Palmitoleic acid on top of HFD ameliorates insulin resistance independent of diacylglycerols and alters gut microbiota in C57BL/6J mice
Qijin Ling,Yn Zheng,Fnli Meng,Xiofn Jing,Qingci Zhen,Zhongting Lu,Shixiu Zhng,Lei Du,Ho Wu,,Xin Guo,
a Department of Nutrition and Food Hygiene, School of Public Health, Cheeloo College of Medicine, Shandong University, Jinan 250012, China
b Research Center of Translational Medicine, Jinan Central Hospital Aff iliated to Shandong First Medical University, Jinan 250013, China
c Department of Hepatology, Qilu Hospital of Shandong University, Jinan 250012, China
d Institute of Hepatology, Shandong University, Jinan 250012, China
Keywords: Palmitoleic acid High fat diet Insulin resistance Gut microbiota
ABSTRACT
1.Introduction
Obesity due to over-nutrition has been on the rise over the past few decades.Obesity increases the risk of many diseases,especially insulin resistance,type 2 diabetes and cardiovascular disease[1].Insulin resistance,also known as impaired insulin sensitivity,occurs when cells in muscle,fat and liver do not respond properly to insulin.Insulin is a hormone secreted by pancreatic β cells after food ingestion and is essential for regulating blood glucose levels[2].Chronic inflammation in metabolic related organs such as liver and adipose tissue is received much attention as the mediator between obesity and insulin resistance[3].Excessive storage of triacylglycerol in adipocytes damages the function of adipocytes and induces macrophages to inf iltrate into adipose tissue,leading to inf lammation,thus changing the expression and secretion of adipokines[4-5].Over nutrition also causes macrophage infiltration and activation of inflammatory pathways in the liver,such as nuclear factor kappa light chain enhancer of activated B cells (NF-κB) and c-Jun N-terminal kinases (JNK)pathways,as well as increasing the expression of inflammatory cytokines such as tumor necrosis factor alpha (TNF-α),interleukin 6 (IL-6),and interleukin 1β (IL-1β),leading to hepatic insulin resistance[6-7].
In humans,the ultimate cause of obesity is that long-term energy intake is greater than energy output,that is,overeating and inactivity lifestyle.The causes of overeating and inactivity lifestyle are complicated and related to psychological,social,environmental,and genetic factors[8].Rodents,such as diet induced obesity (DIO)mice,are widely used to study the pathogenesis of human obesity and obesity related diseases.A normal rodent’s diet contains about 10%kcal of fat.In order to induce the generation of obesity,45% kcal fat diet or 60% kcal fat diet is used in mice.Mice fed 45% fat diet become obese,while mice fed 60% fat diet become more obese and more quickly to do so.This reduces the time and costs for rodents to become obese.Therefore,many researchers often use 60% highfat diet to induce obesity in rodents from the perspective of economy and convenience.However,60% fat diet has a shortcoming,that is,the high-fat diet of human beings,such as the typical American or European diet (about 36%-40% fat in terms of energy),cannot achieve 60% of energy from fat.Therefore,45% fat diet for rodents may be closer to the high-fat diet of humans than 60% fat diet[9].Researchers also found that although 60% fat diet produces more exaggerated metabolic reactions than 40% fat diet,the difference in obesity,physiology and metabolism between rodents fed with 45% fat diet and rodents fed 60% fat diet is relatively small[9-11].
The gastrointestinal tract is the first organ to be exposed to dietary components,such as saturated fat and refined sugar,and their excessive intake can increase the incidence of obesity.Intestinal inflammation is found in both obese humans and mice[12-13].Many evidence suggest that the composition of gut microbiota is associated with obesity and obesity related insulin resistance[14].Gut microbiota is altered to promote reduction of adiposity after bariatric surgery[15].Transplantation of fecal microbiota from lean donor to overweight or obese recipient promotes weight loss and ameliorates metabolic syndrome for the latter both in human and mice[16-18].Several phyla of bacteria are identified to significantly associate with metabolic diseases.For example,the abundance of Firmicutes was positively correlated with the obesity level[19].A rise in the Firmicutes/Bacteroidetes ratio is related to increasing of a low-grade inflammation and insulin resistance[20].Akkermansia muciniphilais negatively correlated with obesity,diabetes,and cardiovascular disease[21].Supplementation ofA.muciniphilaimproves metabolic syndrome,thereforeA.muciniphilais a potential probiotics for treatment of type 2 diabetes and obesity[22].Gut microbiota regulates inflammation through a variety of mechanisms.Gut microbiota contribute to health and integrity of the human gut barrier.In chronic diseases and states of chronic inflammation,there is a diminished gut barrier function.Lipopolysaccharide(LPS) from the outer membrane of Gram-negative bacteria may transfer through the intestinal boundary and lead to systemic inflammation.In addition,metabolites from gut microbiota,such as short chain fatty acids (SCFAs),ethanol,and secondary bile acids,have been shown to regulate the host immune system[23].
Dietary fats may affect host physiological function through interaction with gut microbiota.A study compared metabolism and gut microbiota in mice fed with different fats-rich diets,namely,low fat diet,high saturated fat diet,highn-6 polyunsaturated fatty acid (n-6-PUFA) diet or highn-3 PUFA diet.Mice fed with high saturated fat diet or highn-6-PUFA diet significantly increased fat accumulation and body weight,while mice fed with high saturated fat diet increased insulin resistance,colonic permeability,and adipose tissue inflammation.Mice fed with high saturated fat diet and mice fed with highn-6-PUFA diet had a similar gut microbiota composition,which were very different from the gut microbiota in mice fed with low fat diet and mice fed with highn-3 PUFA diet[24].Compared with highn-3 PUFA diet,high saturated fat diet decreased the abundance ofA.muciniphila,LactobacillusandBifidobacteriumin gut and increased white adipose tissue inflammation and insulin resistance via LPS/toll-like receptor (TLR)/C-C motif chemokine ligand 2 (CCL2) pathway[25].The effects of olive oil,which is rich in oleic acids (a type of monounsaturated fatty acid),on gut microbiota are inconsistent.Mice fed with HFD (olive oil,45% kcal) for 3 weeks increased the abundance of Bacteroidetes and decreased the abundance of Firmicutes[26].However,another study fed mice with HFD (olive oil,45% kcal) for 16 weeks got the opposite results in the abundance of Bacteroidetes and Firmicutes[27].Although studies indicate that fatty acids may have antibacterial activity or may be used as metabolic substrates by gut microbiota,the mechanism by which dietary fatty acids affect gut microbiota remains unclear[28-30].
