A ratiometric f luorescent probe for hypoxanthine detection in aquatic products based on the enzyme mimics and f luorescence of cobalt-doped carbon nitride
Xin Wang,Chengyi Hong,Zhengzhong Lin,Zhiyong Huang
College of Ocean Food and Biological Engineering, Jimei University, Xiamen 361021, China
Keywords: Co doped g-C3N4 Ratiometric f luorescent probe Hypoxanthine Freshness Aquatic products
ABSTRACT A ratiometric f luorescent probe for hypoxanthine (Hx) detection was established based on the mimic enzyme and f luorescence characteristics of cobalt-doped graphite-phase carbon nitride (Co doped g-C3N4).In addition to emitting strong fluorescence,the peroxidase activity of Co doped g-C3N4 can catalyze the reaction of O-phenylenediamine and H2O2 to produce diallyl phthalate which can emit yellow fluorescence at 570 nm.Through the decomposition of Hx by xanthine oxidase,Hx can be indirectly detected by the generating hydrogen peroxide based on the measurement of fluorescent ratio I (F570/F370).The linear range was 1.7-272.2 mg/kg(R2=0.997),and the detection limit was 1.52 mg/kg (3σ/K,n =9).The established method was applied to Hx detection in bass,grass carp,and shrimp,and the data were verif ied by HPLC.The result shows that the established probe is sensitive,accurate,and reliable,and can be used for Hx detection in aquatic products.
1.Introduction
Graphite-phase carbon nitride (g-C3N4) is a two-dimensional lamellar structure similar to graphene and is composed of tri-s-triazine(C6N7) and s-triazine (C3N3) rings[1].g-C3N4has the advantages of good chemical stability[2],evident physical properties,stable f luorescence[3],and adjustable band gap.Its inherent enzyme activity and spectral absorption characteristics are widely used in sensors[4],catalysis[5-7],and cell imaging[8].However,given the stacking between layers,the original g-C3N4has a wide energy band gap,small specific surface area,high photogenerated charge recombination rate[9-10],and low catalytic activity.In addition,the van der Waals force between lamellae results in the low solubility of g-C3N4[11],leading to its challenge in application.For further development and application,researchers have changed the preparation methods,including high-temperature and high-pressure[12],solvothermal[13],and thermal polycondensation methods[14].And element doping[15],heterojunction composites[16],and adjustment of specific surface[17]were also used to improve the properties of g-C3N4.For example,the catalytic activity was increased by controlling the treatment time of g-C3N4in a high-pressure hydrogen atmosphere at 400 °C[18].Ni/CM-C3N4nanocomposite was synthesized with Ni as co-catalyst by a simple solvothermal method,and its hydrogen evolution activity was signif icantly better than that of unmodif ied CM-C3N4[13].g-C3N4was doped with phenyl ring-C3N4to prepare a strong green photoluminescence[19].In the present experiment,melamine was used as the precursor to improve the dispersion of g-C3N4in water by the molten-salt method.Cobalt (Co) has been used as a doping element to prepare cobalt-doped g-C3N4(Co doped g-C3N4) with a strong peroxidase-like activity and strong f luorescence[20].
Aquatic products play very important roles in human health with rich nutritions and unique f lavor[21].However,aquatic products easily may deteriorate under improper storage conditions,affecting the f lavor and even posing potential health risks to human body[22-23].Thus,the accurate assessment of the freshness of aquatic products is the basic requirements for food safety[24].As one of the decomposition product of adenosine triphosphate (ATP),hypoxanthine (Hx) is regarded as an important index to judge the freshness of aquatic products[25].Hx has been used to reflect the freshness of aquatic products in early stage[26].However,traditional Hx detection methods are time-consuming,laborious,and cost effective[27-28].Accurate,reliable,sensitive,and fast Hx detection methods are desired to ensure the edible safety of aquatic products and improve the edible quality of aquatic products.
