The human-derived novel gut commensal Luoshenia tenuis regulates body weight and food intake in mice
Yu Jing,Mengxun Du,Lisheng Xie,Minzhi Jing,Yokun Zhng,Mingxi Bi,Chng Liu,b,,Hongwei Liu,Shungjing Liu,b,
a State Key Laboratory of Microbial Technology, Shandong University, Qingdao 266237, China
b State Key Laboratory of Microbial Resources, Institute of Microbiology, Chinese Academy of Sciences, Beijing 100101, China
c College of Life Science, Hebei University, Baoding 071000, China
d State Key Laboratory of Mycology, Institute of Microbiology, Chinese Academy of Sciences, Beijing 100101, China
Keywords: Luoshenia tenuis Gut microbe Feeding behavior Obesity Next-generation probiotic
ABSTRACT Many studies have revealed that gut microbes modulate host metabolism.In this study,we characterized the therapeutic effects of a novel gut commensal Luoshenia tenuis against host metabolic disorders.First,by in silico analysis,we demonstrated that the L.tenuis was prevalent in the gut microbiomes of healthy humans but were depleted specif ically in obesity cohorts.Further in vitro cultivation revealed that L.tenuis produced short chain fatty acids that were verif ied to modulate host metabolism and some other volatile metabolites to benef it hosts by anti-inf lammation and anti-tumor.Second,gavage of the L.tenuis signif icantly decreased the body weight gain and food intake of high-fat diet-feeding C57BL/6J mice,which was in parallel with the changed expression level of genes related to satiety and feeding behavior.We then performed the gavage trial using diet induced obese mice,and it revealed that the administration of L.tenuis alleviated signif icantly the abnormal glucose and lipid metabolisms and reduced the inf lammatory response.In summary,this study revealed a previously-unknown human gut commensal microbe that benef ited host metabolism,and set the stage for the development of novel next-generation probiotic applicable for treatment of obesity and related metabolic disorders.*Corresponding authors at: State Key Laboratory of Microbial Technology,Shandong University,Qingdao 266237,China.
1.Introduction
During the past decades,the incidence of obesity and related metabolic disorders increased drastically and has become a worldwide major health concern imposing a heavy burden on the economy and public health[1-4].Anti-obesity medications such as glucagon-like peptide-1 (GLP-1),leptin,ghrelin and peptide tyrosine tyrosine (PYY)were designed based on gut-brain axis hormones and functioned by regulation of appetite and energy metabolism[5].However,the long-term pharmacotherapy of these chemical medications would cause adverse cardiovascular effects,increase suicidal risk and give rise to drug dependence[6].Alternatively,microecological therapy including probiotic,prebiotic and synbiotic has become a promising strategy for intervention of chronic diseases such as obesity,type 2 diabetes and constipation in respect of its low side effects[7-9].The microecological agent is basically comprised of viable microbes and the components and/or metabolites of those microbes which are prevalent and abundant in the normal gastrointestinal tracts of healthy people[9-13].As a result,the microecological therapy is well-tolerated for most of the patients and long-term application of which is rarely found to cause serious adverse effects.Thus,it is of practical value to develop more effective microecological therapies for chronic diseases such as obesity.We believe that as we have more in-depth knowledge about the compositional and functional differences of gut microbiomes between health and metabolic diseases,more microecological therapies will be developed.
Gut microbiome,as an ecosystem comprising large numbers of bacteria living in the intestinal tract,plays a pivotal role in maintaining host metabolism homeostasis,and its dysbiosis would cause or aggravate metabolic diseases as obesity,diabetes,atherosclerosis and fatty liver[14].Culture-independent gut metagenomic cohort studies concerning metabolic diseases have revealed a vast number of correlations between host pathologies and specific gut commensal microbial taxa,behind which might lie new biotherapies or pathogeneses for metabolic diseases.Functional study of these phenotype-associated taxa by culture-dependent studies will help us to understand host-microbe interactions and to develop new functional gut microbes into next-generation probiotics (NGPs)and clinical biotherapies.One successful example isAkkermansia muciniphila,one of the most promising NGPs,which has been demonstrated to counteract diet-induced obesity and related metabolic disorders such as insulin resistance,glucose intolerance and hepatic steatosis both using murine models[15-16]and in clinic trial[17].Another example isChristensenellaceae minuta,as its therapeutic potential on obesity and abnormal metabolism was experimentally validated in rodent models[18].Some biotech companies turn to developC.minutainto new biotherapy for management of obesity and associated metabolic disorders[19].Besides,Faecalibacterium prausnitzii,Clostridiumbutyricum,Bacteroidesxylanisolvens,ParabacteroidesdistasonisandDysosmobacter welbioniswere experimentally proven to contribute to host metabolism via known or unknown mechanisms[20-24].However,due to the heavy workload and lack of culturable microbial resource,very limited number of commensal microbial taxa associated with host metabolic phenotypes have ever been functionally verified by wet-lab experiments with pure cultured strains to date[25].There are still a large number of gut commensal microbes that are negatively correlated with metabolic diseases are functionally unknown.As increasing gut commensal bacteria associated with host health are cultured and studied,more functional candidates of NGPs will be unravelled.
