The role of a novel antibacterial substance,cyclic opine-producing Lacticaseibacillus rhamnosus LS8 in ameliorating ulcerative colitis:a fecal microbiota transplantation study
To Wng,Shung Wng,Shuhen Dong,Ruiling Wng,Shuxun Wng,Jie Yng,Xin Wng,,Xin Lü,
a College of Food Science and Engineering, Northwest Agriculture and Forestry University, Yangling 712100, China
b Faculty of Food Science and Engineering, Kunming University of Science and Technology, Kunming 650500, China
c School of Life Science and Technology, Xinjiang University, Urumqi 830046, China
Keywords: Gut barrier Gut microbiota Fecal microbiota transplantation Lacticaseibacillus rhamnosus Oxidative stress Ulcerative colitis
ABSTRACT Intestinal microbiota imbalance may worsen the progression of ulcerative colitis (UC).Lacticaseibacillus rhamnosus LS8 (LR) has the potential ability to regulate microbiota through producing a novel antibacterial substance,cyclic opine: cycloalanopine.This study aimed to investigate whether LR could ameliorate dextran sulfate sodium-induced UC in mice via modulating intestinal microbiota using fecal microbiota transplantation(FMT) experiment.The results showed that both LR and FMT attenuated UC as evidenced by 1) alleviating disease activity index and colonic pathology;2) up-regulating MUCs and tight junction proteins;3) increasing oxidative mediators and decreasing antioxidant mediators;4) down-regulating proinflammatory cytokines and chemokines.These results were mainly attributable to the microbiota-regulating effect of LR,including increasing benef icial bacteria (like Akkermansia) and its related SCFAs,while decreasing harmful bacteria(like Proteobacteria) and its related LPS,thereby suppressing the hyperactivation of TLR4/NF-κB pathway.Consequently,LR can alleviate UC and is a potential dietary supplement to attenuate UC.
1.Introduction
Ulcerative colitis (UC) is one of the inf lammatory diseases that occur in colonic tissue,and its incidence is still increasing in newly industrialized countries[1].The pathological symptoms of UC mainly include weight loss,abdominal pain,persistent diarrhea,and rectal bleeding[2].In addition to genetic predisposition,some other factors,such as intestinal barrier damage,immune disorders,oxidative stress,gut microbiota imbalance,and environmental changes may also exacerbate or even cause the progression of UC[1].Numerous studies have reported that maintaining intestinal microbiota balance,strengthening the intestinal barrier,and inhibiting oxidative stress and inf lammatory responses are promising ways to ameliorate UC[2-3].
The intestinal epithelium is predominantly composed of the microvilli,epithelial cell tight junctions (TJs),and mucus layer.It is an important barrier between the host and commensal bacteria[4].The integrity of the gut barrier is essential for the symbiotic effect of gut microbiota because it can prevent bacteria translocation[1,5].The gut leak may result in exposure of intestinal contents to the host periphery and further lead to an inf lammatory response[6].Numerous studies reported that the gut barrier,especially the expression of TJs,would be blocked in the UC mice[5,7-8].In addition to the intestinal barrier,oxidative stress is also an important factor associated with the pathophysiological process of UC[9-10].The excessive accumulation of reactive oxygen species (ROS) within the immune cells could cause the hyperactivation of the inf lammatory response,and further lead to intestinal tissue damage[11].In addition,the degradation of ROS in the intestinal lumen could cause the accumulation of molecular oxygen and promote the aerobic respiration ofEscherichiacoli,which could in turn exacerbate inflammatory response[12].Therefore,preventing gut barrier damage and inhibiting oxidative stress are potential methods to ameliorate UC.
Nearly 100 trillion microorganisms inhabit in the host’s gastrointestinal tract and play an important role in maintaining health,such as homeostasis,immunity,and metabolism[13].Previous studies indicated that the imbalance of intestinal microbiota may be involved in the occurrence and progression of UC[14-15].Once the occurrence of dysbacteriosis,the relatively high levels of harmful bacteria (e.g.,ClostridiumdifficileandShigellaspecies) or its related harmful substances (e.g.,LPS and microbial toxins) could trigger the inflammatory response and aggravate the progression of UC[15-18].For example,pathogenic bacteria (e.g.,Bacteroidesfragilisand enteropathogenicE.coli) could directly disrupt the integrity of the gut barrier,leading to an increase in intestinal permeability,and further promoting an inflammatory response[19].The increase in Proteobacteria (mostly belonging to LPS-producing bacteria)could cause colonic inflammation and trigger oxidative stress,and in turn exacerbate intestinal microbiota disturbance by promoting the enrichment of facultative anaerobic bacteria[20].In recent years,fecal microbiota transplantation (FMT) has been considered as a potential method to ameliorate UC[21-22].Furthermore,FMT was also an effective way to study the effects of dietary supplement-modulated intestinal microbiota on patients with UC[7,9].
Probiotics are vital components of intestinal microbiota and play an essential role in attenuating inflammatory bowel diseases.Several studies reported that some probiotics could alleviate dextran sulfate sodium (DSS)-induced UC in mice through modulating gut microbiota,such asLactobacillusrhamnosus[7]andLactobacillus paracasei[23].LacticaseibacillusrhamnosusLS8 (LR),previously isolated from homemade fermented milk,is a potential intestinal microbiota-modulating strain as it can secrete a novel small molecule antibacterial substance-cyclic opine: cycloalanopine (4,6-dimethyl-1,2,5-triazepane-3,7-dione,C6H11N3O2,157.09 Da) and inhibit the growth of food-borne and multidrug-resistant pathogens[24].Moreover,LR showed a potential amelioration effect on colitis in mice by preventing the increase of disease activity index (DAI)[25].Nevertheless,it is not clear whether the amelioration effect of LR on colitis is attributable to its modulation of intestinal microbiota.Thus,this study aimed to further investigate the effects of LR-modulated intestinal microbiota on gut barrier integrity,inflammation,and oxidative stress in DSS-induced UC mice via the FMT experiment.The present study could promote the application of LR as a potential dietary supplement to alleviate UC.