Palmitoleic acid (16:1) is an omega-7 monounsaturated fatty acid found in plants and seafood.It can also be synthesized in adipocytede novofatty acid synthesis.Many evidence suggests that palmitoleic acid serves as a lipokine providing beneficial effects for prevention and management of metabolic diseases[31-32].Palmitoleic acid reduces hepatic steatosis,the level of toll-like receptor 4 and inflammatory cytokines such as TNF-α and IL-6 induced by HFD,as well as decreasing macrophage infiltration via peroxisome proliferator activated receptor (PPAR)-γ activation[33].In low-density lipoprotein receptor knockout (LDLR-KO) mice,palmitoleic acid retards atherosclerosis development by improving lipid and glucose metabolism and reducing inflammation[34-35].Palmitoleic acid increases glucose uptake in adipocytes via AMPactivated protein kinase (AMPK) activation[36]and reduces muscle insulin resistance induced by palmitic acid through p38 mitogenactivated protein kinase (p38 MAPK) pathway[37].Palmitoleic acid is also reported to regulate pancreatic β-cells apoptosis and insulin secretion[32,38].Although there are many evidence showed that palmitoleic acid is associated with reducing inflammation and increasing insulin sensitivity,the mechanism of the beneficial effects of palmitoleic acid,especially related to gut microbiota,is still unclear.Therefore,we investigated the beneficial phenotype of palmitoleic acid and the alteration of gut microbiota in HFD-induced obese mice.
2.Methods and materials
2.1 Animal experiment
Four-week-old male C57BL/6J mice were obtained from Beijing Vital River Laboratory Animal Technology Corporation (Beijing,China).Mice in the same cage share similar gut microbiota due to coprophagia,resulting in inter-cage differences.This is called “cage effects”[39-40].To eliminate cage effects,2 mice were housed in a cage instead of 4 mice in a cage.Beginning at 6 weeks age,mice were fed with high-fat diet (HFD) or Chow diet (CD) for 12 weeks.HFD(Research diet,#D12492) contains 20% kcal energy from protein(Casein),20% kcal energy from carbohydrate that the main ingredient is sucrose,and 60% kcal energy from fat that the main ingredient is lard.In this experiment,we fed mice with 60% fat HFD to induce obesity model,because compared with 45% fat HFD,mice fed with 60% fat HFD can become more obese in a short time.In HFD or CD,mice were randomly divided into 3 groups (in each group,n=8)and were treated with palmitoleic acid (600 mg/day·kg body weight,conjugated with BSA and suspended in phosphate-buffered saline),oleic acid (600 mg/day·kg body weight,conjugated with BSA and suspended in phosphate-buffered saline),or BSA via oral gavages for 6 weeks,respectively.Before dissection,glucose tolerance test (GTT)was conducted.After mice were recovered for at least one week,mice were fasted for 8 h and collected blood samples under anesthesia by isoflurane.Then mice were sacrificed by snapping the neck and severing the spine,liver,adipose tissue,muscle,and fecal samples were collected.Bloods samples were centrifuged at 12 000 r/min and 4 °C for 5 min.Serums were collected to measure fasting blood glucose and insulin.Liver,adipose tissue,muscle,and fecal samples were frozen in liquid nitrogen and then stored at -80 °C for further analyses.All procedures were approved by the Institutional Animal Care and Use Committee at Shandong University and performed in conformance with the guide.
2.2 GTT
GTT was used to detect glucose metabolism in CD and HFD-fed mice.Mice were fasted with sani-chip (hard wood) bedding (without wire floor to reduce stress) overnight with water and lids.Bring the mice to the procedure room in the morning for at least 2 h so that they can be accustomed to the environment to reduce stress.D-glucose(sigma,Cat#: G7528) solution (2 g/kg body weight) was injected intraperitoneally followed by blood sampling at 0,30,60,90,120 min using TRUEresult glucose meter.
2.3 HOMA-IR
Serums from fasting blood were collected to measure fasting blood glucose and insulin.Fasting blood glucose was measured by glucose assay kit (Jiancheng,Nanjing,#F006-1-1).Insulin level was detected by mouse insulin ELISA kit (Invitrogen,#EMINS 96 test).The calculation of HOMA-IR was that the level of fasting blood glucose (mmol/L) multiplied by the level of fasting insulin (mU/mL),then divided by 22.5.
2.4 Diacylglycerol (DAG) measurement
The levels of DAGs in liver and white adipose tissue were measured by a DAG assay kit (Abcam,#Ab242293).In short,liver and adipose tissue were homogenized in PBS and centrifuged at 12 000 ×gfor 15 min at 4 °C.And the supernatants were collected to extract DAGs following the manufacturer’s instructions.The extracted DAG was phosphorylated by a kinase to yield phosphatidic acid,which was hydrolyzed to glycerol-3-phosphate.Finally,glycerol-3-phosphate was oxidized by glycerol-3-phosphate oxidase (GPO),producing hydrogen peroxide which reacted with a fluorometric probe for the quantification of DAGs.The final levels of DAGs were normalized by protein concentration.