In this study,we confirmed byin-silicoanalysis of gut metagenomic datasets that a novel gut commensalLuosheniatenuisgen.nov.sp.nov.described in our previous study is widely existed in over 86% of the assessed healthy cohorts and significantly decreased in obese humans compared with the healthy counterparts[26].We then verified that gavage ofL.tenuisto high-fat diet (HFD) mice significantly decreased the body weight gain and food intake by modulation of genes involved in feeding behavior,and improved the glucose and lipid metabolism of diet induced obese (DIO) mice.Our study demonstrates that the novel human gut commensalL.tenuisis a promising candidate of NPGs for treatment of obesity and related metabolic disorders.
2.Materials and methods
2.1 Ethical statements
All mice experiments in this study were approved by the ethics committee of Institute of Microbiology,Chinese Academy of Sciences (IMCAS).The protocols were approved by the Committee on the Ethics of Animal Experiments of IMCAS (permit SQIMCAS2021051).The experiments were conducted according to the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals (NIH publications No.8023,revised 1978).
2.2 Mice, housing and diets
All C57BL/6 J male mice were purchased from Beijing Vital River Laboratory Animal Technology Co.,Ltd.(Beijing,China).The mice were housed in a specific-pathogen-free (SPF) environment under controlled conditions,a 12-h light/dark cycle,a temperature of 20-22 °C,and (45 ± 5)% humidity,with free access to food and water.All mice were acclimated for one week before the experiment.
For theL.tenuisefficiency assay on HFD-feeding mice,5-weekold C57BL/6J male mice fed with HFD (D12492,60% calories from fat,Research Diets,USA) for 16 weeks were sorted into 3 groups(n=15 each) based on their initial blood glucose levels and body weight.One group was treated daily with 1 × 109CFU of livedL.tenuissuspended in 0.1 mL of sterile anaerobic PBS by mouth.Another group was given the same amount ofL.tenuiscells that were inactivated by autoclaving at 121 °C for 15 min.The control group was given an equivalent volume of sterile anaerobic PBS by gavage.
For theL.tenuisefficiency assay on DIO mice,5-week-old C57BL/6J male mice fed with HFD for 10 weeks to induce obesity by company (GemPharmatech LLC,Jiangsu,China),and the DIO mice with body weight > 35 g were purchased and delivered to our P2 animal house with a controlled environment for an acclimation period of one-week.And then they were sorted into four groups based on their body weight and blood glucose levels and treated for another 9 weeks.The DIO_LT group (n=10) was treated daily with 1 × 109CFU of livedL.tenuissuspended in 0.2 mL of sterile anaerobic PBS by mouth.The DIO_LTpa group (n=5) was given the same amount ofL.tenuiscells that were inactivated by autoclaving at 121 °C for 15 min.The control groups (n=5 each) DIO_CK and ND_CK were given an equivalent volume of sterile anaerobic PBS by gavage.But the diet of ND_CK group mice was changed from HFD to normal diet (ND).
2.3 Microbial strains
L.tenuiswas cultured in modified mGAM broth medium at 37 °C in an anaerobic tube for 48 h[26].For thein vivoefficacy assay,cells were obtained by centrifuging at 8 000 ×gfor 10 min at 4 °C.The cells suspension for oral administration was prepared by suspending the cultured bacterial cells in sterile anaerobic PBS with a final cell density of 1 × 109CFU/mL.100 µL of the bacterial suspension was given daily.
2.4 Body weight and food intake
The body weight and food intake were measured every 3 days.Food intake per mouse per day was determined by the following equation: (total food intake each cage)/[(5 mice per cage) × (days of food consumption)].
2.5 Fecal samples
Each mouse was kept in an empty compartmentalized storage boxes without bedding for 30 min,and then collected fresh fecal samples into sterile tubes.Tubes were stored at -80 °C for further analysis.
