Comparative study of the effects of Tartary buckwheat seed and sprout consumption on the physiological indices and gut microbiota of C57BL/6J mice
Guohui Nn,Hixi Zho,Qiong Wu,Lisong Liu,Ziho Gun,Chenglei Li,Hul Wu,Dbing Xing,Qi Wu,
a College of Life Science, Sichuan Agricultural University, Ya'an 625014, China
b Faculty of Quality Management and Inspection &Quarantine, Yibin University, Yibin 644000, China
c College of Pharmacy and Biological Engineering, Chengdu University, Chengdu 610106, China
Keywords: Tartary buckwheat Tartary buckwheat sprouts Antinutritional factors Gut microbiota Health effect
ABSTRACT Tartary buckwheat (Fagopyrum tataricum) is a well-known pseudocereal for its health and economic value.However,abundant antinutritional factors (ANFs) reduces its health benefits.As reported,germination can improve the nutritional prof ile of grains.In this study,we systematically evaluate the safety of Tartary buckwheat seeds (TB) and Tartary buckwheat sprouts (TBS) used as high active ingredients.After evaluating nutrition levels,bioactive compounds and ANFs in TBS during germinating,5th-day TBS were selected as the raw material.C57BL/6J mice were gavaged daily with distilled water,TB,or TBS for 6 weeks.The physiological indices related to ANFs were determined.Results showed that the TB intake tends to generate negative effects on the gut microbiota,and organs.Additionally,upon TB intake,the Fe3+ content in serum,trypsin activity in pancreas and jejunum decreased,while the cytokine,IgE,and histamine levels in serum,water content in faeces,cytokine levels in liver and jejunum increased.Conversely,TBS did not induce any obvious negative effects on the above relevant indices and showed better lipid-lowering effect.Altogether,TBS are safer and more effective as a raw material to produce the functional food for long-term consumption with the intention of preventing and treating hyperlipidaemia.
1.Introduction
Tartary buckwheat (Fagopyrum tataricumGaertn,TB) belongs to the Polygonaceae family,which is a traditional pseudo-grain and mainly cultivated in the Himalayas region and east Asia[1-2].Compared to other grains,TB is rich in various nutrients,such as starch,proteins,vitamins,f ibre,and minerals including iron,zinc,and selenium[1,3-4].Importantly,TB contains numerous bioactive components,including phenolic compounds (rutin,quercetin) andγ-aminobutyric acid (GABA)[5].Besides,TB has been reported to function in preventing and treating cardiovascular and cerebrovascular diseases (CVD)[6].Animal experiments have shown that TB flavonoids significantly reduced triglyceride (TG) levels in hyperlipidemic mice,TB polyphenols and proteins could prevent hardening of the blood vessels,and TB anthocyanins enabled to prevent the deposition of lipid peroxides in blood vessels[7-10].Hence,TB has been widely used as long-term usage of nutritional supplement for people who have the prevention demand from the CVD.
H owever,TB contain antinutritional factors (ANFs),that can cause health problems such as allergies and diarrhoea,especially to sensitive people.Tr ypsin inhibitor (TIs),ph ytic acid (PA),and allergens are the major anti-nutritional factors[11-12].As reported,TIs can reduce protein digestibility,cause pancreatic hypertrophy,decrease pancreatic trypsin activity and change intestinal tissue,while PA can inhibit the absorption of most minerals[13-14].Allergen proteins are commonly found in grains and most potent allergens are thermally stable,possibly causing adverse effects[15-16].Thus,allergen proteins are also regarded as ANFs.Research shows that allergen proteins in buckwheat can lead to increased levels of antibodies and cytokines in serum[17].
Studies have demonstrated that germination as a biochemical technique can effectively improve nutrition profile in cereals[18-19].The variation trend of nutrients in TB during germinating has also been reported.After germination,the nutrient contents in TB,such as proteins,amino acids,reducing sugar,vitamin C (VC),and vitamin B are constantly accumulating[2].Meanwhile,the active ingredients,including rutin,quercetin,GABA as well as total antioxidant capacity in TB sprouts (TBS) were significantly higher than those in the TB[2,20].Importantly,a decrease in ANFs,including TIs,PA and allergens,in germinated cereals has been elucidated[21-23].Therefore,TBS seems to be more abundant in active bioingredients than TB and safer for long-term consumption.
However,studies to compare and evaluate the health effects of TB and TBSin vivostill need to be accomplished.To more fully and safely utilize TB as a functional food resource,this study systematically evaluated the effects of TB and TBS on health.The nutrition and active ingredients in the germination process of TB were detected to identify the change trend of main ANFs and determine the appropriate germination time.Then,the health effects of TB and TBS were compared through the evaluation of physiological indices in mice.Additionally,results related to TIs,PA and allergens,including trypsin activity,microelements,immunoglobulin E (IgE) and other indicators,were discussed.Moreover,changes in the intestinal microorganisms of mice were also evaluated.
2.Materials and methods
2.1 Materials
‘Mi Qiao No.1’,a variety of TB was used in this study.The original seeds were the latest harvest and obtained in Ya’an City,Sichuan Province,China.The latest harvest seeds were dried and stored at room temperature.