2.Materials and methods
2.1 Preparation of LR
The fresh LR (GenBank no.KJ152776) bacterial suspension was prepared by culturing LR in the MRS medium (37 °C,16 h),collecting the bacterial cells by centrifugation (7 500 ×g,5 min),and then resuspended in 0.9% NaCl physiological saline solution (PSS,approximately 5 × 109CFU/mL) after washing twice with sterile PSS.The prepared fresh LR bacterial suspension was immediately administered to mice by gavage within 1 h.
2.2 Animals and experimental design
In this study,the Animal Ethics Committee of Xi’an Jiaotong University approved all animal protocols (permission no.SCXK 2018-001).Eight-week-old C57BL/6 male mice were provided by Hunan SJA Laboratory Animal Co.Ltd.(Changsha,Hunan,China).All mice were housed under controlled environmental conditions with humidity of (55 ± 5)%,temperature of (23 ± 2) °C,and 12 h light/dark cycles.
2.2.1 LR-treated UC mice
Thirty mice were randomly separated into 3 groups (n=10) as follows: healthy control group (CON),DSS-induced UC group (DSS),and LR-treated UC group (DSS+LR).After 1 week of adaptive feeding,the mice were subjected to different treatments according to the protocol shown in Fig.1A.During the days of 1-24,mice in the DSS+LR group were given LR bacterial suspension by oral gavage(200 µL/mouse/day,approximately 109CFU),while mice in the CON and DSS groups were orally supplemented with PSS (200 µL/mouse/day).To establish a UC mouse model,the mice in the DSS and DSS+LR groups were induced with 2.5% DSS (dissolve in drinking water,MW 36-50 kDa,MP Biomedicals,Aurora,OH,USA) during the days of 14-21.All mice were anesthetized after 3 days of recovery by supplementing with normal drinking water.
2.2.2 FMT-treated UC mice
Donor mice:Ten 8-week-old male C57BL/6 mice were divided into DSS and DSS+LR groups (n=5).After the establishment of UC and LR-treated UC mice as described above,mouse fresh fecal samples were collected and immediately preserved in liquid nitrogen until FMT intervention.Before transplantation administration,the fecal samples were processed including homogenization in PSS (feces/PSS=1:10,m/V),removal of solid fecal residues (centrifugation at 2 000 ×gfor 1 min),obtaining bacterial pellets (centrifugation at 12 000 ×gfor 3 min),washing twice in PSS,and then resuspension in PSS to initial volume.The prepared fecal bacteria suspension was immediately administered to recipient mice within 10 min.
Recipient mice:Twenty 8-week-old male C57BL/6 mice were randomly separated into T+DSS and T+LR groups (n=10).The mice in the T+DSS group were given fecal bacteria from the DSS group by oral gavage,while the mice in the T+LR group were given fecal bacteria from the DSS+LR group.The detailed experimental scheme is shown in Fig.S1A.After 1 week of adaptive feeding,all mice were administered multiple antibiotics (0.1 g/L vancomycin,0.2 g/L metronidazole,0.2 g/L neomycin,and 0.2 g/L ampicillin)in drinking water for 2 consecutive weeks.From the days of 14-33,mice in the T+DSS and T+LR groups were treated with FMT.During the days of 28-33,all mice were supplemented with drinking water containing 2% DSS for 5 consecutive days to induce lowgrade UC.
During the days of DSS induction period,mouse DAI was calculated according to the previously proposed standard[25],including fecal occult blood,body weight loss,and fecal consistency.A commercial occult blood kit (Nanjing Jiancheng Technol,Jiangsu,China) was utilized to assess the severity of mouse fecal occult blood.
At the end of mouse feeding,all mice were anesthetized by intraperitoneal injection of xylazine and ketamine (10 and 100 mg/kg,Sigma-Aldrich).Serum samples were collected by centrifuging blood samples at 3 000 ×gfor 10 min and then stored at -80 °C until analysis.Mice thymus and spleen tissues were excised and weighed for organ index analysis (organ weight/body weight).After being excised for length measurement,all colon tissues were washed in ice-cold PSS to remove the contents and separated into two parts for histological assessments (fixed in the 4% paraformaldehyde solution)or biochemical analyses (stored at -80 °C).
2.3 Histological assessment of colonic tissue
After fixing overnight in the 4% paraformaldehyde,mice distal colonic tissues were embedded in the paraffin and stored at 4 °C.For the Alcian blue or hematoxylin and esion (H&E) analyses,all colonic blocks were sliced into 5 µm thickness and stained with the relevant reagents.A microscope from Olympus Corporation (Shinjuku,Tokyo,Japan) was used to visualize and photograph all stained areas.The goblet cells in Alcian blue-stained areas were counted by Image J(National Institutes of Health,Bethesda,MD,USA).
2.4 Biochemical parameters analysis
The homogenized supernatants of colon tissues were prepared as described previously[26].The levels of inflammatory cytokines(IL-6,IL-1β,and TNF-α) and serum lipopolysaccharides (LPS)were measured by the relevant ELISA kits (Jingmei Biotech,Yancheng,Jiangsu,China).The levels of myeloperoxidase (MPO),malondialdehyde (MDA),reduced glutathione (GSH),superoxide dismutase (SOD),and total mercapto (T-SH) were measured using the commercial kits provided by Nanjing Jiancheng Technol.
2.5 mRNA expression analysis
The colonic tissues were processed including total RNA extraction,cDNA synthesis,and target genes quantification according to the method described previously[27].The relative mRNA levels of target genes were normalized to GAPDH using the 2-ΔΔCtmethod[28].The primer sequences used for qPCR analyses are shown in Table S1.
2.6 Protein expression analysis
2.6.1 Immunofluorescence
The protein levels of TJs,including ZO-1,occludin,and claudin-1,were quantified by immunofluorescence as the method described previously[22].Except for the primary antibody of ZO-1(catalog no.PB9234),which was purchased from the Wuhan Boster Biological Technology (Hubei,China),other antibodies including occludin (catalog no.WL01996),claudin-1 (catalog no.WL03073) and FITC-conjugated secondary antibody (green,catalog no.WLA032) were provided by Wanleibio (Shenyang,Liaoning,China).