2.5 16S rDNA Sequence
Total genome DNA from fecal samples was extracted using CTAB method.DNA concentration and purity was monitored on 1%agarose gels.According to the concentration,DNA was diluted to 1 ng/µL using sterile water.16S rRNA/18S rRNA/ITS genes of distinct regions (16S V4/16S V3/16S V3-V4/16S V4-V5,18S V4/18S V9,ITS1/ITS2,Arc V4) were amplified used specific primer (e.g.,16S V4: 515F806R,18S V4: 528F-706R,18S V9: 1380F-1510R,et al.)with the barcode.All PCR reactions were carried out with 15 µL of Phusion® High-Fidelity PCR Master Mix (New England Biolabs);2 µmol/L of forward and reverse primers,and about 10 ng template DNA.Thermal cycling consisted of initial denaturation at 98 °C for 1 min,followed by 30 cycles of denaturation at 98 °C for 10 s,annealing at 50 °C for 30 s,and elongation at 72 °C for 30 s.Finally,72 °C for 5 min.Mix same volume of 1X loading buffer (contained SYB green) with PCR products and operate electrophoresis on 2%agarose gel for detection.PCR products was mixed in equidensity ratios.Then,mixture PCR products was purified with Qiagen Gel Extraction Kit (Qiagen,Germany).Sequencing libraries were generated using TruSeq®DNA PCR-Free Sample Preparation Kit(Illumina,USA) following manufacturer’s recommendations and index codes were added.The library quality was assessed on the Qubit@ 2.0 Fluorometer (Thermo Scientific) and Agilent Bioanalyzer 2100 system.At last,the library was sequenced on an Illumina NovaSeq platform and 250 bp paired-end reads were generated.
2.6 Bioinformatic analysis
Paired-end reads was assigned to samples based on their unique barcode and truncated by cutting off the barcode and primer sequence.Paired-end reads were merged using FLASH (V1.2.7)[41].Quality filtering on the raw tags were performed under specific filtering conditions to obtain the high-quality clean tags according to the QIIME (V1.9.1) quality-controlled process[42-43].The tags were compared with the reference database (Silva database) to detect chimera sequences,and then the chimera sequences were removed.Then the Effective Tags finally obtained.Sequences analysis were performed by Uparse software (Uparse v7.0.1001)[44].Sequences with ≥ 97% similarity were assigned to the same operational taxonomic units (OTUs).Representative sequence for each OTU was screened for further annotation.For each representative sequence,the Silva Database[45]was used based on Mothur algorithm to annotate taxonomic information.In order to study phylogenetic relationship of different OTUs,and the difference of the dominant species in different samples (groups),multiple sequence alignment was conducted using the MUSCLE software (Version 3.8.31)[46].OTUs abundance information were normalized using a standard of sequence number corresponding to the sample with the least sequences.Subsequent analysis ofα-diversity andβ-diversity were all performed basing on this output normalized data.α-Diversity is applied in analyzing complexity of species diversity for a sample through 6 indices,including Observed-species,Chao1,Shannon,Simpson,abundance-based coverage estimators (ACE),Good-coverage.All the indices in our samples were calculated with QIIME (Version 1.7.0)and displayed with R software (Version 2.15.3).β-Diversity analysis was used to evaluate differences of samples in species complexity,β-diversity on both weighted and unweighted unifrac were calculated by QIIME software (Version 1.9.1).
2.7 Immunohistochemistry (IHC) and hematoxylin-eosin(H&E) staining
Liver,colon,and adipose tissue were fixed in neutral formalin for 72 h and made into slices with a thickness of 5 μm for H&E staining and 8 μm for IHC.After that,H&E staining or IHC were performed and observed under microscope.For IHC,the antibody was F4/80(1:400) (Cell Signaling Technology,#70076).
2.8 Western blot analysis
Lysates prepared from frozen tissue samples were used for Western blot.The level of phospho-NF-κB p65,NF-κB p65 (Cell Signaling Technology,#8214),the stress-activated protein kinase/Jun-amino-terminal kinase (SAPK/JNK) (Cell Signaling Technology,#9252S),phospho-SAPK/JNK(Thr183/Thr185) (Cell Signaling Technology,#9251S),glucose transporter type 4 (GLUT4) (Cell Signaling Technology,#2213),Occludin (Affinity Biosciences,# DF7504),Claudin-1 (Affinity Biosciences,# AF0127),and Tubulin(Cell Signaling Technology,#2125) were analyzed as described[4].
2.9 Real-time quantitative polymerase chain reaction(RT-PCR)
Total RNA was extracted from colon,adipose tissue,and liver with TRIzol and purified by miRNeasy Micro Kit (Qiagen.#217084).cDNA was obtained by reverse transcription from total RNA using SuperScript IV Reverse Transcriptase (Invitrogen,#18090010),and mRNA expression levels were analyzed by Roche LC480 fluorescence quantitative PCR (#28424) using Platinum™ Taq DNA Polymerase(Invitrogen,# 15966005)[47].In colon,the expressions of TNF-α,IL-1β,IL-6,and monocyte chemoattractant protein 1 (MCP-1) were measured.In adipose tissue,the levels of adiponectin and MCP-1 were measured.In liver,MCP-1 was measured.Primers of those genes were listed in Table S1.
2.10 Statistical analysis
Data are presented as mean ± standard error of the mean (SEM).One-way ANOVA and least significance difference (LSD) method as a suitable post-hoc test was used to determine the differences among groups by using SPSS 26 (IBM,SPSS,USA) and aP-value < 0.05 was considered statistically significant.For data from 16s rDNA sequencing,Wilcoxon rank sum test,Tukey test,t-test,or MetaStat method was used.Data are presented as mean ± standard deviation (s.d.).
3.Results
3.1 Palmitoleic acid reduces HFD-induced insulin resistance
Six weeks old mice were fed with HFD or CD for 12 weeks.In HFD or CD,mice were randomly divided into 3 groups and were treated with palmitoleic acid,oleic acid,or BSA as control via oral gavages for 6 weeks on top of CD or HFD feeding,respectively.Insulin resistance was significantly increased in HFD-fed mice compared to CD-fed mice (Figs.1A-C).Palmitoleic acid significantly increased insulin sensitivity and glucose clearance in HFD-fed mice from GTT (Figs.1A and B) and HOMA-IR (Fig.1C),compared to BSA control and oleic acid treated mice.However,in CD-fed mice,palmitoleic acid did not exhibited the effect of increasing insulin sensitivity (Figs.1A-C).This data indicated that palmitoleic acid could reduce insulin resistance in HFD-induced obese mice.