2.6 Tissue sampling
After treatment,each mouse was anesthetized with pentobarbital sodium (TRC,Canada),and blood was sampled from the fundus portal and cava veins.After exsanguination,mice were euthanized by cervical dislocation.The intestines and the liver were precisely dissected,weighed and photographed.One piece of tissue (0.5 cm ×0.5 cm square) in the middle of the largest lobe of liver were cut off and fixed in 4% paraformaldehyde for 24 h at room temperature and the other liver tissues were frozen in liquid nitrogen and stored at-80 °C for further analysis.
2.7 Insulin tolerance test (ITT) and oral glucose tolerance test (OGTT)
An ITT was performed by injecting insulin (0.6 U/kg)intraperitoneally after 6 h of fasting.An OGTT was performed by gavage of glucose solution (2 g/kg) after overnight fasting.The level of blood glucose was measured by tail vein bleeding using a glucose meter (EA-18,Sinocare,China) before oral glucose load (0 min) and at 30,60,and 120 min after oral glucose load.The AUCs generated from the data collected during the ITT/OGTT were calculated with GraphPad v8.2.1.441.
2.8 Histological analysis
Liver tissue was sampled promptly after euthanasia,and fixed in 4% paraformaldehyde until paraffin embedding.Colorectum was emptied and subsequently rolled around needle after which the generated Swizz rolls were carefully preserved in 4% paraformalin until paraffin embedding.The paraffin sections of liver,and colorectum tissues were stained with H&E.The slides were scanned under a light microscope using Imaging Sys (Nikon).Liver sections were prepared by company (Servicebio Co.Ltd.,Hubei,China) and subsequently assessed by two independent observers in a blinded fashion,using the liver damage score for evaluation of H&E staining liver sections as described by previous study[27].For colorectum intestinal injury score,morphologic examinations were performed using inverted fluorescence microscope,and colorectum injury was analysed by a blinded,and experienced investigator for absent,mild,moderate,or severe injury,according to Chiu’s intestinal injury pathological grading (score 0-5)[28].
2.9 Real-time qPCR analysis
Total RNA was extracted and purified from stomach,ileum and colon tissues following the protocol described in the Blood and Tissue Kit and TRIzol reagent (Vazyme).Concentration of total RNA was tested by Qubit (Invitrogen,Q33226).The cDNA was synthesized using a HiScript III RT SuperMix reverse transcription kit (Vazyme).Gene expression levels were detected using SYBR Green I (Vazyme)and pre-designed primer (Table S3).The relative mRNA expression level normalized to the internal controlβ-actin gene was calculated by the comparative threshold cycle (Ct) method[29].Experiments were performed in triplicate and repeated at least two times.The qPCR mixture contained 10 µL SYBR green DNA-binding dye,1 µL cDNA of each tissue and 0.8 µL primers,then replenish with DEPC water to 20 µL PCR amplification was performed using the following cycling parameters: 3 min at 95 °C,40 cycles of 3 s at 95 °C,and 30 s at 60 °C.
2.10 In-silico analysis of publicly-accessible metagenomic datasets
The 1 129 gut metagenomes used for analysis of the prevalence and relative abundance ofL.tenuisspecies in gut microbiomes of healthy humans were collected as described in our previous study in which the accessions of each run was available[26].The publicly available metagenomic dataset form the cohort of obese patients and its healthy counterparts was available under Bioproject PRJEB4336,and was achieved directly from GMrepo[30].The prevalence and relative abundance was analysed by kraken2-based annotation of quality-controlled raw metagenomic data using a customized database as described in our previous study[26].
2.11 16S rRNA gene sequencing and analysis
DNA for amplicon sequencing of gut microbiota was extracted from approximately 50 mg of cecum contents of mouse.The V3-V4 region of 16S rRNA was amplified using the primers F341 (CCTACGGGRSGCAGCAG) and R806(GGACTACHVGGGTWTCTAAT) by PCR and sequenced in the HiSeq 2500 by company (Megagene,Guangzhou,China).The clean reads were denoised (command: -unoise3) and analyzed by Usearch 64-bit v11 following the recommended pipeline (https://drive5.com/usearch/).A representative sequence of each ASV (amplicon sequence variants) was assigned to the taxa at genus level in the ltp_vhGMB constructed in our previous work[26].Statistical analysis of differentially abundant sequences and taxa were performed by MicrobiomeAnalyst (https://www.microbiomeanalyst.ca/MicrobiomeAnalyst/)[31].