2.2 Cultivation of TBS
The TB seeds were cleaned and soaked in 42 °C distilled water for 3 h.Then,they were evenly spread on a seedling plate which was constructed with two layers,the upper layer for seeding and the lower layer for storing distilled water.The soaked seeds were covered with a layer of filter paper and incubated at 25 °C in the dark for 2 days.On the 3rdday,the filter paper was removed and the seeds were incubated in a greenhouse (25 °C,16 h light and 8 h darkness) for 6 days.During germination,the seeds was kept moist using distilled water.The germinated TB were harvested daily from the first day of pre-soaking and immediately dried in a vacuum-freeze drier.Finally,TB marked as 0 and TBS marked as 1-8 were dehulled and ground into powder.The powder of TB and TBS were respectively sifted through a 60-mesh sieve and stored at -80 °C for further use.
2.3 Determination of biochemical indices of germinated TB
2.3.1 Observation and measurement of the growth index
From the first day after planting,10 TBS were collected for capturing pictures,and measuring the length and weight each day.
To visualize the microscopic morphology of TBS powder,0.1 mg TBS powder was uniformly pasted on carbon conductive tape to create a sample table.The sample table was placed into a gold spraying machine (Hitachi MC1000,15 mA,100 s) for gold spraying,following the visualization with a scanning electron microscope(HITACHI UHR FE SEM SU8020,SEM).
2.3.2 Determination of biochemical indices in TBS
To confirm the appropriate time with respect to nutrient levels and activity changes in the germination process of TB,we first detected the contents of main nutrients.The content of water-soluble protein was detected by Coomassie bright blue G250 assay[24],while the content of water-soluble amino acids was detected by the colorimetric method of Ninhydrin[25].Additionally,the direct spectrophotometry was used for determining the VC contents[26].The contents of total sugar and reducing sugar were detected by 3,5-dinitrosalicylic acid colourimetry[27].The content of crude fiber was determined using the apparatus Hanon F800 Fiber Analyzer (YiKe Technology,China).
Subsequently,we detected bioactive components and antioxidant capacity.The total flavonoids (TF) were extracted with 80% ethanol and detected by sodium nitrite-aluminium nitrate colourimetric method[28].The GABA content of the TBS powder was detected using the GABA assay kit of Shanghai Enzyme-linked Biotechnology Co.,Ltd.,following with the instructions.The total antioxidant capacity and superoxide dismutase activity were detected using the Total Antioxidant Capacity Assay Kit (FRAP method) and Total Superoxide Dismutase (T-SOD) Assay Kit (Hydroxylamine method) of Nanjing Jiancheng Bioengineering Research Institute (China) according to the method described in manual instructions.DPPH radical scavenging rate of TBSP was carried out according to the method described by previous studies[29].
2.3.3 Determination of rutin and quercetin contents
Rutin and quercetin contents in TBS were analysed using quantitative high-performance liquid chromatography (HPLC,1260 Infinity II system,Agilent,California,USA) fitted with a C18column(250 mm × 4.6 mm,5 μm;RStech,Korea)[30].Water containing 0.2% phosphoric acid (A) and acetonitrile (B) were used as a gradient mixture solvent.The solvent programme was as follows:the proportion of solvent B at 0 min was 10%,40% at 10 min,60%at 18 min,and 10% at 20 min,which was pumped at a flow rate of 1 mL/min.The UV detector was operated at 290 nm.Compounds were identified and quantified by a comparison with authentic standards.
2.3.4 Evaluation of trypsin inhibitor activity and determination of phytic acid
The TBS powder (200 mg) and TB powder (200 mg) were ground with 5.0 mL distilled water.The homogenate was centrifuged at 4 000 r/min for 10 min,and the residue suspended with 2.0 mL distilled water was centrifuged again at the same speed and time.The volume of combined supernatant was constant to 10 mL.To detect the TI activity,we determined the activity of trypsin catalysing the substrate benzoyl-DL-argininep-nitroanilide (BAPNA) by measuring the amount ofp-nitroanilide originated from BAPNA via its spectrophotometric reading at 410 nm[31].
The PA was extracted for 2 h from TBS powder (500 mg) and TB powder (500 mg) with 10.0 mL 1.2% HCl and 10% Na2SO4.After centrifugation at 4 000 r/min for 30 min,the supernatant was placed at 4 °C for 12 h.Then,the extract was mixed with 15%trichloroacetic acid (TCA) at ratio of 1:1 and kept in 4 °C for 2 h.The mixture was centrifuged at 4 000 r/min for 30 min,and the PH of the obtained supernatant was adjusted to 6.0-6.5 with 0.75 mol/L NaOH.Subsequently,this extract was diluted with distilled water to 30.0 mL,from which 3.0 mL was taken and mixed with 1.0 mL 0.03% FeCl3and 1.0 mL 0.3% sulfosalicylic acid to prepare the analysis sample.The absorbance of this analysis sample was measured at 410 nm.PA content in TB and TBS powder was calculated according to the PA standard curve,respectively[32].