Ratiometric fluorescent method presents good self-calibration capability,which can reduce the interference caused by the background and improve the accuracy and reliability of measurement[29].Based on the mimic enzyme and the fluorescence of Co doped g-C3N4,a ratiometric fluorescent probe for the rapid detection of Hx in aquatic products was established in the present study.A fluorescence peak at 570 nm was observed because of the diallyl phthalate (DAP) generated by the reaction ofO-phenylenediamine (OPD) and H2O2.And a fluorescence peak at 370 nm was also observed which might be due to the spectral blue-shift (from 420 nm to 370 nm) of Co doped g-C3N4in the detection system.Under the excitation at 310 nm,the fluorescent intensities at 570 nm increased with the increases of H2O2concentrations,while the fluorescent intensities at 370 nm decreased.Good linear relationships between H2O2concentrations and the fluorescent ratiosI(F570/F370) were constructed.Hx in aquatic products could be rapidly and accurately measured by mean of the decomposition of Hx with xanthine oxidase (XOD) (Fig.1).
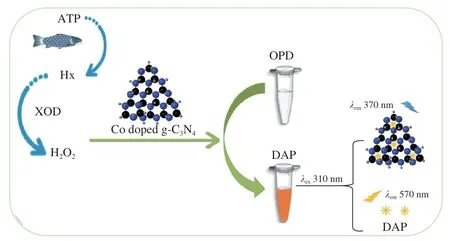
Fig.1 Ratiometric fluorescent probe for Hx measurement based on the mimic enzyme and fluorescence characteristics of Co doped g-C3N4.
2.Experimental
2.1 Reagents and apparatus
Melamine (C3H6N6),cobalt chloride hexahydrate (CoCl2·6H2O),sodium acetate trihydrate (CH3COONa),monopotassium phosphate(KH2PO4),uric acid (C5H4N4O3,UA),andO-phenylenediamine(C6H8N2,OPD) were purchased from Aladdin (Shanghai,China).Potassium chloride (KCl),sodium chloride (NaCl),sodium hydroxide(NaOH),zinc sulfate heptahydrate (ZnSO4·7H2O),magnesium sulfate heptahydrate (MgSO4·7H2O),hydrogen peroxide (H2O2,30% (m/m)),trichloroacetic acid (CCl3COOH),and dipotassium hydrogen phosphate (K2HPO4) were purchased from Sinopharm Chemical Reagent Co.,Ltd.(Shanghai,China).Glucose (C6H12O6,Glu),sucrose (C12H22O11),ascorbic acid (C6H8O6,AA),dimethyl sulfoxide,acetic acid,and hydrochloric acid (HCl) were purchased from Xilong Scientific Co.,Ltd.(Guangdong,China).XOD (50 U/mg)and Hx (C5H4N4O) were purchased from Shanghai Yuanye Bio-Technology Co.,Ltd.(Shanghai,China).ATP was purchased from Shanghai Macklin Biochemical Co.,Ltd.(Shanghai,China).A dialysis bag was purchased from Union Carbide Company (100 Da,USA).Acetate buffer (100 mmol/L,pH 5.0) and phosphate buffer (10 mmol/L,pH 7.4) were used in the present study,and ultrapurewater was used throughout the experiment.
A rotary evaporator (DLAB RE-100S,China),a box resistancefurnace (SX2-12-10,Jinan Precision &Scientific Instrument Co.,Ltd.,China),and a clinical centrifuge (H2050R,Hunan Xiangyi Laboratory Instrument Development Co.,Ltd.,China) were used in the experiment.A fluorescence spectrometer (LS 55,USA),an enzyme labeling instrument (SpectraMax Plus 384,USA),a Lambda 265 ultraviolet-visible spectrophotometer (PerkinElmer,USA),and a high-performance liquid chromatography (HPLC,Agilent 1260,USA) were the main detection instruments.Transmission electron microscopy (TEM,JEOL JEM2010,Japan) was used to characterize the morphology and size of Co-doped-gC3N4.The elemental composition was measured using X-ray photoelectron spectroscopy(XPS,Thermo ESCALAB 250xi,USA).