2.12 Short-chain fatty acid (SCFA) and volatile metabolites analysis
The levels of SCFAs (acetic acid,propionate,isobutyrate,butyrate,isopentanoic acid,and pentanoic acid) were measured by GC-MS according to a previously described method[23].Briefly,the samples were extracted with equal volume of ethyl acetate by vortex for 3 times.All content transferred to 15 mL centrifuge tube,centrifuged for 10 min at 8 000 r/min.Supernatants were collected in automatic injection bottle (2 mL,9 mm,ND9) for further analysis.The extracts were analysed using a QP 2010 Ultra GC-MS system with a Rtx-Wax capillary column (60 m × 0.25 mm ×0.25 mm),and the injection was performed at 230 °C with 2 mL of samples.Helium at a flow rate of 1.2 mL/min as used as the carrier gas.Electronic impact was recorded at 70 eV.Oven temperatures were programmed from 60 °C to 100 °C at 5 °C/min,with a 1-min hold;to 150 °C at 5 °C/min,with a 5-min hold;and to 225 °C at 30 °C/min,with a 20-min hold.
For the measurement of volatile metabolites,the headspace-solid phase micro-extraction was performed as described by Chen et al.[32].The samples were analysed using a QE 2104324S GC-MS system with a DB-5MS capillary column (30 m × 0.25 mm × 0.25 µm),and the injection was performed at 240 °C.Helium at a flow rate of 1 mL/min as used as the carrier gas.Electronic impact was recorded at 70 eV.Oven temperatures were programmed from 60 °C to 240 °C at 5 °C/min,with a 15-min hold;and to 300 °C at 30 °C/min,with a 5-min hold,and data were collected in full scan mode (m/z32-500).
2.13 Statistical analysis.
All data are expressed as the mean ± SEM.Mouse groups were compared using one-way or two-way ANOVA,followed by either the Kruskal-Wallis or the LSD post-hoc test.Statistical analysis was performed using SPSS v20,and visualized using GraphPad Prism v8.2.1.441.Statistical significance is indicated by asterisks (*);*P< 0.05,**P< 0.01,***P< 0.001,****P< 0.000 1.
3.Results
3.1 Biological and bioinformatical profiling of L.tenuis
L.tenuis(NSJ-44T),a strictly anaerobic microbe isolated from fecal sample of a healthy adult,was described and denominated in our recent study[26].The phylogenetic tree and genomic analysis revealed thatLuosheniawas a new genus,closest toChristensenellaminuta,and the 16S rRNA gene sequence similarity betweenL.tenuisandC.minutawas 87.07%.The bacterial colonies on modified mGAM agar plates were extremely small,showing pinpoint,whitish to semitranslucent,convex colonies.Image achieved by transmission electron microscope (Fig.1A) displayed that the bacterial cells are ovoid with pointed ends (1.3-1.6 µm long and 0.7-0.9 µm wide),and occur singly or in pairs.
To investigate the prevalence ofL.tenuisin the populations,1 129 gut metagenomes of healthy cohorts with various ages,regions and dietary structures were collected from GMrepo for kraken2-based taxonomic analysis as described in methods section.The results revealed that theL.tenuiswas widely existed in healthy humans as it was found in over 86% of the analysed samples (Fig.1B) with a mean relative abundance (RA) of 0.008% (Table S1).To further evaluate the association betweenL.tenuisand host obese pathology,we revisited and analysed the gut metagenomes from an obese cohort(n=316) and its healthy counterparts (n=156).As shown in Fig.1C,theL.tenuiswas significantly depleted in obese cohort compared with the healthy counterparts.The observed negative correlation suggested a protective potential ofL.tenuisagainst obesity.
We then profiled the metabolic characteristics ofL.tenuisby gas chromatography-mass spectrometry (GC-MS) analysis of the production of volatile metabolites including SCFAs duringinvitrofermentation.As shown in Fig.1D and Table S2,L.tenuisproducedtrans-farnesol (v22) and hexahydrofarnesyl acetone (v28),which exerted anti-inflammatory effects as proved by previous studies[33-35].Moreover,2-acetylpyrazine (v30) and 4-vinylguaiacol (v24) were reported to possess anti-tumor activity[36-37].We also verified that theL.tenuiswas a producer of host-beneficial SCFAs,as propionic acid,butyric acid,isobutyric acid,pentanoic acid and isovaleric acid were identified after 24-h fermentation ofL.tenuis(Fig.1E).
3.2 L.tenuis administration reduces body weight gain and food intake of HFD mice
We then performed an animal trial as illustrated in Fig.2A to experimentally verify the potential effects ofL.tenuison hosts.In brief,5-week male C57BL/6J mice were daily administrated with viableL.tenuiscells (HFD_LT,n=10),pasteurizedL.tenuis(HFD_LTpa,n=10) and vehicle PBS (HFD_CK,n=10) for 17 weeks to monitoring the changes of body weight and the other metabolic features.First,compared with the control group,the body weight gain,daily food intake and inguinal fat weight in HFD mice were reduced significantly afterL.tenuisadministrations,which suggested a weight control effect ofL.tenuiscells (Figs.2B-D).Besides,we noticed that the HFD mice treated with pasteurizedL.tenuisalso exhibited significant decrease on body weight gain and food intake,implying that the weight control effect was independent of cell viability.