2.3.5 Determination of the expression level of ANFs genes in TBS
Total mRNA was extracted from TBS grown for 0-8 days using RNAout kit (Tianze,China),and cDNA was synthesized for gene expression analysis using RevertAid™ First Strand cDNA Synthesis Kit (MBI,USA) with an oligo (dT) 18 primer,according to the methods described in the instructions.The gene sequences ofTB10(JK729379.1),TB16(GO496294.1),TB22(AY044918.1),TBa(DQ849083.1),TBb(DQ849083.1) andTIs(FtTI,KC417045.1) were downloaded from the National Center for Biotechnology Information Search database (NCBI).The gene sequence of phytase (FtPAP,FtPinG0007957900.01.T01) was obtained from the TB genome.The specific primers (Table S1) were designed for qPCR.qPCR was performed using the histone gene H3 (HM628903) as an internal reference,and results were calculated by the 2-ΔΔctmethod.
2.4 Animals and the experiment in vivo
2.4.1 Animals
Four-week-old male C57BL/6J mice were purchased from the Laboratory Animal Centre,Sichuan University (Chengdu,China) with ethical approval from the Ethical Committee for Animal Experiments,Sichuan Agricultural University,and maintained following the Guide for the Care and Use of Laboratory Animals (NIH Publication No.8023,revised 1978).Four mice were housed in each stainless-steel cage in a room with a 14/10-h light/dark cycle,(20 ± 1) °C and(40 ± 10)% relative humidity (RH).The mice were fed a standard commercial pelleted grower diet (Slac Laboratory Animal Co.,Ltd.,Shanghai,China),and distilled water was provided byadlibitum.
2.4.2 Experimental design
The TBS was germinated for 5 days and TB was ground into powder using the method introduced in section 2.2.The TBS and TB powder were suspended with distilled water in a mass to volume ratio of 1:4,respectively.The suspension was stored at -80 °C for later use.After a 7-day adaptation period under laboratory conditions,the animals were randomly divided into three groups with 12 animals in each and fed a standard diet for 6 weeks.The major nutrients were shown in Table S2.Daily oral gavage was performed to create the following experiment groups with 300.0 µL of corresponding diets per mouse: the CON group with distilled water,the TB group with TB powder,and the TBS group with TBS powder.During this period,food and water intake as well as body weight were recorded weekly.On the day before the end of the experiment,the animals were subjected to overnight fasting,and fresh faeces from each group were collected after gavage for 30 min.At the end of the experiment,the mice were euthanized with isoflurane after fasting and gavaged in the same way as the day before.The liver,pancreas,spleen,kidneys,and intestine were weighed,respectively,continuing with a calculation of the organ weight/body weight as the organ index.Serum was collected by cardiac puncture for further analysis.Partial pancreatic and jejunal content were collected for determining trypsin activity.Additionally,partial liver and jejunal samples were collected for RNA isolation.The above samples were immediately immersed in liquid nitrogen and stored at -80 °C.Partial liver,pancreatic,duodenal,jejunal and ileal samples were collected and immersed in 4% paraformaldehyde solution for histopathological examination.
2.4.3 Serum index analysis
Total cholesterol (TC),triglycerides (TGs),low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C) were quantified from serum lipids following the standard protocols mentioned in the kits (Jiancheng Bioengineering Co.,Nanjing,China).IgE,histamine (His),interleukin-6 (IL-6) and interleukin-10 (IL-10) were detected in serum using commercial reagent kits purchased from Enzyme-linked Biotechnology Co.,Ltd.(Shanghai,China) in terms of the standard protocols provided.Serum trace elements including K+,Ca2+,Mg2+,and Fe3+,were determined by a biochemical analyser.The metal element digestibility was evaluated with a value obtained from the calculation (value=the trace element content in percentage/the metal element content in the feed).
2.4.4 Trypsin activity determination
The pre-treatment of pancreatic and jejunal contents had been described in previous studies[33-34].Lyophilized pancreata were homogenized in cold physiological saline and centrifuged at 1 100 ×gfor 20 min.Trypsinogen in the supernatant were activated by mixing equal volumes of 2% enterokinase (in 0.04 mol/L Tris containing 0.01 mol/L CaCl2,pH 8.1) and incubating at 37 °C for 90 min.Jejunal contents were collected by flushing with cold physiological saline (5.0 mL).The trypsin activity was detected by using commercial reagent kits bought from Jiancheng Bioengineering Co.(China),following the manufacturer’s recommendations.
2.4.5 Determination of gene expression levels of inflammatory cytokines
Total mRNA was extracted from the liver and jejunum using an RNAout kit (Tianze,China),and cDNA was synthesized with a RevertAid™ First Strand cDNA Synthesis Kit (MBI,USA).The gene sequences of interleukin-4 (IL-4,ID: M25892.1),IL-6(ID:DQ788722.1),IL-10(ID: NM_010548.2) and tumour necrosis factor alpha (TNF-α,ID: M11731.1) were downloaded from NCBI.Specific primers were designed for qPCR (Table S1).qPCR was performed using the 18S rRNA gene as an internal reference,and the results were calculated by the 2-ΔΔCtmethod.