2.2 Preparation of Co doped g-C3N4
Co doped g-C3N4was prepared using our previous method(Supplementary material).A typical Michaelis-Menten curvewell described the kinetics of Co doped g-C3N4was shown in Fig.S1.TheKmvalues were 0.045 and 0.678 mmol/L using TMB and H2O2as substrates,respectively.TheKmvalues were much lower than those of HRP as shown in Table S1,indicating that the Co doped g-C3N4presented a high peroxidase-like activity.
2.3 Detection of H2O2 and Hx
The experimental conditions affecting the detection of H2O2were optimized (Fig.S2).A total of 20 µL 5 mmol/L OPD,20 µL 0.8 mg/mL Co doped g-C3N4,140 µL 100 mmol/L acetate buffer solution (pH 5.0),and 20 µL H2O2of different concentrations were mixed.After incubation at 60 °C for 15 min,the mixture was diluted to 400 µL with ultrapure water.Then,the fluorescent spectra of the solution were recorded at the excitation wavelength of 310 nm(emission slit: 15 nm,excitation slit: 20 nm).
By mean of the decomposition by XOD,Hx was degraded to produce H2O2,and detected indirectly by the ratiometric fluorescent probe.Briefly,Hx standard solutions or sample solutions were mixed with 5 µL 5 U/mL XOD dissolved in 55 µL 10 mmol/L phosphate buffer (pH 7.4).After incubation at 37 °C for 10 min,the solutions were mixed with 20 µL 0.8 mg/mL Co doped g-C3N4,20 µL 5 mmol/L OPD,and 100 µL 100 mmol/L acetate buffer (pH 5.0).After incubation again at 60 °C for 15 min,the solutions were diluted to 400 µL with ultrapure water,and the fluorescent spectra of the solution were recorded as described above.
2.4 Sample pretreatment
Fresh grass carp,sea bass,and prawn,purchased from the RT-MART supermarket in Xiamen,China,were selected as real samples for Hx detection.After removing the head,scales and internal organs,and rinsing with water,the back muscle of fish was collected.Similarly,the meat of prawns was collected after removing the head,shell,and glands.All samples were homogenized and stored at 25 °C.2 g of samples were fully mixed with 5 mL of 10 g/mL CCl3COOH by vortex oscillation[30].After centrifugation at 4 °C 8 000 r/min for 10 min,the supernatants were collected and adjusted to pH 6.0 with 1 mol/L NaOH for Hx measurement.HPLC method[31]was also used to validate the detection results.The chromatographic conditions were described as follows: Eclipse plus C18(4.6 mm × 150 mm,5 µm) of chromatographic column,0.02 mol/L KH2PO4-0.02 mol/L K2HPO4(V/V=50:50) of mobile phase,1 mL/min of flow rate,and 254 nm of detection wavelength.
2.5 Statistical analysis
A pairedt-test method and a least significant difference method were used for the significant difference of the experimental data using SPSS 17.0 software package.
3.Results and discussion
3.1 Characterization of Co doped g-C3N4
Fig.2A shows the TEM (JEOL JEM2010,Japan) diagram of the prepared Co doped g-C3N4.From the detailed figure,the morphological characteristics of Co doped g-C3N4are evident,close to regular hexagon,uniform in size with an average particle size of 11.85 nm.The XPS (Thermo ESCALAB 250xi,USA) spectrum in Fig.2B shows the structural composition of Co doped g-C3N4which is mainly composed of C,N,O,and doped with Co.As shown in Fig.2C,the XPS peak of C1s can be divided into 4 components(284.8,286.3,287.7,and 288.9 eV),which are attributed to C-C/C=C,C-N,C-O/C=N,and C=O bonds,respectively[32].The existence of C=O might play an important role in the fluorescence characteristics of Co doped g-C3N4[33].The N1s spectra display three peaks at 398.4,399.5,and 400.9 eV,which are assigned to pyridinic,pyrrolic,and graphitic N,respectively (Fig.2D).The cobalt signal in the XPS spectrum shows that Co is successfully doped into the carbon nitride.The two fitting peaks in Fig.2 revealed that the binding energies of Co2p are 779.8 and 781.8 eV,respectively,which means that Co in Co doped g-C3N4exists in the forms of Co2+and Co3+.The existence of high valence state of Co further improves the peroxidase activity of Co doped g-C3N4.