Fig.1 Palmitoleic acid reduces HFD-induced insulin resistance.6 weeks old mice were fed with HFD or CD for 12 weeks.Mice were treated with palmitoleic acid,oleic acid,or BSA as control via oral gavages for 6 weeks.(A) GTT.(B) Area under curve (AUC) of GTT.(C) HOMA-IR.Calculated based on fasting glucose level (mmol/L) and fasting insulin level (mU/mL).n =6-7 mice per group.The data are mean ± SEM (error bars).*P < 0.05,**P < 0.01,***P < 0.001,CD BSA vs HFD BSA;†P < 0.05,††P < 0.01,HFD BSA vs HFD palmitoleic acid;‡P < 0.05,‡‡P < 0.01,HFD oleic acid vs HFD palmitoleic acid;§P < 0.05,HFD BSA vs HFD oleic acid.
3.2 Palmitoleic acid does not alter body weight, liver weight,and fat content in HFD-induced obese mice
Over-nutrition leads to obese.In our study,HFD significantly increased body weight and fat content in mice (Figs.2B,D,and E).Moreover,HFD-fed mice exhibited impaired glucose tolerance and insulin resistance (Fig.1).In many cases,the enhancement of insulin sensitivity is due to the decrease of body weight and fat content via reducing energy intake[48].In our study,mice fed with HFD significantly increased average daily energy intake compared to mice fed with CD.However,there was no significant difference in the average daily energy intake (from diet and treatment) between groups with different treatments (BSA,oleic acid,and palmitoleic acid) either in mice fed with CD or in mice fed with HFD,although in CD-fed mice treated with palmitoleic acid,energy intake had an upward trend(Fig.2A).In addition,palmitoleic acid did not alter the body weight and liver weight in both HFD-fed and CD-fed mice (Figs.2B and C).In CD-fed mice,palmitoleic acid increased fat content including total visceral fat (the sum of epididymal fat,perinephric fat,and mesenteric fat) and inguinal fat,indicating that palmitoleic acid can promote adiposity in CD-fed mice (Fig.2D).However,palmitoleic acid did not change the fat content in HFD-fed mice (Fig.2E).Therefore,the data showed that although palmitoleic acid can increase adiposity in CD-fed mice,it did not alter body weight,liver weight,and fat content in HFD-induced obese mice.
3.3 Palmitoleic acid increases the diversity of gut microbiota in HFD-fed mice
Since palmitoleic acid had beneficial effects of increasing insulin sensitivity only in HFD-fed mice in this study,we analyzed 16S rDNA sequence from the fecal samples of HFD-fed mice.According to OTUs results obtained by clustering,the common and unique OTUs among different groups were analyzed.Venn Graph showed that palmitoleic acid increased the number of OTUs in the gut microbiota of HFD-induced obese mice (Fig.3A).Rarefaction curve and rank abundance curve are common curves describing the diversity of samples within groups.Rarefaction curve can directly reflect the rationality of the sequencing data amount and indirectly reflect the richness of species in the samples.Rank abundance curve can intuitively reflect the richness and uniformity of species in the sample.The result from rarefaction curve and rank abundance curve exhibited that palmitoleic acid increased the richness and uniformity of gut microbiota species compared to BSA control and oleic acid treatment in HFD-fed mice (Figs.S1A and B).Wilcoxon rank sum test showed that observed species of gut microbiota from palmitoleic acid treated HFD-fed mice were significantly higher than BSA and oleic acid treated HFD-fed mice (Fig.3B).Palmitoleic acid increased gut microbiota in HFD-fed mice fromα-diversity of 3 indices including observed-species,Chao1,and ACE (Figs.S2A-C),while did not show differences among groups for Shannon,Simpson,and Good-coverage(Data not shown).Therefore,our data indicated that in HFD-fed mice,palmitoleic acid significantly enhanced the number of OTUs and the richness of species for gut microbiota.
3.4 Palmitoleic acid decreases the abundance of Firmicutes and increases the abundance of Bacteroidota in HFD-fed mice
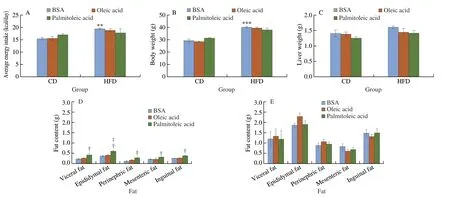
Fig.2 Palmitoleic acid does not alter body weight,liver weight,and fat content in HFD-induced obese mice,but increase adiposity in adipose tissue of CD-fed mice.6 weeks old mice were fed with HFD or CD for 12 weeks.Mice were treated with palmitoleic acid,oleic acid,or BSA as control via oral gavages for 6 weeks.(A) Average daily energy intake.Calculation based on food intake (g/day),CD (3.82 kcal/g) or HFD (5.24 kcal/g) from diet and body weight,8% BSA,8%BSA with 600 mg/kg/day oleic acid or palmitoleic acid from treatment.(B) Body weight.(C) Liver weight.(D) Fat content for CD-fed mice.(E) Fat content for HFD-fed mice.n =6-7 mice per group.The data are mean ± SEM (error bars).**P < 0.01,***P < 0.001,CD BSA vs HFD BSA;†P < 0.05,HFD BSA vs HFD palmitoleic acid;‡P < 0.05,HFD oleic acid vs HFD palmitoleic acid.

Fig.3 Palmitoleic acid increases the diversity of gut microbiota in HFD-fed mice.6 weeks old mice were fed with HFD for 12 weeks.Mice were treated with palmitoleic acid,oleic acid,or BSA as control via oral gavages for 6 weeks.Fecal samples were collected for 16s rDNA sequence.(A) Venn graph.(B) Observedspecies.n =5-6 mice per group.*P < 0.05,**P < 0.01.