2.4.6 Histopathological examination
For histopathological examination,the harvested liver,pancreatic,duodenal,jejunal and ileal samples were immediately fixed in 4%paraformaldehyde and processed using a series of fractionated ethanol solutions.Next,the samples were embedded in paraffin,cut into 5 μm thick sections,stained with haematoxylin and eosin,and observed under a digital triocular camera microscope (Motic China Group Co.,Ltd.,Xiamen,China) for routine morphological evaluation at 10× and 40× magnification.Villus height,crypt depth and muscle layer thickness in the duodenal,jejunal and ileal samples were measured on three pictures of each sample.
2.4.7 DNA extraction,and 16S rRNA gene sequencing
16S rRNA sequencing (including DNA extraction and PCR)was subcontracted to Biomarker Technologies Co.,Ltd.(Beijing,China).The V3 and V4 regions of the 16S rRNA were amplified by PCR using composite-specific bacterial primers (Table S2).High-throughput pyrosequencing of the PCR products was performed on an Illumina MiSeq platform at Biomarker Technologies.The raw paired-end reads from the original DNA fragments were merged using FLASH and assigned to each sample according to unique barcodes[35].After denoising and quality filtering,the resulting dereplicated sequences were termed amplicon sequence variants (ASVs)[36].A rarefaction curve was calculated based on the relative proportion of each ASV.Forα-diversity analysis,the Shannon diversity index and Faith_pd estimator were calculated using Quantitative Insights Into Microbial Ecology (QIIME 2) software (https://qiime2.org/).Forβ-diversity analysis,weighted and unweighted UniFrac distance matrices were calculated using Mothur and visualized by principal coordinate analysis (PCoA) with R statistical software.Differences in gut microbiota compositions among the three groups were analysed by linear discriminant analysis effect size (LEfSe) and one-way analysis of variance (ANOVA).Bacteria taxa that were significantly altered by different treatments could be considered as a biomarker taxa.
2.5 Statistical analysis
All determinations were performed in 6 replicates,which were selected randomly from each group,and the results were shown as the mean ± standard deviation (SD).The statistical analysis was performed using Statistical Product and Service Solutions (SPSS,version 26).Differences between groups were statistically analysed using ANOVA followed by Tukey’s multiple comparison tests and unpairedt-test.The differences were considered statistically significant at a level ofP< 0.05.
3.Results
3.1 Macromorphology and micromorphology changes in TB during germination
During the germination period,the biomass and morphology of TBS changed (Figs.S1A-C).Its hypocotyl became longer and its colour got red from the third germination day.With the development of cotyledons,the seed coat easily fell off,and green cotyledons can be observed from the 4thday.
To understand micro-morphological variations in TBS powder,the electron microscopy scanning was performed.The pulverization degree of lyophilized powder increased,but the particle size decreased over germination time (Figs.1A-E).Moreover,starch granules were tightly clustered under the adhesion of proteins (Fig.1F).As the germination time increased,the starch granules became dispersed and their proportions decreased,whereas the proportions of other plant structures increased.Further analysis showed that the starch granules were tightly arranged with regular and round shapes (Fig.1K).With increasing germination time,the starch granules were perforated on the surface,and their shapes became irregular as their size increased(Figs.1L-O).These visible changes suggest that TBS is easier to be digested and absorbed than TB.
3.2 The 5th-day TBS with high bioactive ingredients and low ANFs
To determine the appropriate germination time,we examined the variation trends of nutrients,bioactive ingredients and ANFs in TB over germination process.After germinating for 8 days,the contents of total sugar decreased by 30.0%,while the reducing sugar and cellulose contents increased 7.88 and 5.60 folds,respectively(Fig.S2A).Similarly,the water-soluble protein content was decreased by 30.0%,whereas the amino acid content was increased 0.04 folds(Fig.S2B).The contents of VC and flavonoids increased to 14.26 and 46.7 mg/g in the 5-day-old sprouts and then gradually reached the stabilization phase,whose tendency was closely associated with the antioxidant activity (Figs.S2D,S2E,and Table S3).Additionally,the GABA content was increased to 29.4 mg/g in sprouts on the 8thday(Fig.S2C).For ANFs,TI activity and the PA content in the 8-day-old sprouts were decreased by 42% and 58.1%,respectively.However,the contents of ANFs were not completely eliminated (Figs.S3A,S3B).qRT-PCR analysis showed that the highest expression level of the phytase-associated gene presented on the 3rdday of sprouting.Differently,the allergen-responding genes respectively encoding 10,16,22,24 and 34 kDa proteins were mainly expressed in seeds(Figs.S3C-H).Notably,theTIgene was not expressed in sprouts.The significance analysis suggested that nutrients,active ingredients and antioxidant capacity of TBS exhibited the rapidest change on the 4th-5thday of germination (Table S4).
The changes in nutrients can be inferred to improve bioavailability,while the increase in active substances improves the health effect,and the decrease in ANFs reduces the health risk.Given the various trends of physical and chemical indices,TB germinated for 4-5 days can be potentially developed into corresponding products.Therefore,the freeze-dried powder was prepared using 5-day germinated TBS as the food resource for gavage treatment of mice.