2.6.2 Western blot
The expressions of NF-κB and TLR4 were analyzed by western blot according to the method described previously[25].The antibodies used in this study were purchased from Wanleibio,including primary antibodies (NF-κB p65,catalog no.WL01980;TLR4,catalog no.WL00196;andβ-actin,catalog no.WL01372) and HRP-conjugated secondary antibody (catalog no.WLA024).
2.7 Gut microbiota analysis
The total bacterial DNA was extracted from mice cecal contents using a PowerSoil DNA isolation kit (Mo Bio Laboratories,Carlsbad,CA,USA).The V3-V4 hypervariable regions of bacterial 16S rRNA genes were amplified by PCR using the universal primers of 338F,5’-ACTCCTACGGGAGGCAGCA-3’ and 806R,5’-GGACTACHVGGGTWTCTAAT-3’.The purified and pooled PCR products were conducted next-generation sequencing using the Illumina HiSeq 2500 platform (Illumina Inc.,San Diego,CA,USA).All sequencing data were analyzed and visualized at the Biomarker Cloud platform (http://www.biocloud.net/).The detailed analytical procedures were referred to the methods described previously[27].
2.8 Short-chain fatty acids (SCFAs) analysis
The levels of SCFAs in mice fecal samples were detected by gas chromatography (GC) according to the method described previously[27].Briefly,fecal samples were homogenized in distilled water,acidified with 50% H2SO4,extracted by diethyl ether,and then analyzed by GC.
2.9 Statistical analysis
Data were presented as the mean ± standard deviation (SD).Graphpad Prism 8 (GraphPad Software Inc.,San Diego,CA,USA)was used for significance testing and plotting.Significant differences between two groups (T+DSS and T+LR) were evaluated by Student’st-test or between three groups (CON,DSS,and DSS+LR)were evaluated by ANOVA,followed by Tukey’s test for multiple comparisons.AP-value < 0.05 was statistically significant.The relationships between gut microbiota and UC-related parameters,or between gut microbiota-related substances (LPS and SCFAs) and UCrelated parameters were analyzed by calculating the Pearson’s correlation coefficients using RStudio software (version 2021.09.2+382).
3.Results
3.1 Effects of LR on the pathological symptoms in DSSinduced UC mice
From Fig.1B,there was no significant difference in water consumption containing 2.5% DSS between DSS and DSS+LR groups.Compared with the CON group,DSS induction led to severe UC-related pathological symptoms,such as body weight loss(Fig.1C),increased DAI (Fig.1D),colon length shortening (Figs.1E and F),increased spleen index and decreased thymus index (Fig.1G).However,treatment with LR ameliorated all these adverse changes(Figs.1C-G).In addition,H&E staining of colonic tissues showed that the DSS-induced UC mice had obvious crypt loss,inflammatory cells infiltration,and intestinal mucosal damage,while LR-treated mice showed attenuation of these alterations and had relatively intact colon structure (Fig.1H).
Similarly,the results of FMT also suggested that the UC-related pathological symptoms in the T+LR group were lighter than those in the T+DSS group,mainly including body weight loss (Fig.S1B),increased DAI (Fig.S1C),colon length shortening (Fig.S1D and E)and decreased thymus index (Fig.S1F).H&E staining of colonic tissues also indicated that the colon structure in the T+LR group was relatively intact and they had less crypt loss and inflammatory cell infiltration (Fig.S1G).Taken together,all results showed that LR and LR-modulated intestinal microbiota could alleviate the UC-related pathological symptoms in mice.
3.2 Effects of LR on the intestinal barrier integrity in DSSinduced UC mice
The results of Alcian blue staining suggested that compared to the CON group,DSS induction resulted in a significant loss of goblet cells,while LR supplementation prevented this adverse change(Figs.2A and B).Compared with the healthy mice,the mRNA levels ofMUC2,MUC3,andTff3in the UC mice were significantly decreased but significantly reversed in the LR-treated mice (Fig.2D).Immunofluorescence analysis of colonic tissues suggested that the expressions of TJs (ZO-1,occludin,and claudin-1) in the DSS group were significantly decreased compared with the CON group,but ameliorated in the DSS+LR group (Figs.2F and G).
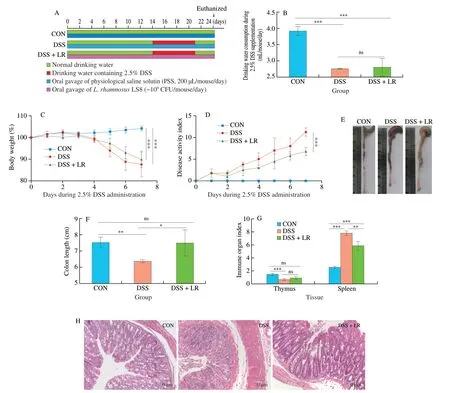
Fig.1 LR supplementation alleviated the pathological symptoms in UC mice.(A) LR supplementation experimental scheme;(B) daily drinking water consumption,(C) body weight and (D) DAI score during 2.5% induction;(E) representative macroscopic images of colonic tissue;(F) colon length;(G) immune organ indexes,including thymus and spleen tissues;(H) representative H&E-stained images of colonic tissue,scale bars,50 µm.Data in B-G (n=8) and H (n=3)are expressed as mean ± SD.Significant differences between different groups were evaluated by Tukey’s test for multiple comparisons.*P < 0.05,**P < 0.01 and***P < 0.001;while ns indicates no statistical significance.
Similarly,FMT results verified that the changes in the intestinal barrier showed a consistent trend (Fig.S2).Briefly,the intestinal barrier in the T+LR group was superior to the T+DSS group,as evidenced by more goblet cells (Fig.S2A and B),higher mRNA levels ofMUC2,MUC3,andTff3(Figs.S2C-E),and higher protein levels of ZO-1,occludin,and claudin-1 (Fig.S2F) in the T+LR group.Therefore,treatment with LR and LR-modulated intestinal microbiota could alleviate the intestinal barrier damage in DSSinduced UC mice.