To confirm if the reduced food intake was a microbe-mediated regulation of host behavior,we screened the marker genes/signalling pathways that were known to regulate or participate host food intake by qPCR-based quantification of gene transcriptions in gastrointestinal tissues of HFD mice.As shown in Fig.2E,we found that the administration of viable and pasteurizedL.tenuisincreased the transcription level of gastricleptinandnucb2,respectively encoding the LEPTIN and NESFATIN-1 that were responsible for the increase of satiety and suppression of appetite in mice[38-39].Besides,we observed that the transcription level ofghrlencoding GHRELIN,a stimulant for feeding behavior,was decreased in HFD mice treated with viable and pasteurizedL.tenuiscompared with the control group (Fig.2E).In ileum,the transcription level ofnucb2was increased significantly as in stomach.The transcription level ofpyyandgcgwere increased clearly afterL.tenuisintervention,although not statistically significant.The PYY encoded bypyy,a gut hormone that regulates appetite and inhibits pancreatic secretion.The GLP-1 encoded bygcg,reducing food intake and inhibits glucagon secretion,which could further lead to the inhibition of pancreatic secretion and appetite (Fig.2F).We also observed the transcription level ofpyywas increased significantly in colon (Fig.2G).
We next quantified the colonization ofL.tenuisin HFD mice before and after gavage trial by qPCR analysis.Before gavage,theL.tenuiswas not detected in the fecal samples of mice in each group.After 17-week gavage,the amount ofL.tenuiswas still undetectable in fecal samples of the HFD_CK and HFD_LTpa groups but significantly increased to (1.58 ± 1.01) × 106CFU/g dried faeces in HFD_LT group (Fig.2H).
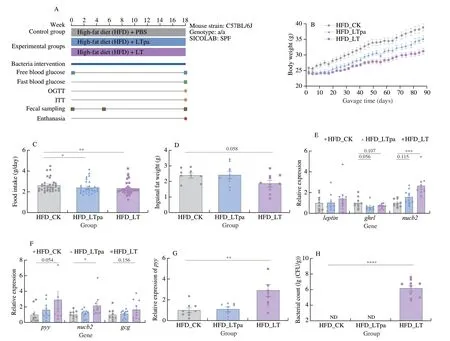
Fig.2 The intervention of L.tenuis alleviated body weight gain and food intake in high fat diet mice.(A) Experimental layouts and diet composition.(B) Body weight of mice treated during 17 weeks by daily oral gavage.(C) Average daily food intake at intervals of 3 days.(D) Inguinal fat weight of each group.(E)Transcription level of leptin,ghrl,and nucb2 in stomach.(F) Transcription level of pyy,nucb2 and gcg in ileum.(G) Transcription level of pyy in colon.(H) The colonization test of L.tenuis in mice.*P < 0.05;**P < 0.01;***P < 0.001,****P < 0.000 1.
3.3 L.tenuis gavage alleviates the metabolic disorders of HFD mice
We then assessed the therapeutic effects ofL.tenuiswith respect to the pathoglycemia and liver damage of experimental mice induced by long-term HFD feeding[40].As shown in Figs.3A and B,comparing with the HFD_CK group,the HFD_LT group showed significant hypoglycemic effect on OGTT,and exhibited a marginal effect on the ITT (Figs.3C-D).And the free and fasted glycemia was decreased significantly in HFD_LT group comparing with the HFD_CK group(Figs.3E-F),showing that viableL.tenuiscells clearly improved blood glucose metabolism in HFD mice.
Further histological evaluation of liver tissue by the H&E staining revealed that the fatty-liver like hepatic lesions as macrosteatosis,hepatocyte ballooning and fat deposition was resorted afterL.tenuistreatment comparing with the control group (Fig.3G).The lower liver index and liver damage score in HFD_LT group also collaborated that the liver lesion was significantly improved byL.tenuisgavage(Fig.3H and I).Recent evidence supported that high fat diet affected intestinal mucosal barrier function[41].Therefore,we investigated the effects ofL.tenuison the colorectum tissue.The results revealed that the gavage of livingL.tenuisincreased the average colorectum length (Figs.3J-K) and improved the gut barrier integrity as the mucosa damage score was decreased (Fig.3L) and the transcription level oftjp1andcldn1encoding the tight junction protein zonula occludens 1 (ZO-1)and CLAUDIN-1 were increased (Figs.3M and N).The results above suggested that the administration ofL.tenuisalleviated liver and intestinal mucosa damage in HFD mice.