3.3 Effects of TB and TBS on the body weight, organ indices,and food and water intake of mice
Throughout the experimental period,the mice from all groups showed normal behaviour and activity.Food and water intake were similar among all groups (Table 1).However,the body weight and organ indices of mice in the TB and TBS groups altered compared to those of the CON group after feeding for 6 weeks.The body weight of the mice in the TB and TBS groups were lower than those in the CON group and the lightest mice were from the TBS group.Besides,the liver index of mice in the TB and TBS groups was significantly increased.Compared to the CON and TBS groups,the mice from the TB group had significantly higher index of the pancreas and spleen.Furthermore,the bowel index of mice in TB and TBS groups showed a significant increase relative to that in the CON group.Significantly,this bowel index in the mice of TBS group was much higher than that in the TB group.The kidney index of mice in the TB group was significantly higher than that in the CON group.

Table 1 Effects of TB and TBS on food and water intake,body weight,organ indices,and serum indices*.
3.4 Impacts of TB and TBS on serum lipids, inflammatory factors and microelements
As shown in Table 1,serum indices can be adjusted through TB and TBS intake.Compared to the CON group,TB intake had little effect on the TC,TG,HDL-C and LDL-C contents.In contrast,the TC and LDL-C contents in TBS group decreased by 13.0% and 26.3%,respectively.The TG contents in the TBS group were not significantly lower than those in the CON group,while it significantly decreased about 15.9% when compared to the TB group.Additionally,the contents of inflammatory factors including IL-6,IL-10 and IgE were significantly upregulated by 28.4%,35.6%,and 8.4% in the TB group,respectively.However,the IL-6,IL-10 and IgE in the TBS group remained normal,while the His content was significantly downregulated about 18.2%.Furthermore,the absorption of microelements such as K+,Ca2+and Fe3+was inhibited in the TB group but not in the TBS mice group (Tables 1 and S5).
3.5 Effects of TB and TBS on liver inflammatory factor and histopathological changes
To clarify the effects of TB and TBS intake on the liver,the histopathological changes and liver inflammatory factor genes were examined (Fig.2).Results showed that the liver cells of mice in the TB group was exhibited vacuolar degeneration (Figs.2B and E),while the CON (Figs.2A and D) and TBS groups (Figs.2C and F)showed regular histological structures.Histopathological changes in the liver sections of mice were correlated with changes in liver inflammatory factors in each group.As shown in Figs.2G-J,the gene expression levels ofIL-4,IL-6,IL-10andTNF-αwere significantly increased in the TB and TBS groups,whereas these gene expression in the TBS group were relatively lower.
3.6 Analysis of TB and TBS influences on pancreas trypsin activity and histopathology