3.3 Effects of LR on the inflammatory response in DSSinduced UC mice
Compared with the healthy mice,DSS induction increased the mRNA levels of proinflammatory cytokines (e.g.,TNF-α,IL-1β,IL-6,andIL-17a) and C-X-C chemokine receptor type 2 (CXCR2) ligands chemokines (e.g.,Cxcl1,Cxcl2,Cxcl3,Cxcl5,andCcl7),while these alterations were all alleviated in the LR-treated mice (Figs.3A and B).Consistent with the changes in mRNA,the protein levels of TNF-α,IL-1β and IL-6 measured by ELISA were also significantly increased in the DSS group and ameliorated in the DSS+LR group (Figs.3C-E).Western bolt results showed that DSS administration caused an overexpression of the TLR4/NF-κB pathway,as manifested by the high levels of TLR4 and NF-κB in the DSS group than those in the CON group.However,these adverse changes were prevented by LR supplementation (Figs.3F and G).
FMT results suggested that the mRNA or protein levels of these proinflammatory cytokines and CXCR2 ligands chemokines mentioned above in the T+DSS group were all significantly higher than those in the T+LR group,except for the mRNA levels ofIL-1βandCxcl5,which were not significantly different between the T+DSS and T+LR groups (Figs.3H-L).Moreover,Western bolt results also showed that supplementation of the gut microbiota from LRtreated UC mice could prevent the overexpression of TLR4/NF-κB as compared with the T+DSS group (Figs.3M and N).Therefore,LR and LR-regulated gut microbiota could ameliorate DSS-induced colonic inflammation,which might be related to the suppression of the TLR4/NF-κB pathway.
3.4 Effects of LR on the oxidative stress response in DSS-induced UC mice
From Fig.4,compared with the healthy mice,DSS administration resulted in an increase in oxidative mediators (serum MDA and MPO)and a decrease in antioxidant mediators (GSH,SOD,and T-SH),while these adverse alterations were all significantly ameliorated in the LR-treated mice (Figs.4A-E).Similarly,FMT results also showed that the T+LR group had lower levels of oxidative mediators(serum MDA and MPO) and higher levels of antioxidant mediators(GSH,SOD,and T-SH) than those in the T+DSS group (Figs.4F-J).Therefore,treatment with LR and LR-modulated intestinal microbiota could attenuate the oxidative stress response in the DSS-induced UC mice.
3.5 Effects of LR on the intestinal microbiota in DSS-induced UC mice
Compared with the healthy mice,DSS induction increased Shannon and Simpson indexes,while these changes were reversed in the LRtreated mice (Figs.S3C and D).Moreover,ACE and Chao indexes in the LR-treated mice were all lower than those in the UC mice(Figs.S3A and B).However,the alpha diversity between T+DSS and T+LR groups had no significant difference (Figs.S3A-D).The UPGMA clustering tree results showed that there were significant differences between different treatment groups,indicating that the beta diversities of gut microbiota among different groups were different (Fig.S3E).The results of PCoA based on the Bray-Curtis distance also showed significant differences in the beta diversity of intestinal microbiota between different groups,as evidenced by the obvious clustering separation between different groups (Fig.S3F).
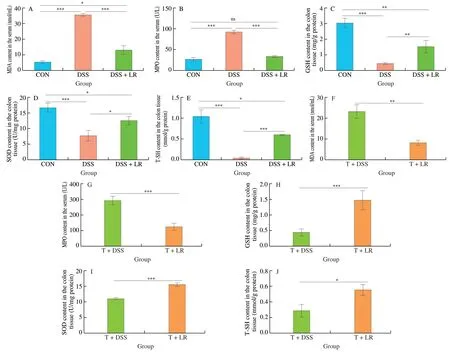
Fig.4 LR and FMT relieved the oxidative stress response in UC mice.Levels of serum (A and F) MDA and (B and G) MPO;Levels of colonic (C and H) GSH,(D and I) SOD,and (E and J) T-SH.Data are expressed as mean ± SD (n=5);Significant differences between two groups (T+DSS and T+LR) were evaluated by Student’s t-test or between 3 groups (CON,DSS,and DSS+LR) were evaluated by Tukey’s test for multiple comparisons.*P < 0.05,**P < 0.01 and***P < 0.001;while ns indicates no statistical significance.
The relative abundances of intestinal microbiota at the phylum level showed that DSS induction led to an increase in Proteobacteria and Firmicutes/Bacteroidetes,and a decrease in Verrucomicrobia,while the changes of Proteobacteria and Verrucomicrobia were all significantly reversed in the LR-treated mice (Figs.S3G-J).However,these phyla were not significantly different between the T+DSS and T+LR groups (Figs.S3G-J).In the genus level analysis (top 50,Table S2 and Fig.S4),results showed that compared with the healthy mice,there were 16 genera were increased and 10 genera were decreased in the DSS-induced UC mice.After treatment with LR,10 DSS-increased genera (ASF356,Desulfovibrio,Parabacteroides,Ruminococcaceae_UCG-004,Rikenellaceae_RC9_gut_group,Ruminiclostridium,Ruminococcaceae_UCG-005,Ruminiclostridium_6,uncultured_bacterium_f_Clostridiales_vadinBB60_group,and uncultured_bacterium_f_Desulfovibrionaceae),and 2 DSS-decreased genera (Akkermansiaand Ruminococcaceae_UCG-014) were significantly reversed.Among these 12 significant reversal genera,Desulfovibrio,Parabacteroides,Ruminiclostridium_6,Akkermansia,and Ruminococcaceae_UCG-014 had a relatively higher abundance in the gut microbiota,ranking in the top 30 (Figs.5A-F).The results of FMT showed that compared with the T+DSS group,the relative abundances of 8 genera (Alistipes,Enterorhabdus,Faecalibaculum,Family_XIII_AD3011_group,Ruminococcaceae_UCG-005,[Eubacterium]_nodatum_group,Parasutterella,and uncultured_bacterium_o_Mollicutes_RF39) were higher,while 2 genera (Dubosiellaand Ruminococcaceae_UCG-013)were lower in the T+DSS group (Figs.5A-F,Table S2,and Fig.S4).