The composition of gut microbiota was analysis to further investigate the effects of palmitoleic acid in the gut microbiota of HFD-fed mice.According to the annotation results of species,the top 10 species with maximum abundance in each group at each classification level (phylum,class,order,family,genus) were selected to generate the column accumulation diagram of relative abundance,to visually check the species with high relative abundance and their proportion at different classification levels of each sample.Relative abundance of phylum level showed that both palmitoleic acid and oleic acid treatment increased the abundance of Bacteroidota (also called Bacteroidetes) and Proteobacteria,and decreased the abundance of Firmicutes (Fig.4A).According to the species annotation and abundance information of all samples at the order level,the top 35 genera with the highest abundance were selected.Palmitoleic acid increased the abundance ofBacteroidalesandCytophagales,which belonged to Bacteroidota,and the abundance ofPseudomonadales,Alteromonadales,Oceanospirillales,etc.,which belonged to Proteobacteria(Fig.4B).Palmitoleic acid decreased the abundance ofErysipelotrichales,Lactobacillales,Bacillales,Staphylococcales,andLachnospirales,which is belonged to Firmicutes (Fig.4B).In order to further study the phylogenetic relationships of genus level species,the representative sequences of top 100 genera were obtained by multisequence alignment.The result further indicated that HFD facilitated the evolution of Firmicutes,while palmitoleic acid or oleic acid promoted the evolution of Bacteroidota and Proteobacteria(Fig.4C).In order to find the species with significant differences (P< 0.05)at different taxonomic levels,t-test was performed between groups.At phylum level,oleic acid significantly increased the abundance of Bacteroidota,while palmitoleic acid not only significantly increased the abundance of Bacteroidota,but also significantly decreased the abundance of Firmicutes(Fig.5A).At class level,palmitoleic acid or oleic acid enhanced the abundance of Bacteroidia,while only palmitoleic acid reduced the abundance of Desulfovibrionia (Fig.S3).In order to analyze the species with significant differences between study groups,the species abundance data between groups was analyzed using MetaStat method based on the species abundance tables of different levels.The results also showed at phylum level,palmitoleic acid significantly increased the abundance of Bacteroidota and reduced the abundance of Firmicutes in HFD-fed mice (Fig.5B).

Fig.4 Palmitoleic acid decreases the abundance of Firmicutes and increases the abundance of Bacteroidota in HFD-fed mice.6 weeks old mice were fed with HFD for 12 weeks.Mice were treated with palmitoleic acid,oleic acid,or BSA as control via oral gavages for 6 weeks.Fecal samples were collected for 16S rDNA sequence.(A) Relative abundance at phylum level.(B) Heatmap cluster of relative abundance.(C) Phylogenetic relationship of species at genus level.n =5-6 mice per group.
3.5 Palmitoleic acid promotes intestinal tight junction integrity and reduces HFD-induced colon inflammation

Fig.5 Palmitoleic acid decreases the abundance of Firmicutes and increases the abundance of Bacteroidota in HFD-fed mice.6 weeks old mice were fed with HFD for 12 weeks.Mice were treated with palmitoleic acid,oleic acid,or BSA as control via oral gavages for 6 weeks.Fecal samples were collected for 16S rDNA sequence.(A) Species difference at phylum level by t-test.(B) Species difference at phylum level by MetaStat method.n =5-6 mice per group.The data are mean ± s.d.(error bars).*P < 0.05.

Fig.5 (Continued)
Because the richness and composition of gut microbiota were associated with intestinal function which influenced the systemic metabolic homeostasis[49],the colon was collected and analyzed for intestinal tight junction integrity and inflammation.Occludin is an enzyme that services as an integral plasma-membrane protein localized at the tight junctions.Claudin-1 is one of the claudins that plays important structural and functional roles in tight junctions.Claudin-1 and occludin regulate the formation,maintenance,and function of tight junction in intestine[50].In HFD-fed mice,palmitoleic acid significantly increased the level of occludin (Figs.6A and B) and claudin-1 (Figs.6A and C) compared to BSA and oleic acid treatment.Oleic acid also showed the enhancement of claudin-1 level compared to BSA control (Figs.6A and C).The result indicated that both oleic acid and palmitoleic acid had beneficial effect on maintaining intestinal tight junction integrity,but palmitoleic acid played a more important role.In addition,palmitoleic acid reduced HFD-induced macrophage infiltration (Fig.6D) and the levels ofMCP-1,TNF-α,andIL-1β(Fig.6E) in colon,indicating downregulating inflammatory response.Together,the results found that palmitoleic acid promoted intestinal tight junction integrity and reduced HFD-induced colon inflammation.

Fig.7 Palmitoleic acid decreases HFD-induced adipose tissue macrophage infiltration and inflammation,and improves the function of adipose tissue.6 weeks old mice were fed with HFD for 12 weeks.Mice were treated with palmitoleic acid,oleic acid,or BSA as control via oral gavages for 6 weeks.Adipose tissue was collected for further analysis.(A) Macrophage infiltration by IHC for F4/80.(B) mRNA levels of MCP-1 by RT-PCR.(C) mRNA levels of adiponectin by RT-PCR.(D) Level of GLUT4 by Western blotting.(E) Quantification of GLUT4. n =4 mice per group.The data are mean ± SEM (error bars).†P < 0.05,††P < 0.01,†††P < 0.001,BSA vs palmitoleic acid;‡P < 0.05,‡‡P < 0.01,oleic acid vs palmitoleic acid;§P < 0.05,BSA vs oleic acid.
3.6 Palmitoleic acid decreases HFD-induced adipose tissue macrophage infiltration and inflammation, and improves the function of adipose tissue
Adipose tissue stores energy after eating and releases it during starvation.When blood glucose is increased,the body secretes insulin to stimulate glucose uptake by adipocytes to reduce glucose in circulation.Glucose enters adipocytes for glycolysis,and the metabolites are used to synthesize triglycerides and stored in adipocytes.Adipose tissue also has an endocrine function and can control metabolic balance by secreting adipokines.Therefore,adipose tissue plays an important role in metabolic diseases[4,51].Palmitoleic acid significantly reduced HFD-induced macrophage infiltration in adipose tissue (Fig.7A).Further study found that HFD-fed mice treated with palmitoleic acid exhibited significantly lowered mRNA level ofMCP-1,which is a major mediator of monocyte/macrophage infiltration at the inflammatory sides,compared to HFD-fed mice treated with BSA or oleic acid (Fig.7B).Both palmitoleic acid and oleic acid increased the level of adiponectin,which is an important adipokine that enhances insulin sensitivity and metabolic balance(Fig.7C).However,only palmitoleic acid enhanced the expression of glucose transporter 4 (GLUT4),which is the insulin-regulated glucose transporter found primarily in adipose tissues (Figs.7D and E).Therefore,palmitoleic acid decreased HFD-induced adipose tissue macrophage infiltration and inflammation,as well as improves the function of adipose tissue.