Since the pancreas is an important digestive gland in the digestive system,its histopathological changes and trypsin activity were also analysed (Fig.3).In the TB group,the interstitium of the pancreas widened,oedema was obvious,and the pancreatic acinus and epithelial cells showed slight degeneration and necrosis (Figs.3B and E).Differently,only epithelial cells of the mice in the TBS group were slightly degraded and necrotic (Fig.3F).In addition,the trypsin activity in mice from the TB group was significantly inhibited compared to that in the CON and TBS groups (Fig.3G).
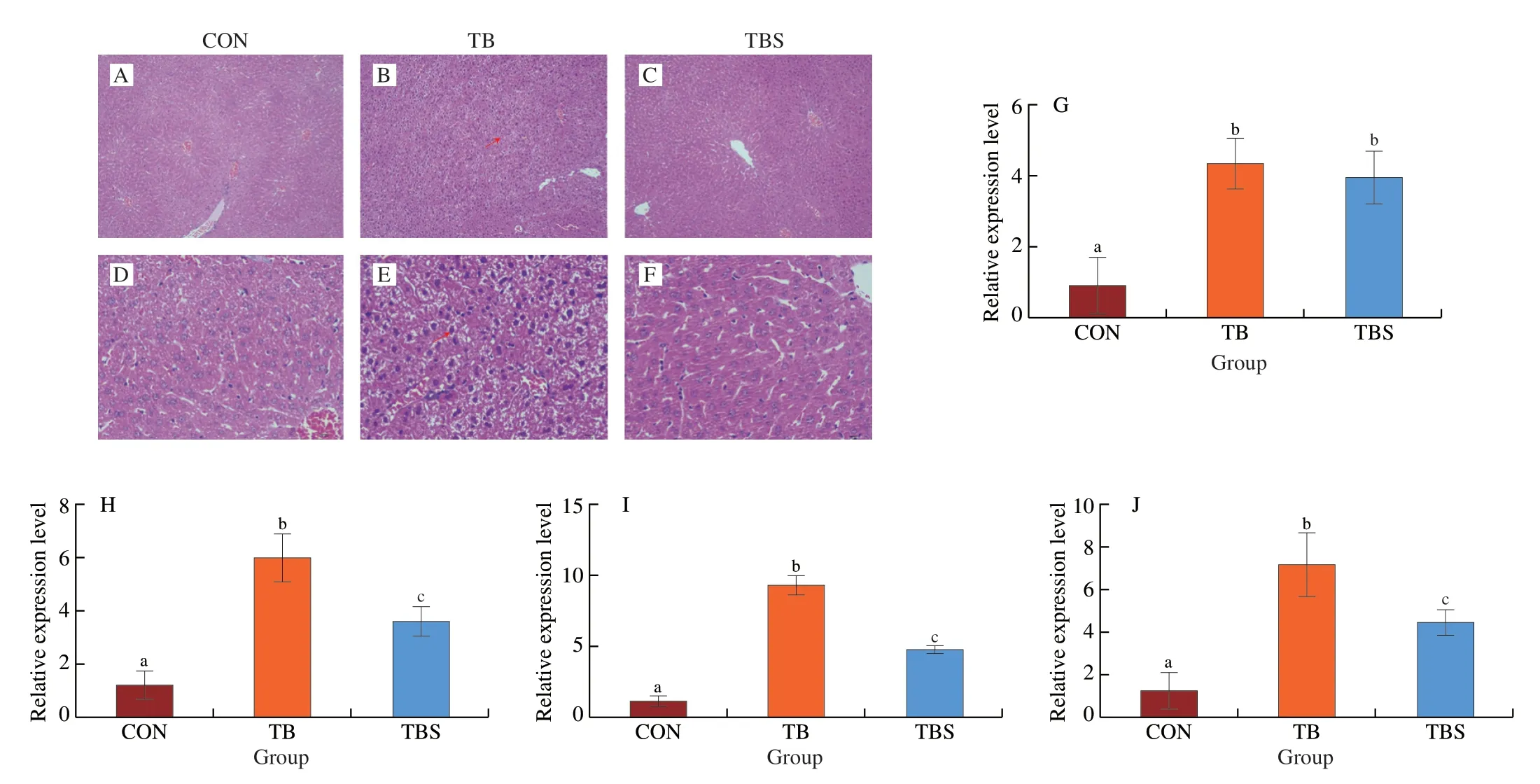
Fig.2 Liver indices of mice in each group after 6-week feeding.(A-F) Histopathological liver sections of mice in different groups.Red arrows indicate the hepatocytes degeneration.A-C,Scale bar: 100 µm;D-F,Scale bar: 10 µm.(G-J) Expression levels of liver inflammatory cytokine genes including IL-4,IL-6, IL-10 and TNF-α,respectively.Error bars indicate mean ± SD.Different lowercase letters represent significant differences at P < 0.05.

Fig.3 Pancreas indices of mice in each group.(A-F) Histopathological pancreatic sections of mice.Red arrows represent the degenerated and necrotic epithelial cells.A-C,Scale bar: 100 µm;D-F,Scale bar: 10 µm.(G) Trypsin activity in the pancreas.Error bars indicate mean ± SD.Different lowercase letters mean significant differences at P < 0.05.
3.7 Comparison of TB and TBS effects on intestinal inflammatory factor, trypsin activity, histopathological changes and faeces
As a major site for food digestion and absorption,physiological changes in the small intestine were also tracked in this study (Figs.4 and S4-S6).After 6-week feeding,TB and TBS intake influenced the morphology of the intestine,which was indicated as follows:1) the intestinal villi of mice in the TB and TBS groups were significantly increased (Figs.S5A-C);2) the crypt depths of the jejunum and ileum in the TBS group were significantly decreased,whereas the TB group did not show significant changes (Figs.S5D-F);3) the intestinal muscular thickness in the TB group was decreased,while that in the TBS group remained normal(Figs.S5G-I).These changes suggested that TBS enabled improving intestinal digestion and absorption capacity.Moreover,we conducted histopathological observations of the duodenum,jejunum and ileum in the mice from three groups.The duodenum,jejunum and ileum of mice in the CON and TBS groups maintained normal histological structures (Fig.S4).However,in TB mice group,the duodenum,ileum and jejunum all showed lesions to varying degrees,including lytic necrosis of intestinal villi (Fig.4D),focal necrosis of the mucous epithelium (Fig.4A) and intestinal glands (Figs.4D-F).Meanwhile,the gene expression of jejunal inflammatory factor showed thatIL-4,IL-6,IL-10andTNF-αlevels in the TB mice group were significantly increased,while those in the TBS group remained normal.Furthermore,TB intake inhibited the trypsin activity in the jejunum.Changes in intestinal physiology can affect faecal status.Fig.S6 addressed that the faeces from mice of the TB group had obviously higher moisture contents than those from the CON and TBS groups.The presence of TIs and allergen proteins might cause the above change in faeces of mice from the TB group,which might be more obvious with the extension of feeding time.