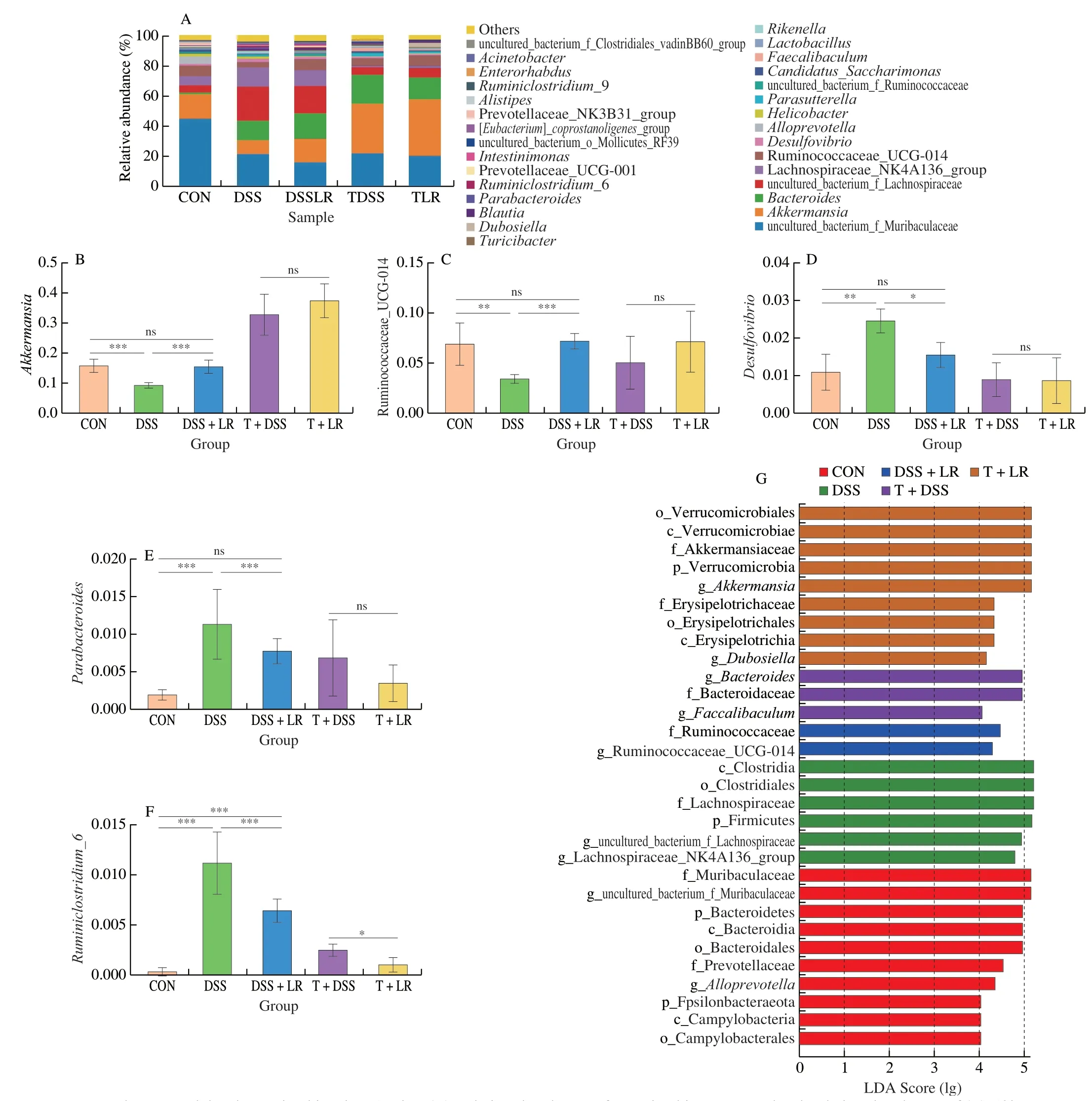
Fig.5 LR and FMT modulated gut microbiota in UC mice.(A) Relative abundances of gut microbiota at genus level;relative abundances of (B) Akkermansia,(C) Ruminococcaceae_UCG-014,(D) Desulfovibrio,(E) Parabacteroides,and (F) Ruminiclostridium_6;(G) LEfSe score plot,only the taxa with LDA score > 4.0 are shown.Data in B-F are expressed as mean ± SD (n=6);Significant differences between two groups (T+DSS and T+LR) were evaluated by Student’s t-test or between 3 groups (CON,DSS,and DSS+LR) were evaluated by Tukey’s test for multiple comparisons.*P < 0.05,**P < 0.01 and ***P < 0.001;while ns indicates no statistical significance.