3.2 Fluorescent and peroxide-like properties of Co doped g-C3N4
Co doped g-C3N4had stable and strong fluorescence characteristics with a blue fluorescence at 420 nm under the excitation at 310 nm.As shown in Fig.3A,under different pH conditions,the fluorescent intensity of Co doped g-C3N4decreases with the increase of solution acidity,and the fluorescent peak showed a slight blue-shift.Fig.3B shows the fluorescent spectra of Co doped g-C3N4,Co doped g-C3N4+H2O2,Co doped g-C3N4+OPD,and Co doped g-C3N4+OPD+H2O2at the excitation wavelength of 310 nm.At pH 5.0,the addition of H2O2alone causes no significant effect on the fluorescent peak of Co doped g-C3N4.However,after the addition of OPD or OPD+H2O2the fluorescent spectra result in significant blue-shift ranging from 420 nm to 370 nm.Under the catalysis of Co doped g-C3N4with high peroxidase,the reaction of OPD and H2O2produced DAP.Through the electrostatic attraction based on the zeta potentials of Co doped g-C3N4and DAP as shown in Fig.S3,the generated DAP might adsorb on the surfaces of Co doped g-C3N4,leading to the fluorescent quenching at 370 nm due to the photo-induced electron transfer effect[34].In addition,DAP is a photosensitive substance with fluorescent properties which can emit fluorescence at 570 nm.As shown in Fig.3C,under the excitation