3.7 Palmitoleic acid decreases HFD-induced hepatic macrophage infiltration and inflammation
The liver is an important organ in glucose metabolism,which influences systemic glucose metabolism[52].In HFD-fed mice,palmitoleic acid significantly decreased macrophage infiltration compared to BSA control and oleic acid treatment (Fig.8A).HFD-fed mice treated with palmitoleic acid exhibited significantly lowered mRNA level ofMCP-1compared to HFD-fed mice treated with BSA or oleic acid (Fig.8B).In addition,palmitoleic acid significantly reduced the phosphorylation of JNK1 and NF-κB in HFD-fed mice,indicating reducing HFD-induced pro-inflammatory response (Figs.8C-E).Together,palmitoleic acid decreases HFD-induced hepatic macrophage infiltration and pro-inflammatory response.
3.8 Palmitoleic acid does not alter the levels of DAGs in liver and adipose tissue
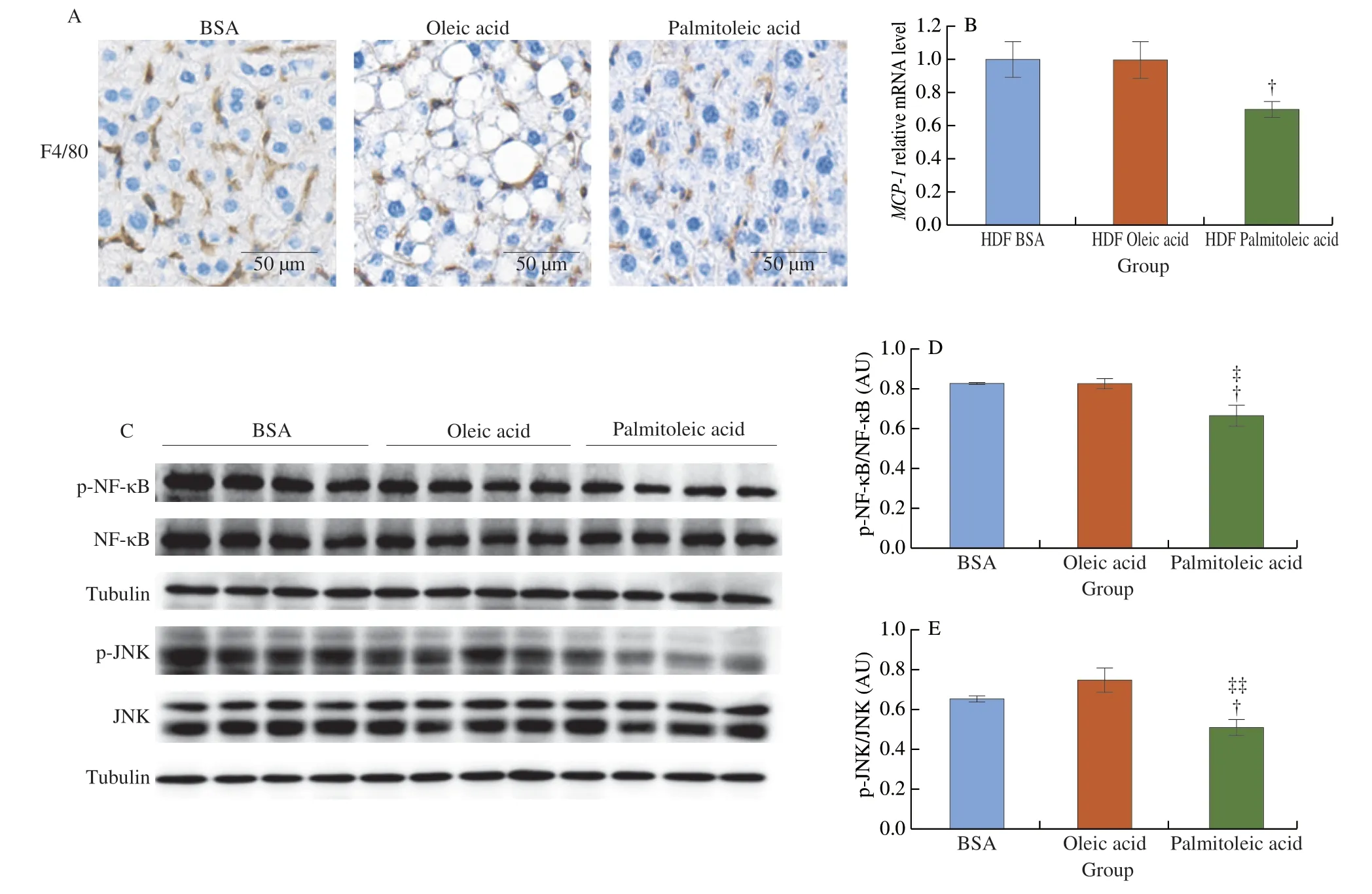
Fig.8 Palmitoleic acid decreases HFD-induced hepatic macrophage infiltration and inflammation.6 weeks old mice were fed with HFD for 12 weeks.Mice were treated with palmitoleic acid,oleic acid,or BSA as control via oral gavages for 6 weeks.The liver was collected for further analysis.(A) Macrophage infiltration by IHC for F4/80.(B) mRNA levels of MCP-1 by RT-PCR.(C) Level of phosphorylation of NF-κB and JNK by Western blotting.(D) Quantification of NF-κB phosphorylation.(E) Quantification of JNK phosphorylation.n =4 mice per group.The data are mean ± SEM (error bars).†P < 0.05,BSA vs palmitoleic acid;‡P < 0.05,‡‡P < 0.01,oleic acid vs palmitoleic acid.