3.8 Gut microbiota diversity upon the consumption of TB or TBS
To determine whether gut microbiota was affected after TB and TBS consumption,the gut microbiota was analysed.After quality filtering,we acquired 1 434 380 high-quality filtered reads from 18 faecal samples,which corresponded to 79 688 reads for each individual mouse.The rarefaction curves for all samples had reached a plateau at this sequencing depth,suggesting that the sequencing was sufficiently complete (Fig.S7).To evaluate gut microbiota diversity in the samples,theα-diversity within the gut microbiota communities was estimated using the ACE,Simpson,Shannon,Chao,and Faith’s PD index (Fig.S8 and Table S6).Only the Faith’s PD index showed the TBS group with a significant difference from the CON group,neither the other 4 indexes did.It highlighted that the intake of TB and TBS had little effect on theα-diversity of gut microbiota in mice.

Fig.4 Intestine indices of mice in each group.(A-F) Histopathological intestine sections of mice in the TB group.The degenerated and necrotic intestinal gland was indicated by red arrows,while dissolved and necrotic intestinal villi by the green one,and denaturised and necrotic intestinal mucosa by the yellow one.A-C,Scale bar: 100 µm;D-F,Scale bar: 10 µm.(G-J) Expression levels of jejunum inflammatory cytokine genes including IL-4,IL-6,IL-10 and TNF-α.Error bars mean mean ± SD.Significant differences were indicated with different lowercase letters at P < 0.05.(K) Trypsin activity in the jejunum.Error bars represent the ± SD.
The distributions ofβ-diversity (weighted and unweighted UniFrac distances) were compared among groups.Through anoism analysis based on the unweighted (R=0.259,P=0.001) and weight UniFrac (R=0.340,P=0.001) method between groups,we confirmed that inter-group differences were greater than intra-ones(Fig.S9).PCoA was used to show the patterns of separation with different treatments.The unweighted UniFrac PCoA (R2=0.210,P=0.001) and the weighted UniFrac PCoA (R2=0.309,P=0.001)showed significant separation between the TBS group and the CON group,while the gut microbiota in the TB group was overlapped with those in the other two groups (Fig.5A).Altogether,the intake of TB and TBS changed theβ-diversity of gut microbiota in mice.
To further ascertain which taxa were responsible for the observed community differences induced by dietary supplementation in this study,the linear discriminant analysis (LDA) effective size (LEfSe)algorithm was employed to compare the differences at the class and phylum levels (Fig.5C).This analysis identified 7 taxa,including 3 phyla and 4 classes that were differentially abundant in each diet group.Firmicutes (LDA score=4.77,P=0.019) and Clostridia (LDA score=4.83,P=0.026) were identified as biomarker taxa for TBS group mice.The phylum Deferribacteres (LDA score=4.18,P=0.018),class Deferribacteres (LDA score=4.18,P=0.018),and class Erysipelotrichia (LDA score=4.07,P=0.024) were biomarker taxa for TB.For the CON group,Bacteroidetes (LDA score=4.90,P=0.011) and its annotated subclass Bacteroidia(LDA score=4.90,P=0.011) were identified as biomarkers by LEfSe analysis.
Fig.5D shows the three phyla with significant differences among three groups by ANOVA at the phylum level.Compared to CON,TB consumption significantly increased the abundance of Firmicutes and decreased the abundance of Bacteroidetes,while TBS consumption only significantly increased the abundance of Firmicutes,which was consistent with the histogram of specie distribution (Fig.S9 and Table S7).Moreover,the Firmicutes/Bacteroidetes ratio was significantly different between the CON and TB groups (Fig.5E).Supplementation with TBS did not cause a significant increase in this ratio compared with CON treatment.Fig.S10 shows the effects of TB and TBS on the gut microbiota balance at the family level in mice in the TB and TBS groups.Compared with those in the CON group,the relative abundance levels of Muribaculaceae,Christensenellaceae and Xanthobacteraceae were significantly reduced,and the Lachnospiraceae abundance was increased in the TB group.TBS intake significantly improved the abundance of Lachnospiraceae and reduced the abundance levels of Pedosphaeraceae,Christensenellaceae,Xanthobacteraceae,Xanthomonadaceae,Thermoanaerobaculaceae,and uncultured_bacterium_o_Mollicutes_RF39.Furthermore,the relative abundance levels of Lachnospiraceae and Pedosphaeraceae in the TB group were significantly higher and lower,respectively,than those in the TBS group.
4.Discussion
At present,studies on the physiological effects of TB and TBS usually focus on regulating lipid and glucose metabolism disorders caused by a high-fat diet.However,these studies did not consider the physiological effects of ANFs.In this study,the changes in ANFs over the TB germination process was determined,and TBS germinated for 5 days were selected for gavage administration.The results illustrate that TB and TBS consumption has distinct effects on body weight,organ indices,serum indices,faecal indices and the microbiota in healthy mice without differences in daily food and water intake.This study may provide guidance for optimal utilization of TB resources.

Fig.5 Gut microbiota composition in mice after the 6-week intervention among three groups.PCoA was used to show patterns of separation by TB and TBS consumption.(A) Variations based on the unweighted UniFrac distance.(B) Variations based on the weighted UniFrac distance.(C) LEfSe analysis of gut microbiota compositions in different groups of mice (LDA > 4,P < 0.05).LDA scores derived from LEfSe analysis showed the biomarker taxa (class level and above).Column configurations showed the effects of TB and TBS on the gut microbiota balance in mice (phylum level).(D) Differences in bacterial community composition at the phylum level among three groups.Error bars represent the ± SD.Different lowercase letters indicate the significant differences at P < 0.05.(E) Firmicutes/Bacteroidetes ratios in the CON,TB,and TBS groups.Error bars represent mean ± SD.Different lowercase letters mean the significant differences at P < 0.05.