at 310 nm the fluorescent intensities at 370 and 570 nm decrease and increase respectively with the increases of H2O2concentrations.By mean of the decomposition by XOD,Hx was quantitatively detected by measuring the fluorescent ratio ofI(F570/F370).Fig.3D shows the absorption spectra of the detection system in the visible-light range.Under the catalysis of Co doped g-C3N4,the DAP generated by the reaction of OPD and H2O2exhibits an evident absorption peak at 450 nm.Furthermore,the reaction is accompanied by the production of a yellow solution (inset in Fig.3D).Thus,the levels of Hx can also be realized through colorless to yellow solutions.
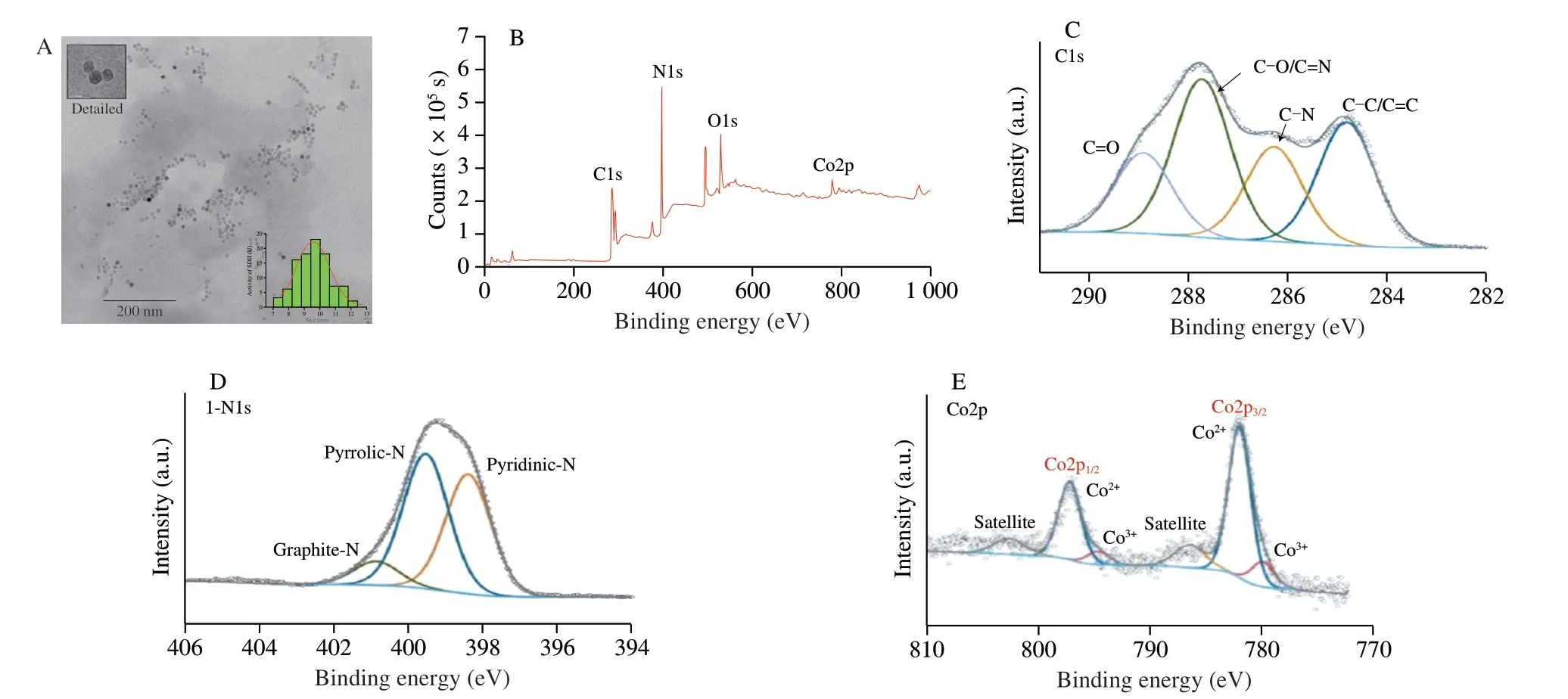
Fig.2 TEM image and size distribution (A) and XPS spectrum of Co doped g-C3N4 (B),XPS spectra with fitting peaks of C1s (C),N1s (D),and Co (E).
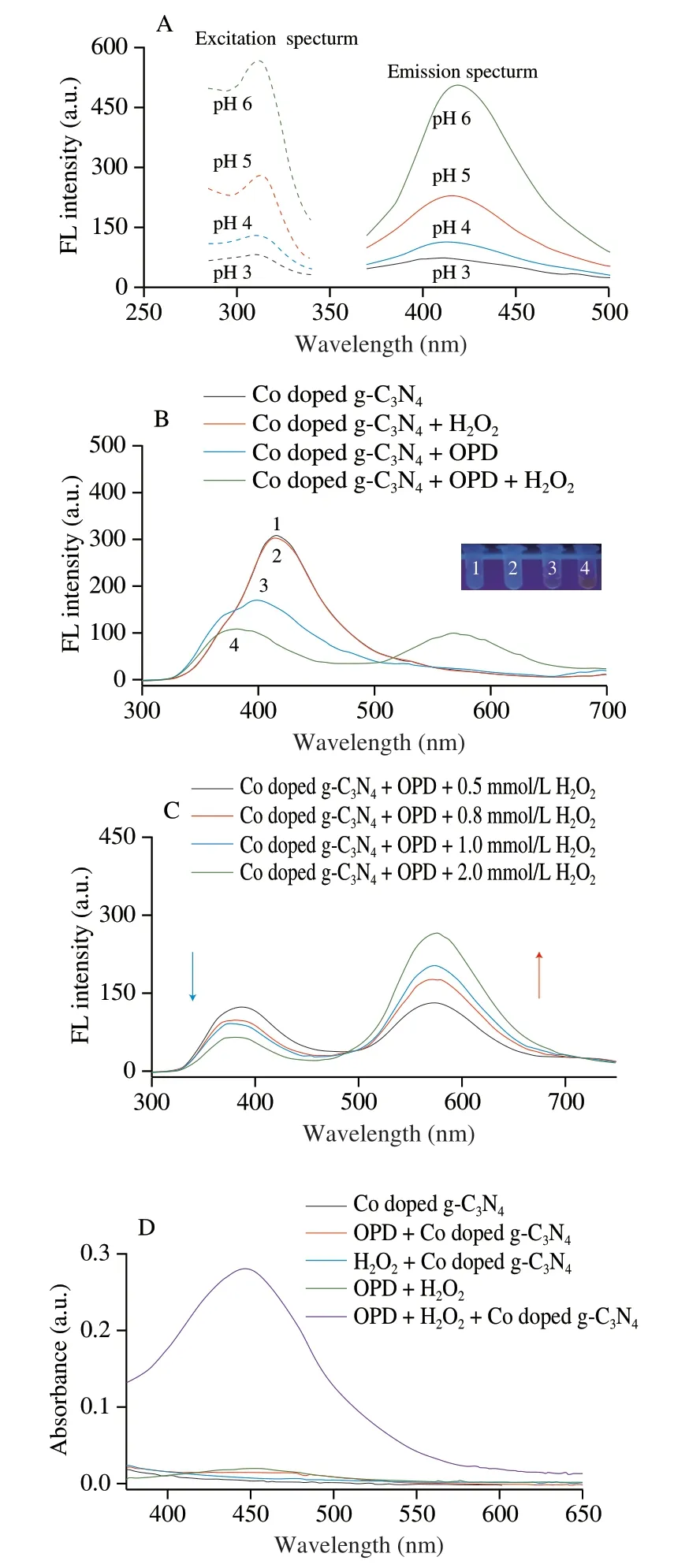
Fig.3 (A) Fluorescence spectra of Co doped g-C3N4 at different pH,0.8 mg/mL Co doped g-C3N4;(B) fluorescence spectra of catalytic system,100 mmol/L acetate buffer (pH 5.0),0.8 mg/mL Co doped g-C3N4,0.5 mmol/L OPD,and 0.5 mmol/L H2O2;(C) fluorescence spectra of detection system at different H2O2 concentrations,100 mmol/L acetate buffer (pH 5.0),0.8 mg/mL Co doped g-C3N4,and 0.5 mmol/L OPD;(D) absorption spectra of detection system,0.8 mg/mL Co doped g-C3N4,0.5 mmol/L OPD,and 0.5 mmol/L H2O2.