DAG,which is a bioactive signaling lipid,in tissue (for example,liver) is found to positively correlate with HOMA-IR[53].DAG activates protein kinase Cε,which subsequently inhibits the insulin receptor kinase and then reduces insulin-stimulated tyrosine phosphorylation of insulin receptor substrate-1 and -2,leading to impair insulin signaling[54].In our study,to identify whether DAG was related to increase insulin sensitivity by palmitoleic acid,DAGs were measured in liver and adipose tissue (epididymal fat)of both CD-fed and HFD-fed mice.The results showed that HFD significantly increased the level of DAGs in liver,but not in visceral fat (Fig.9).Palmitoleic acid had a trend to increase DAGs in liver of CD-fed mice,but no significance (Fig.9A).Both oleic acid and palmitoleic acid had a trend to increase DAGs in adipose tissue of HFD-fed mice,but no significance (Fig.9B).Palmitoleic acid or oleic acid did not alter the levels of DAGs in liver and adipose tissue of CD-fed or HFD-fed mice,compared to BSA control (Fig.9).Therefore,palmitoleic acid did not reduce the levels of DAGs in liver and adipose tissue of HFD-fed mice.It indicated that palmitoleic acid reduced insulin resistance in HFD-fed mice by an independentmanner of DAGs.

Fig.9 Palmitoleic acid does not alter the levels of DAGs in liver and adipose tissue.6 weeks old mice were fed with HFD or CD for 12 weeks.Mice were treated with palmitoleic acid,oleic acid,or BSA as control via oral gavages for 6 weeks.Liver and adipose tissue (epididymal fat) were collected for analyzing the levels of DAGs.(A) The level of DAGs in liver.(B) The level of DAGs in white adipose tissue.n =5-6 mice per group.The data are mean ± SEM(error bars).*P < 0.05,CD BSA vs HFD BSA.
4.Discussion
The gut microbiota plays an important role in normal intestinal function and maintenance of the health of the host.The gut microbiota is closely related to the onset and development of metabolic diseases,such as obesity,type 2 diabetes,nonalcoholic fatty liver disease,cardiovascular disease and so on[55].Obesity is associated with decreased bacterial diversity,changes in bacterial gene expression and metabolic pathways.Obese people had different proportions of specific phyla in their gut microbiota: fewer Bacteroidetes and more Firmicutes than lean controls[56].In obeseob/obmice and DIO mice,the phylum Firmicutes is the bacteria with a great increase[57-58].HFD-fed germ-free mice gain less body weight than control mice fed with HFD and are protected from insulin resistance.In addition,transplanting the gut microbiota of conventionally fed mice into germ-free mice led to increased body weight and fat content,as well as increased insulin resistance and glucose tolerance[59].Antibiotics also can influence systemic glucose homeostasis by altering gut microbiota[60].Therefore,the gut microbiota regulates systemic metabolism,especially glucose intolerance and insulin sensitivity.
In this study,we fed mice with 60% fat HFD for 12 weeks to induce obesity model,because mice fed with 60% fat HFD can become more obese in a short time compared to mice fed with 45%fat HFD.However,the typical American or European diet contains about 36%-40% fat by energy.Thus,our study with a 60% fat content may be potentially not relevant to human physiology.After 12 weeks HFD feeding,mice were treated with palmitoleic acid(600 mg/day·kg body weight,conjugated with BSA and suspended in phosphate-buffered saline),oleic acid (600 mg/day·kg body weight,conjugated with BSA and suspended in phosphate-buffered saline),or BSA via oral gavages for 6 weeks while continuing HFD feeding.We found that HFD significantly increased body weight,fat content,and insulin resistance in mice,while palmitoleic acid reduced HFDinduced insulin resistance but did not change the body weight and fat content.Palmitoleic acid also did not affect energy intake either in CD-fed mice or HFD-fed mice,although in CD-fed mice treated with palmitoleic acid,energy intake had an upward trend.In addition,palmitoleic acid did not show beneficial effects on insulin sensitivity in CD-fed mice.This might due to CD-fed mice had better insulin sensitivity,palmitoleic acid couldn’t show the improvement effects.However,palmitoleic acid can promote adiposity in CD-fed mice.This may be due to the slightly higher energy intake of this group of mice.Moreover,it is found in a population study that palmitoleic acid level in plasma is positively correlated with fat deposition[61].Intragastric administration of palmitoleic acid to mice can increase the level of palmitoleic acid in blood circulation[62].In order to further investigate the mechanism of the effects of palmitoleic acid on glucose homeostasis and insulin resistance in HFD-fed mice.We did 16S rDNA sequence for HFD-fed mice fecal samples to detect the alteration of gut microbiota.We found that palmitoleic acid increased gut microbiota diversity in HFD-induced obese mice fromα-diversity of 3 indices including observed-species,Chao1,and ACE.HFD increased the abundance of Firmicutes at phylum level.Oleic acid significantly increased the abundance of Bacteroidota (Bacteroidetes),while palmitoleic acid not only significantly increased the abundance of Bacteroidota,but also significantly decreased the abundance of Firmicutes.At class level,both palmitoleic acid and oleic acid enhanced the abundance ofBacteroidia,while only palmitoleic acid reduced the abundance ofDesulfovibrionia.Desulfovibrioniabelongs to Desulfobacterota at phylum level.Desulfovibrioniais associated with metabolic disorders,because it metabolizes sulfate to produce hydrogen sulfide,leading to damages of the intestinal epithelial mucosa and enhance chronic inflammation[63].
The gut microbiota composition affects intestinal integrity,which appear to be the key for a mechanism that maintains the gut health[64].Intestinal integrity is the ability of the body to maintain a well-regulated barrier function.The gut microbiota metabolizes dietary components and produces many active microbial metabolites.Studies have found that microbial metabolites directly interacted with intestinal epithelial cells and immune cells,affecting host health[65].Dietary fibers were fermented by gut microbiota such asBifidobacteriumandBacteroidesto SCFA,which was reported to increase the expressions of claudin-1 and occludin and stimulate anion release to maintain osmotic equilibrium in the intestinal lumen for resident microbes[66].Dietary tryptophan is catabolized into indole by gut microbiota.Indole and indole derivatives enhanced gut barrier function via secretion of incretin hormone glucagon-like peptide-1 via the aryl hydrocarbon receptor[67].In addition,the bacterial components and their receptors also participate in regulating intestinal integrity.For example,bacterial lipopeptides binds to TLR2,resulting in activation of protein kinase Cα and protein kinase δ,which increase apical tightening and sealing of the tight junctions[68].Impaired barrier function could result in enhanced intestinal permeability and thereby facilitate the translocation of microbiota-derived endotoxins,such as LPS into the systemic circulation[50].Intestinal barrier dysfunction and the subsequent translocation of bacteria and bacterial products are now considered to be important mechanisms associated with obesity and insulin resistance[69].In this study,we found that palmitoleic acid improved intestinal tight junction integrity by increasing the levels of claudin-1 and occludin.Oleic acid also increased the level of claudin-1,but not occludin level.Moreover,palmitoleic acid reduced HFD-induced macrophage infiltration and the levels of MCP-1,TNF-α,and IL-1β,indicating downregulating inflammation in colon.A study found that seabuckthorn pulp and seed oils,which mainly contained palmitoleic acid had a protective effect on radiation-induced acute intestinal injury by downregulating the levels of TNF-α,IL-1β,IL-6,and IL-8[70].A human study showed that palmitoleic acid decreased the inflammatory activity through the increased expression of hepatocyte nuclear factor (HNF)4α and HNF4γ in patients with ulcerative colitis[71].Therefore,our study and others studies had found that palmitoleic acid reduces inflammation in the gut,and we also found that palmitoleic acid enhances the barrier function of the gut.