3.4 L.tenuis administration does not cause apparent disturbance on gut microbiota

Fig.3 The changes of physiological and biochemical indexes in HFD mice after L.tenuis gavage.(A,B) Plasma glucose profile and mean AUC measured during an OGTT.(C,D) Plasma glucose profile and mean AUC measured during ITT.(E,F) Free and fasting blood glucose level at the end of gavage.(G) Appearance and sections of representative images from liver after H&E staining.Scale bars,100 µm.Measurements were taken from distinct samples.(H) Liver weight and(I) liver damage score of each group.(J) Appearance and sections of representative images of colorectum Swiss roll after H&E staining.(K) Colon length and(L) gut mucosa damage level of each group.The transcription level of (M) tjp1 and (N) cldn1 in colon.*P < 0.05;**P < 0.01.
To evaluate if the gut microbiota composition was targeted and effected byL.tenuisgavage,we sequenced and analysed the 16S rRNA gene amplicons of caecal contents of experimental mice (n=6).As shown in Fig.4A,theL.tenuisadministration marginally increased theα-diversity of gut microbiota as the Chao1 diversity index was increased afterL.tenuistreatment.The subsequent principal coordinate analysis (PCoA) based analysis ofβ-diversity demonstrated that the gavage of viable and pasteurizedL.tenuisslightly altered the overall gut microbiota composition(PERMANOVA or other statistical analysis,P< 0.067) compared with the control group (Fig.4B).Yet,theβ-diversity between two intervention groups (HFD_LT and HFD_LTpa) showed no significant difference.There was no significant difference in the phylum-level microbial composition that was observed (Fig.4C).Yet,further LDA Effect Size (LEfSe) analysis demonstrated that the Lachnospiraceae,Oscillospiraceae and Akkermansiaceae were significantly enriched in HFD mice afterL.tenuistreatment,while the Helicobacteraceae and Streptococcaceae were enriched in the control group (Fig.4D).The results indicated that the impact ofL.tenuisgavage on gut microbiota is very limited.

Fig.4 The impact of gut microbiota composition by L.tenuis gavage.(A) α-Diversity (Chao1 index) and (B) β-diversity of each group in the cecal contents.(C) Microbial composition at phylum level of the cecal contents.(D) The log-transformed LDA scores computed with LEfSe for bacterial taxa differentially abundant between HFD_CK/LT groups.

Fig.4 (Continued)
3.5 L.tenuis improves glucose and lipid metabolism and intestinal barrier function of DIO mice
We have verified in last animal trial that theL.tenuisretarded the weight gain and metabolism deterioration in HFD mice.We ascertained if theL.tenuisexerted weight-loss effect when the obesity is formed.To evaluate the effects ofL.tenuison the developed obesity and metabolic disorders,we used the DIO mice as animal models,and treated which by daily gavage of vehicle PBS(DIO_CK,n=5) and (C57_CK,n=5),viableL.tenuis(DIO_LT,n=10),and pasteurizedL.tenuis(DIO_LTpa,n=5) for 9 weeks(Fig.5A).It revealed that gavage of either the viable or the pasteurizedL.tenuisfailed to decrease the body weight of DIO mice(Fig.5B).However,the administration ofL.tenuissignificantly improved the hyperglycemia of DIO mice.The level of free and fasting blood glucose was significantly decreased after the administration ofL.tenuis(Figs.5C and D).Moreover,the OGTT test revealed that the DIO mice treated withL.tenuisexerted a better glucose tolerance compared with the control group (Figs.5E and F).In parallel,the mice treated with viableL.tenuisexhibited a better insulin sensitivity than the DIO-CK mice did as demonstrated by the ITT test (Figs.5G and H).

Fig.5 The intervention of L.tenuis improved glycolipid metabolism in DIO mice.(A) Experimental layouts and diet composition.(B) Body weight of mice treated during 9 weeks by daily oral gavage.(C) Periodic free blood glucose level.(D) Periodic fasting blood glucose level.(E,F) Plasma glucose profile and mean AUC measured during an OGTT.(G,H) Plasma glucose profile and mean AUC measured during ITT.(I) TG,(J) TCHO,(K) VLDL concentrations of plasma in groups.(L) ALT and (M) AST levels in plasma samples.(N) SOD activity in the liver.Content of (O) ZO-1,(P) TLR-4,(Q) IL-6 and (R) FGF-15 contents in colorectum.*P < 0.05;**P < 0.01;***P < 0.001;****P < 0.000 1.