ANFs in TB has restricted the application and popularization of TB products to a certain extent.Buckwheat seeds generally contain more abundant PA than legumes and cereal grains[37].PA in wheat rolls has been reported to inhibit zinc absorption.Conversely,in the absence of PA,the human body can absorb more zinc and magnesium from food[38].Besides,the mice were administrated for a long-term with germinated barley foodstuff did not inhibit the absorption of minerals compared with the cellulose supplemented group,which is consistent with our study (Table 1)[39].TIs are defence proteins in plants but toxic to mammals and humans[40].TI addition to the diet for mice can increase the pancreas weight,decrease trypsin activity in the intestine and even cause colitis by affecting intestinal microflora[33,41].As reported,germinating can reduce the activity of TIs in TB and improve protein digestibilityin vitro[42],which was consistent with our results in this study.Here,it showed the decreased TIs activity in TBS,which was further confirmed in the mice(Figs.3,4 and S3).Allergen proteins are commonly found in grains,and most people ingesting cereal proteins may develop adverse effects[43].Mice with allergenic sensitization to rice and wheat exhibited increased IgE level,cutaneous anaphylaxis,inflammatory damage to the small intestine and modification of the gut microbiota[44-46].In addition,buckwheat allergy was also mediated by IgE[47].As shown in Figs.2,4,S3 and Table 1,the IgE level of the TBS group was lower than that of the TB group,indicating that TBS showed a lower allergen content and allergy effects.This result further addressed the similar conclusion that germinating has a higher potential to reduce allergen content in soybeans[48].Moreover,during germination,myriad interactions were addressed through activation of endogenous enzymes such asα-amylase,pullulanase,phytase,and other glucosidases.These enzymes degrade ANFs and break down complex macronutrients to their simple and more digestible forms[49].
The metabolites generated by the microbial community influence the host health[50].In this study,TB and TBS intake only significantly affect the abundance of gut microbiota.At the phylum level,the increased levels of Firmicutes and decreased levels of intestinal Bacteroidetes might be related to tissue inflammation[51].Moreover,the decrease in the Firmicutes/Bacteroidetes ratio is correlated to weight loss,and the whole grain can improve this ratio[52-54].The Firmicutes/Bacteroidetes ratio decreased in mice fed a high-fat diet with sprouted adzuki beans,which was consistent with the results in this study(Fig.5E)[55].At the microbiota family level,Lachnospiraceae is the core family of gut microbiota,whose metabolism has been demonstrated.Lachnospiraceae can hydrolyse starch and other sugars to produce butyrate and other short chain fatty acids.Also,they have cellulolytic activity and are associated with inflammatory bowel disease and type 1 diabetes[52,56].Besides,the intake of vegetable foods is positively correlated with the abundance of Lachnospiraceae[57].Muribaculaceae are another dominant family in the gut microbiota and may function in colitis remission[58].Lachnospiraceae and Muribaculaceae are negatively correlated with obesity[59].In this study,the consumption effects of TB and TBS on the inflammatory reaction and body weight of mice may be relevant to the abundant changes of Lachnospiraceae and Muribaculaceae as well as Firmicutes/Bacteroidetes ratio.Briefly,TB and TBS had different effects on gut microbiota.
In summary,due to the existence of ANFs,long-term consumption of TB may have potential side effects on the liver,pancreas,intestine,and absorption of trace elements.Accordingly,it would be better to apply the gemination for properly processing TB to decrease ANFs before consumption.This study revealed that long-term consumption of TBS had no obvious side effects and its biological activity was better compared to TB.Therefore,TBS has greater potential as a raw material for lipid-lowering health food.
Conflicts of interest
There are no any conflicts of interest among all authors or parties involved.
Acknowledgments
Supported by the Opening Project of Key Laboratory of Coarse Cereal Processing of Ministry of Agriculture and Rural Affairs,and Sichuan Engineering and Technology Research Center of Coarse Cereal Industralization,Chengdu University (2022CC013).
Appendix A.Supplementary data
Supplementary data associated with this article can be found,in the online version,at http://doi.org/10.26599/FSHW.2022.9250067.
- 食品科学与人类健康(英文)的其它文章
- Protective effects of oleic acid and polyphenols in extra virgin olive oil on cardiovascular diseases
- Diet and physical activity inf luence the composition of gut microbiota,benef it on Alzheimer’s disease
- Inf luence of nitrogen status on fermentation performances ofnon-Saccharomyces yeasts: a review
- Ganoderma lucidum: a comprehensive review of phytochemistry,eff icacy,safety and clinical study
- Resveratrol combats chronic diseases through enhancing mitochondrial quality
- Demonstration of safety characteristics and effects on gut microbiota of Lactobacillus gasseri HMV18