Obesity is closely related to inflammation of adipose tissue.A HFD damages the integrity or barrier function of the gut and leads to inflammation of adipose tissue[72].SCFAs,which play a role in maintaining intestinal integrity,significantly increased M2 (CD206+)macrophages and Tregs (CD4+CD25+) in a stromal vascular fraction of adipose tissue[73].Microbiota-derived LPS is involved in the transition of macrophages from the M2 to the M1 phenotype[74].In our study,we found palmitoleic acid decreased HFD-induced adipose tissue macrophage infiltration,increased the level of adiponectin and GLUT4,indicating improvement of adipose tissue function.It might be related to alteration of the gut microbiota composition and improve intestinal integrity by palmitoleic acid in HFD-fed mice.Gut microbiota and intestinal barrier are also closely related to liver inflammation.In non-alcoholic fatty liver disease,impaired intestinal barrier caused by nutrition stress increases the translocation of microbes and their products into the blood,leading to hepatic inflammation and even fibrosis/cirrhosis[75].Gut-derived bacterial products,such as LPS and unmethylated CpG DNA,activate the signaling pathways involved in liver inflammation and fibrogenesis through stimulating innate immune receptors,e.g.,toll-like receptors[76].The crosstalk between gut microbiota and liver also mediated by bile acids[30].
DAG,which is a precursor of triglycerides and can be serve as a signal molecule,is related to impaired insulin signaling in liver,adipose tissue,and muscle[53,77-78].In addition,hepatic level of DAG is positively correlated with HOMA-IR[53].Prebiotics such as human milk oligosaccharide 2’-fucosyllactose attenuates the development of liver steatosis and insulin resistance by alleviating gut dysbiosis and gut permeability and reducing hepatic levels of DAGs[79].In our study,we also found palmitoleic acid decreased insulin resistance,increased the abundance of Bacteroidota and decreased the abundance of Firmicutes,and improved gut integrity.Thus,we measured the levels of DAGs in liver and adipose tissue to detect if the phenotypes were related to DAGs.HFD significantly increased the level of DAGs in liver,but not in visceral fat.Other study in rats also found that the DAGs in visceral fat has not changed under HFD feeding[80].Palmitoleic acid or oleic acid did not alter the levels of DAGs in liver and adipose tissue of CD-fed or HFD-fed mice,compared to BSA control.Therefore,palmitoleic acid reduced insulin resistance in HFD-fed mice by a DAG independent-manner.Because palmitoleic acid significantly decreased macrophage infiltration and inflammation in colon,adipose tissue,and liver in HFD-fed mice.Thus,the effects of palmitoleic acid on improving systemic insulin sensitivity in HFDfed mice were due to reduction of local inflammation instead of attenuating lipid-induced insulin resistance.
5.Conclusion
In our study,palmitoleic acid was observed to increase systemic glucose clearance and decrease systemic insulin resistance in HFD-induced obese mice.Palmitoleic acid altered gut microbiota composition by decreasing the abundance of Firmicutes and increasing the abundance of Bacteroidetes,improved intestinal barrier,and reduced colon inflammation in HFD-fed mice.In addition,palmitoleic acid reduced HFD-induced hepatic macrophage infiltration and proinflammatory responses.Palmitoleic acid also decreased adipose tissue inflammation,as well as increased the level of adiponectin and glucose uptake in adipose tissue.However,palmitoleic acid did not reduce DAG levels in liver,which were found to increase under HFD feeding.Therefore,palmitoleic acid ameliorates insulin resistance independent of diacylglycerols,alters gut microbiota,and lowers tissue inflammation.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgments
This research was funded,in whole or in part,by National Natural Science Foundation of China (81803224) and Young Scholars Program of Shandong University (2018WLJH33) to X.G.;National Natural Science Foundation of China (81973031) and Cheeloo Young Scholar Program of Shandong University (21320089963054) to H.W.;Young Scholars Program of Shandong University (2018WLJH34)and the Laboratory for Marine Drugs and Bioproducts of Qingdao National Laboratory for Marine Science and Technology(LMDBKF-2019-05) to L.D.
Institutional review board statement
All experimental procedures were performed according to the Laboratory Animal Welfare Standards and approved by the Subcommittee of Experimental Animal Ethics,Shandong University(Permission number: SYKX20150015).
Appendix A.Supplementary data
Supplementary data associated with this article can be found,in the online version,at http://doi.org/10.26599/FSHW.2022.9250073.
- 食品科学与人类健康(英文)的其它文章
- Protective effects of oleic acid and polyphenols in extra virgin olive oil on cardiovascular diseases
- Diet and physical activity inf luence the composition of gut microbiota,benef it on Alzheimer’s disease
- Inf luence of nitrogen status on fermentation performances ofnon-Saccharomyces yeasts: a review
- Ganoderma lucidum: a comprehensive review of phytochemistry,eff icacy,safety and clinical study
- Resveratrol combats chronic diseases through enhancing mitochondrial quality
- Demonstration of safety characteristics and effects on gut microbiota of Lactobacillus gasseri HMV18

