression,weight control,and lipid metabolism in ovariectomized rats.In contrast,the composition of the BA pool was considerably influenced byβ-oestradiol supplement.Because exogenous estrogen is a key risk factor for cholesterol gallstone formation,long-term use of estrogen in both premenopausal and postmenopausal women is likely to increase the incidence of cholesterol gallstones[60].From this viewpoint,strategies involving probiotics are safer than estrogen supplementation for the treatment of menopausal obesity and lipid metabolism.
5.Conclusion
This study demonstrated the beneficial effects ofL.plantarumCCFM1180 on metabolic disorders caused by estrogen deficiencies.Treatment with CCFM1180 exhibited various metabolic benefits such as appetite suppression,weight control,dyslipidemia alleviation,and reduced liver damage.Different from exogenous estrogen supplementation,CCFM1180 increased the expression of ERα and the secretion of leptin in abdominal adipose tissue,the latter of which could reduce the food intake of ovariectomized rats.The regulation of ERα by CCFM1180 appeared to be tissue-specific as it did not influence the estrogen receptor levels in the liver (protein)and hypothalamus (mRNA).Changes in the gut microbiota and metabolites derived from the gut microbiota (SCFAs and BAs)induced by CCFM1180 are important for enhancing the lipid metabolite content in ovariectomized rats and attenuating estrogen withdrawal-induced blood lipid and liver metabolism disorders.Furthermore,CCFM1180 treatment was found to be safer than estrogen supplementation,especially in terms of its effect on BAs.
Conflicts of interest
Wei Chen is an editorial board member forFood Science and Human Wellnessand was not involved in the editorial review or the decision to publish this article.The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This research was funded by the National Natural Science Foundation of China (31972052,32021005,31820103010),the Fundamental Research Funds for the Central Universities(JUSRP22006,JUSRP51501),the Program of Collaborative Innovation Centre of Food Safety and Quality Control in Jiangsu Province.
- 食品科学与人类健康(英文)的其它文章
- Protective effects of oleic acid and polyphenols in extra virgin olive oil on cardiovascular diseases
- Diet and physical activity inf luence the composition of gut microbiota,benef it on Alzheimer’s disease
- Inf luence of nitrogen status on fermentation performances ofnon-Saccharomyces yeasts: a review
- Ganoderma lucidum: a comprehensive review of phytochemistry,eff icacy,safety and clinical study
- Resveratrol combats chronic diseases through enhancing mitochondrial quality
- Demonstration of safety characteristics and effects on gut microbiota of Lactobacillus gasseri HMV18