Fig.5 (Continued)
Notably,the gavage ofL.tenuisin DIO mouse model also exhibited antihyperlipidemic effect as the concentration of plasma triglyceride (TG),total cholesterol (TCHO),and very low density lipoprotein (VLDL) decreased significantly (Figs.5I-K).We also noticed that the administration of pasteurizedL.tenuisalso exerted hypoglycemic and hypolipidemic effects in DIO mice as the free and fasting blood glucose,TG,TCHO and VLDL were significantly reduced in HFD_LTpa group.Yet,its therapeutic efficacy was much weaker than that of viableL.tenuis.Moreover,we verified that the liver lesions of DIO mice were also alleviated after administration of either viable or pasteurizedL.tenuisas the level of plasma alanine aminotransferase (ALT),plasma aspartate aminotransferase (AST)and hepatic superoxide dismutase (SOD) decreased in the DIO mice treated with viable or pasteurizedL.tenuis(Figs.5L-N).The administration ofL.tenuisexerted beneficial effects on the liver damage of DIO mice.
Moreover,we observed an increased level of intestinal epithelium tight junction protein ZO-1 and a decreased level of inflammatory factor toll-like receptor 4 (TLR-4) and interleukin-6 (IL-6) after administration of the viable or pasteurizedL.tenuiscompared with the control group (Figs.5O-Q),suggesting that the gut epithelium barrier and inflammatory response of DIO mice was markedly improved afterL.tenuis(viable or pasteurized) treatments.Finally,it was noteworthy that the administration of viableL.tenuissignificantly upregulated the expression of fibroblast growth factor 15 (FGF-15) (Fig.5R),a negative regulator of bile acid synthesis,gluconeogenesis and fatty acid oxidation in the liver,which might contribute to the observed improvement of glucose and lipid metabolism in DIO mice[42-43].
4.Discussion
In this study,we demonstrated the novel bacteriumL.tenuisis a promising candidate for development of NGPs for treatment of obesity and related metabolic disorders.Although we have demonstrated thatL.tenuishas beneficial effect on host metabolism in the animal models,optimization of culture conditions and safety assessments are still needed before subsequent clinical trials.It is important to note that the bacterium has been grown in a complex medium,thus all the initial data in mice were obtained using a medium containing animal-derived compounds,not necessarily suitable for administration to humans.Moreover,the expansive role of the gut microbiota in host metabolic health further highlights the need for personalized approaches to treating metabolic diseases.We also noticed that some prebiotics such as oat phenolic compounds could benefit host metabolism via targeted regulation of gut microbiota[44-45].The specifically regulated gut microbial taxa are worthy of further attentions and studies as they might contribute to the regulation of host metabolism as well.These findings and studies will profound the understanding of complex functional interactions between diet/prebiotics,gut microbiota,and host health.
We observed that the glucose metabolism was significantly improved in both model mice.However,the significant weight loss was not observed in DIO mice rather than HFD mice,which is understandable since it is theoretically easier to control weight from gaining too fast than to lose weight after formation of obesity.Though some efforts had been made by us in this study,the mechanisms underpinned the observed beneficial effect ofL.tenuisis not fully understood.Firstly,we identified that theL.tenuisproduced SCFAs clearly (Fig.1E).Hosts rely on their gut microbiota fermenting indigestible dietary components to produce SCFAs for energy,with acetate,propionate,and butyrate being the most abundant[46],and as signalling molecules,but might also enter the systemic circulation and directly affect metabolism or the function of peripheral tissues,such as liver and brain.It is reported that SCFAs involved in the biosynthesis of glucose,cholesterol and fatty acids in hepatocytes and reduced hepatic fat accumulation and inflammatory responses through gut-liver axis,improved glucose homeostasis and reduced hepatic steatosis[47-48].From the H&E staining of liver section,we observed that the intervention of viableL.tenuisimproved hepatic steatosis and reduced lipid accumulation,which might be ascribed to theL.tenuis-mediated production of SCFAsin vivo.In addition,SCFAs were known to play an important role in energy homeostasis,and stimulate the secretion of hormones such as GLP-1 and PYY by gastrointestinal L cells.These hormones were reported to reduce appetite by acting on the hypothalamus and activate brown adipose tissue,inducing gluconeogenesis in the gut and inhibiting gastric emptying,thus reducing hunger and inhibiting feeding activity[49-50].This is quite logical,as many intestinal dominant commensals asBifidobacterium,LactobacillusandBacteroideswere also known to modulate host behaviors by bioproduction of neurotransmitters as gamma-aminobutyric acids[50].However,according to our present evidences,we can only deduce that the production of SCFAs byL.tenuispossibly contributed to the beneficial effects on weight control and glucolipid metabolism observed in the animal trial.To determine the causative role between the efficacy ofL.tenuisand the gut microbial metabolites as SCFAs,further study is needed in the future.