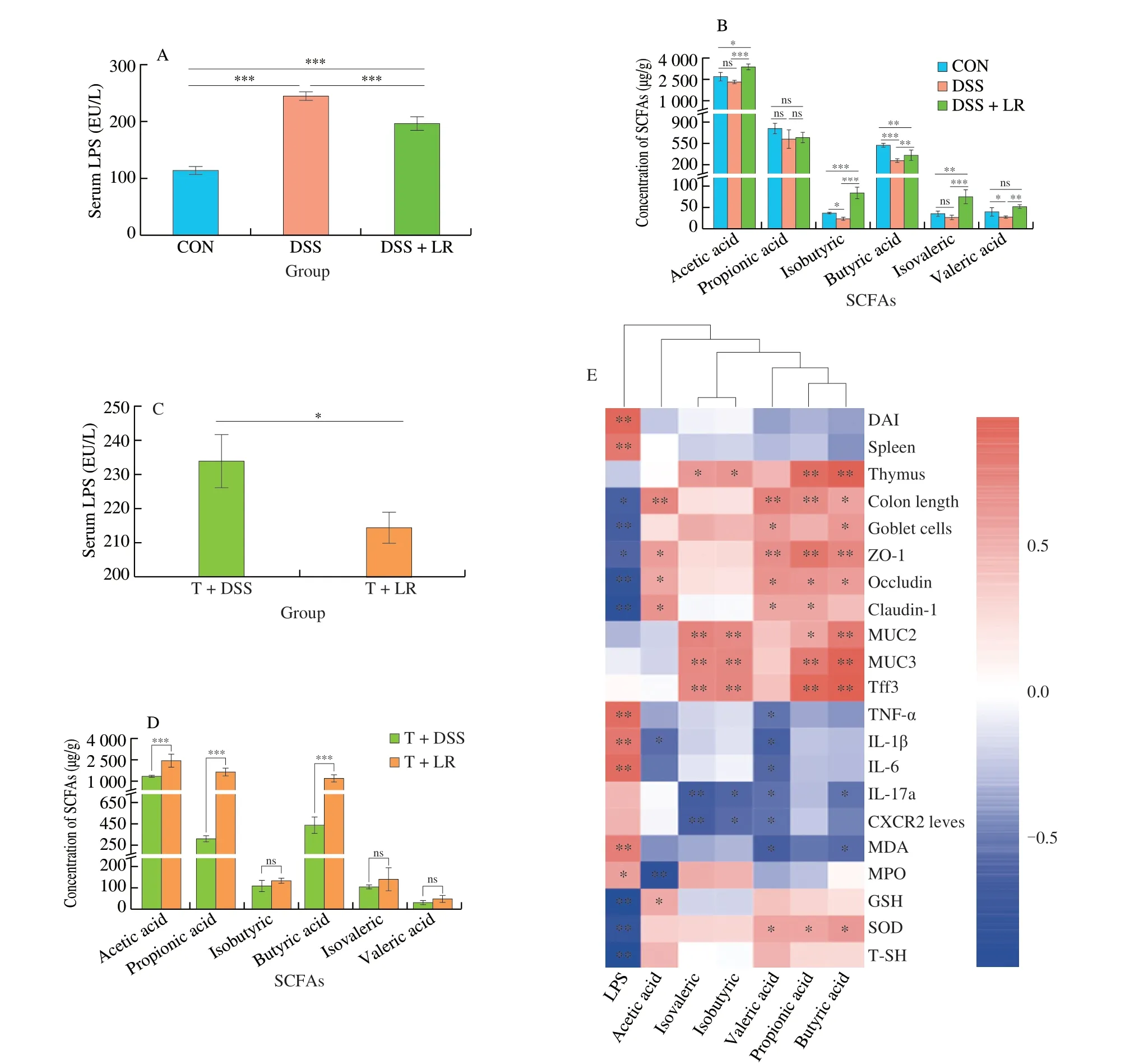
Fig.6 LR and FMT reversed abnormal serum LPS and fecal SCFAs levels in UC mice.(A and C) serum LPS;(B and D) fecal SCFAs;(E) the relationship between the gut bacteria-derived substances (LPS and SCFAs) and UC-related parameters.Data in A-D are expressed as mean ± SD (n=5);Significant differences between two groups (T+DSS and T+LR) were evaluated by Student’s t-test or between three groups (CON,DSS,and DSS+LR) were evaluated by Tukey’s test for multiple comparisons.*P < 0.05,**P < 0.01 and ***P < 0.001;while ns indicates no statistical significance.
LEfSe analysis (Fig.5G) showed that,from the family to the genus levels,2 families (Muribaculaceae and Prevotellaceae) and 2 genera (Alloprevotellaand uncultured_bacterium_f_Muribaculaceae)were the biomarkers in the CON group;1 family (Lachnospiraceae)and 2 genera (uncultured_bacterium_f_Lanchnospiraceae and Lanchnospiraceae_NK4A136_group) were the biomarkers in the DSS group;family Ruminococcaceae and genus Ruminococcaceae_UCG_014 were the biomarkers in the DSS+LR group;family Bacteroidaceae and genusBacteroideswere the biomarkers in the T+DSS group;2 families (Akkermansiaceae and Erysipelotrichaceae)and 2 genera (AkkermansiaandDubosiella) were the biomarkers in the T+LR group.
3.6 Effects of LR on the intestinal microbiota-related substances (LPS and SCFAs) in DSS-induced UC mice
The serum LPS level in the UC mice was higher than in the healthy mice,while it was decreased in the LR-treated mice (Fig.6A).The results of SCFAs analyses showed that DSS administration led to a significant decrease in fecal SCFAs,especially isobutyric,butyric,and valeric acids as compared to the healthy mice (Fig.6B).However,these changes were all reversed after treatment with LR,and the levels of acetic acid,isobutyric,and isovaleric in the LR-treated mice were even higher than those in the healthy control mice (Fig.6B).In the FMT experiment,results showed that the serum LPS level in the T+LR group was lower than that in the T+DSS group,but the levels of acetic,propionic,and butyric acids were higher in the T+LR group than those in the T+DSS group (Figs.6C and D).Taken together,treatment with LR and LR-modulated intestinal microbiota prevented the increase of serum LPS and the decrease of fecal SCFAs in DSS-induced UC mice.
In addition,the relationships between these two intestinal microbiota-derived substances (LPS and SCFAs) and UC parameters were summarized in Fig.6E.The level of serum LPS is positively correlated with the parameters that could exacerbate the progression of UC (e.g.,DAI,spleen index,TNF-α,IL-1β,IL-6,MDA,and MPO),but negatively correlated with the parameters that could ameliorate the development of UC (e.g.,colon length,goblet cells,TJs,GSH,SOD,and T-SH).Oppositely,SCFAs,especially propionic,butyric,and valeric acids,were partially positively correlated with strengthening the gut barrier (e.g.,goblet cells,TJs,MUC2,MUC3,and Tff3) and SOD,but partially negatively correlated with inflammation (TNF-α,IL-1β,IL-6,IL-17a,and CXCR2) and MDA.These results indicated that serum LPS could aggravate inflammation,gut barrier damage,and oxidative damage,while SCFAs could ameliorate these pathological symptoms,especially the gut barrier damage and inflammation.
3.7 Relationship between intestinal microbiota and UC parameters
The potential relationships between intestinal microbiota at the genus level (relative abundance at top 50) and UC-related parameters(mainly including DAI,colon length,gut barrier,inflammation,oxidative stress,and SCFAs) were analyzed by calculating Pearson’s correlations.The heatmap of Pearson’s correlation suggested that the gut bacteria were mainly clustered into nine groups (Fig.S5).The bacteria in groups I-IV,especially in groups II-IV,were partially positively associated with the parameters that could exacerbate UC,including DAI,TNF-α,IL-1β,IL-6,IL-17a,CXCR2,LPS,MDA,and MPO.But these bacteria were partially negatively associated with the parameters that could attenuate UC,including colon length,GSH,SOD,T-SH,and SCFAs (especially propionic,butyric,and valeric acids).The bacteria in groups V and VI had almost no significant correlation with UC parameters,except some bacteria in group VI were positively correlated with isobutyric,isovaleric,valeric,and acetic acids,but negatively correlated with propionic acid.Contrary to the genera in groups II-IV,the bacteria in groups VII and IX were partially negatively associated with DAI,inflammation,and oxidative mediators,but partially positively associated with colon length,antioxidant mediators,and SCFAs.In addition,the bacteria in group VIII also had no significant correlation with UC parameters,except the genus of uncultured_bacterium_f_Ruminococcaceae was positively correlated with colon length,isovaleric,isobutyric,acetic,and valeric acids,but negatively correlated with IL-1β,IL-17a,CXCR2,and MPO.These results indicated that most bacteria in groups I-IV might be aggravating UC,while most bacteria in groups VII and IX might be ameliorating UC.
4.Discussion
Many studies have reported that the imbalance of intestinal microbiota may worsen the pathological symptoms of UC[7,9].LR was a potential intestinal microbiota-modulating strain as it can secrete a novel antibacterial substance (cyclic opine: cycloalanopine),which had an inhibitory effect on both food-borne and multidrug-resistant pathogens[24].Our preliminary experiments also showed that LR had a certain degree of UC attenuating effect on the DSS-induced UC mice[25].In this study,the FMT experiment was used to investigate whether the amelioration effect of LR on UC was attributable to its modulation of the intestinal microbiota.The results indicated that both LR and LR-regulated gut microbiota possessed a superior UC ameliorating effect because both interventions could attenuate increased DAI,shortened colon length,abnormal immune organs indexes,gut barrier damage,oxidative stress,and inflammatory response.These results were partially consistent with the intervention effects ofL.rhamnosusSHA113[7]andCompanilactobacillus crustorumMN047[22].However,LR improved the pathological symptoms of UC better than these two reported probiotics.Taken together,these results indicated that LR could ameliorate UC and the main mechanism of LR against UC was partially attributed to its regulation of the intestinal microbiota.
The intestinal microbiota is essential for the host to maintain gut homeostasis because it can interact with the host’s immune systems[29].Many studies reported that the imbalance of intestinal microbiota could exacerbate the progression of colitis and its related diseases[7,9],and FTM was a promising method to ameliorate these gut microbiota dysbiosis-related diseases[21].Since LR secreted a novel antibacterial substance,cyclic opine: cycloalanopine[24],it had the potential to regulate gut microbiota.Results showed that treatment with LR significantly prevented the DSS-induced gut microbiota dysbiosis,as evidenced by preventing 10 DSS-increased genera (e.g.,ASF356,Desulfovibrio,Parabacteroides,Rikenellaceae_RC9_gut_group,Ruminiclostridium,andRuminiclostridium_6) and 2 DSSdecreased genera (Akkermansiaand Ruminococcaceae_UCG-014).The DSS-increased genera (e.g.,Desulfovibrio,Parabacteroides,andRuminiclostridium) might be positively associated with inflammation[2,21,30],which was also consistent with the results of the relationships between gut microbiota and UC-related parameters(Pearson’s correlation coefficients analysis).On the contrary,the DSS-decreased genera (e.g.,Akkermansiaand Ruminococcaceae_UCG-014) are generally considered beneficial bacteria because they can produce SCFAs[31].In addition,although Lachnospiraceae is a family that also could produce SCFAs,the relative abundance of Lachnospiraceae in the UC mice was higher than that in the healthy and LR-treated mice,which was consistent with the previous findings that this family was higher in the UC patients than the healthy individual[32].Since LR can produce bacteriostatic metabolites (such as cyclic opine and organic acid),it should be emphasized that these antimicrobial substances may also adversely affect the growth of some beneficial bacteria while inhibiting the growth of some harmful bacteria.However,from our experimental results,it appears that the dietary intervention of LR has more advantages than disadvantages for the host’s gut microbiota.
Consistent with the relatively high abundances of SCFAs-producing bacteria (e.g.,Akkermansiaand Ruminococcaceae_UCG-014)in the LR-treated group,the levels of SCFAs (especially acetic and butyric acids) in this group were also higher than those in the DSS group.SCFAs,especially butyric acid,were important for maintaining the host’s health,such as modulating the adaptive immune system,enhancing gut barrier function,inhibiting inflammation,and killing microbial pathogens[31].A prior study reported that butyric acid could attenuate UC via upregulating milk fat globule-EGF factor 8[33],and the butyric acid level in fecal samples was recognized as a potential biomarker to assess the treatment efficacy of FMT in UC mice[30].In this study,the relatively high SCFAs levels (especially butyric acid)in the LR and T+LR groups were corresponding to a better UC ameliorating effect.However,the relationship between gut microbiota and host health is complex.In addition to SCFAs,some other metabolites(such as pro-/anti-inflammatory metabolites) may also be involved in the process of relieving or exacerbating colitis.To further exploit these potential metabolites that may be related to UC,we can use multiple omics techniques,such as metabolome and proteome to mine them,and then supplement or target modulate them to relieve colitis.