We also analyzed the gut microbiota of mice by 16S rRNA gene amplicons and confirmed that feeding of HFD changes the gut microbiota composition.However,we observed only subtle shifts in the gut microbiota composition betweenL.tenuistreated and untreated groups,suggesting a direct effect of the bacteria on host metabolism rather than an indirect effect via targeting the gut microbiota or certain gut microbes.We also investigated whether the lower body weight and fat mass observed onL.tenuistreatment caused by acute inflammation,and the results revealed that the administration ofL.tenuisimproved gut barrier integrity and slightly alleviated intestinal inflammation (Figs.3G,3J and 5O).
Previous studies have revealed that some gut-derived next generation probiotics exerted beneficial effects on host metabolism in a metabolism-dependent manner[51-52].Namely,the cells must be alive and metabolically-active in the host.For example,theP.distasonisalleviates obesity and metabolic dysfunctions via production of succinate and secondary bile acids[23].B.xylanisolvensimproved liver fat deposition via production of folate,whileClostridium butyriumbenefit host by production of butyrate[22,53].The pasteurization of cells counteracted the efficacy.Unlike the mentioned previous works,in our study,we observed that the glucose and lipid metabolism,inflammation levels and intestinal barrier integrity in HFD and DIO mice were improved by both the live and pasteurizedL.tenuiscells,while only the liveL.tenuisgavage exerted efficacy in body weight control.It hinted that theL.tenuisplays a therapeutic role in weight control via a different mechanism from that in the improvement of host glucose and lipid metabolism.The former might be cell metabolism-dependent,while the latter is not,which is more likely to be the effect of the components of cells,such as the compositional biomacromolecules or lysate.Actually,it was not the first report of live and dead gut microbial cells interacted with host via different mechanisms.The representative exampleA.muciniphilaof which both the live and pasteurized cells could improve obesity.However,the live cells functioned by secretion of P9 protein to promote thermogenesis by activating the GLP-1R signalling pathway and IL-6[15,17,54],while the therapeutic effect of pasteurized cells was mainly ascribed to its outer membrane protein Amuc_1100[55].Though,we did not elucidate the definite mechanisms underpinned the differed beneficial effects of live and pasteurizedL.tenuis,we already identified the functional phenotypes of the new taxon and implied that the functional mechanism might be different from those previously reported probiotics.In a word,we provided a potential probiotic candidate for weight control and broadened the understanding of gut bacteria and their potential functions with this work.WhetherL.tenuisas well as other published functional gut microbes can be further developed into the next generation of probiotics needs further validation with clinical trials.
5.Conclusion
In this study,we demonstrated by joint-analysis of the gut metagenomic datasets thatL.tenuisas a newly-described gut commensal bacterium was prevalent among healthy humans but depleted significantly in the cohort of obesity.We then verified that administration ofL.tenuisto C57BL/6J mice significantly decreased the high-fat-diet-induced weight gains and restrained the food intake,which was in parallel with altered expression levels of genes involved in satiety and feeding behavior.Further gavage trial with a metabolic-syndrome murine model demonstrated thatL.tenuisalso exerted therapeutic effects on the pathoglycemia and dyslipidemia of DIO mice.These results provide a rationale for the development ofL.tenuisas a next-generation probiotic for treatment of obesity and associated metabolic disorders.
Conflict of interest
The authors declare that there is no conflict of interest.
Acknowledgements
This work was financially supported by the National Key Research and Development Program of China (2021YFA0717002).
Appendix A.Supplementary data
Supplementary data associated with this article can be found,in the online version,at http://doi.org/10.26599/FSHW.2022.9250071.
- 食品科学与人类健康(英文)的其它文章
- Protective effects of oleic acid and polyphenols in extra virgin olive oil on cardiovascular diseases
- Diet and physical activity inf luence the composition of gut microbiota,benef it on Alzheimer’s disease
- Inf luence of nitrogen status on fermentation performances ofnon-Saccharomyces yeasts: a review
- Ganoderma lucidum: a comprehensive review of phytochemistry,eff icacy,safety and clinical study
- Resveratrol combats chronic diseases through enhancing mitochondrial quality
- Demonstration of safety characteristics and effects on gut microbiota of Lactobacillus gasseri HMV18