On the contrary,as another important gut microbiota-derived substance,LPS is detrimental to human health.LPS is an important activator of TLR4,which could trigger the overactivation of TLR4/NF-κB[34].The high serum LPS level was one of the potential diagnostic markers for inflammation,intestinal barrier damage,and gut microbiota dysbiosis[14].Previous studies suggested that LPS could stimulate intestinal epithelial cells to produce cyclooxygenase-2 and prostaglandin E2,and promote monocyte-like macrophages to secrete IL-1β,thereby exacerbating colonic inflammatory response[35-36].It was found that the serum LPS level in DSS-induced UC mice was higher than in healthy mice[3,37].Similarly,in the present study,DSS induction also resulted in abnormally elevated LPS levels,which might be partly due to the relatively high abundance of Proteobacteria in the UC mice.The serum LPS level in the LR and LR-regulated gut microbiota treatment groups was lower than that in the DSS and T+DSS groups,which was also corresponding to the suppression of the TLR4/NF-κB pathway in the DSS+LR and T+LR groups.Therefore,treatment with LR could ameliorate gut microbiota dysbiosis,which could further increase SCFAs and decrease LPS levels,thereby alleviating inflammation by inhibiting the overexpression of TLR4/NF-κB.Although it can be speculated that the main mechanism of LR against UC was the regulation of gut microbiota and inhibition of microbial pathogens and/or LPS-induced TLR4/NF-κB pathway,it should be emphasized that the expression of phosphorylated NF-κB also needs to be measured to further evaluate the status of the NF-κB pathway.
The pathophysiology of UC was multifactorial[1].In addition to dysbacteriosis,some other factors (e.g.,gut barrier damage,oxidative stress,and inflammation) may also exacerbate the progression of UC[3,10,37].The gut barrier was essential for us to maintain intestinal health,because it not only ensured the selective permeability of nutrients,but also prevented the unrestricted entry of proinflammatory stimuli into the lamina propria or even the circulatory system[1,5].Once the intestinal barrier was damaged (e.g.,the down-expression of TJs,and MUCs),a leaky gut would increase the risk of gut microbiota translocation and further trigger inflammation[6].Some gut microbiota (e.g.,BacteroidesfragilisandEnterococcusfaecalis)and bacteria-related harmful substances (e.g.,LPS and bacterial toxin) could also affect intestinal barrier function and cause inflammation[17-18].Oppositely,Akkermansiacould promote goblet cells to secrete more mucins and further enhance the gut barrier function[38].In this study,LR administration significantly ameliorated the damage of the intestinal barrier in the DSS-induced UC mice,because it prevented goblet cell loss and promoted the expression of ZO-1,occludin,claudin-1,MUCs,and Tff3.These results were partly similar to some previous studies that treatment with probiotics could alleviate gut barrier damage caused by DSS[7-8].Moreover,this study also suggested that the relatively high abundance ofAkkermansiain the LR-treated mice corresponded with the high goblet cell numbers and MUCs expressions.Therefore,LR could alleviate DSS-induced gut barrier damage,which might be partly responsible for its UC ameliorating effect.
Oxidative stress in the colonic tissue would also exacerbate the development of UC[3].The excessive accumulation of ROS within the immune cells could aggravate the inflammatory response and further led to tissue damage[11].The high level of MPO in the UC mice suggested increased neutrophil infiltration,which was associated with gut barrier damage and inflammation[8].In this study,LR supplementation showed an attenuation effect on the DSSinduced oxidative damage,as evidenced by decreasing the level of oxidative mediators (MDA and MPO) and increasing the level of antioxidant mediators (GSH,SOD,and T-SH).These results were partly consistent with the previous results[39].Furthermore,the oxidative stress response in colonic tissue could also exacerbate or even cause the dysbiosis of gut microbiota because it could select for the bacteria that prefer to survive in the microaerobic condition,like Proteobacteria[40].Similarly,in this study,the severe oxidative stress response in the UC mice was accompanied by a relatively high abundance of Proteobacteria.Therefore,treatment with LR could ameliorate the oxidative stress caused by DSS.
The inflammatory response was the main symptom of UC,and preventing colonic inflammation was the most direct way to relieve UC.In this study,treatment with LR ameliorated the severe inflammatory response caused by DSS,such as preventing abnormal elevations of proinflammatory cytokines (e.g.,TNF-α,IL-1β,and IL-6)and CXCR2 ligands chemokines (e.g.,Cxcl1,Cxcl2,Cxcl3,Cxcl5,and Ccl7).The high expressions of these cytokines in the colonic tissue could lead to the recruitment and infiltration of inflammatory cells in inflamed intestinal mucosa tissue[41].Therefore,LR administration could attenuate DSS-induced colonic inflammation.
Based on the FMT experiment,this study suggested that supplementation of LR could attenuate the pathological symptoms of UC induced by DSS via modulating gut microbiota.LR administration could increase the relative abundances of beneficial bacteria (e.g.,Akkermansiaand Ruminococcaceae_UCG-014)and decrease the relative abundances of harmful bacteria (e.g.,Proteobacteria,Desulfovibrio,andRuminiclostridium),which further increased fecal SCFAs levels and lowed serum LPS levels,thereby inhibiting the overexpression of TLR4/NF-κB pathway.In addition,strengthening the gut barrier,ameliorating oxidative stress,and inhibiting abnormally elevated inflammatory response were also conducive to its against UC.Although LR had the potential to prevent or alleviate UC in the DSS-induced mouse model,its clinical effects still need further study.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This research was supported by the National Natural Science Foundation of China (32001652),Chinese Universities Scientific Fund (2452018062),and Keypoint Research and Invention Program of Shannxi Province (2021ZDLNY05-06).
Appendix A.Supplementary data
Supplementary data associated with this article can be found,in the online version,at http://doi.org/10.26599/FSHW.2022.9250066.
- 食品科学与人类健康(英文)的其它文章
- Protective effects of oleic acid and polyphenols in extra virgin olive oil on cardiovascular diseases
- Diet and physical activity inf luence the composition of gut microbiota,benef it on Alzheimer’s disease
- Inf luence of nitrogen status on fermentation performances ofnon-Saccharomyces yeasts: a review
- Ganoderma lucidum: a comprehensive review of phytochemistry,eff icacy,safety and clinical study
- Resveratrol combats chronic diseases through enhancing mitochondrial quality
- Demonstration of safety characteristics and effects on gut microbiota of Lactobacillus gasseri HMV18