3.3 Working curveand detection limit
For investigating the feasibility of the probe,H2O2was firstly and directly detected based on the fluorescent ratio ofI(F570/F370).As shown in Figs.4A and B,the concentrations of H2O2ranging from 2 µmol/L to 2 000 µmol/L are linearly correlated with theI(F570/F370)values with two equations in which the equation ofy=0.002x+0.122 3 is fit for 2-350 µmol/L of H2O2andy=0.001 8x+0.429 8 is fit for 400-2 000 µmol/L of H2O2.The detection limit (LOD) of H2O2was 1.5 µmol/L based on 3α/K(whereαis the standard deviation of nine reagent blanks,andKis the slope of the linear equation).
The decomposition conditions of XOD were optimized as shown in Fig.S4.The decomposition degree of Hx was enhanced with the increase of XOD dosage.However,the decomposition effect decreased when the XOD dosage exceeded 5 µL × 5 U/mL.Therefore,the appropriate dosage of XOD was set as 5 µL × 5 U/mL in the detection solution.Similarly,the decomposition time was chosen as 10 min.After the decomposition,Hx was indirectly detected as that of H2O2measurement.Fig.4B showed that theI(F570/F370) values linearly correlated with Hx concentrations ranging from 1.7 mg/kg to 272.2 mg/kg (R2=0.997).The LOD tested with nine sample blanks was 1.52 mg/kg (3α/K).
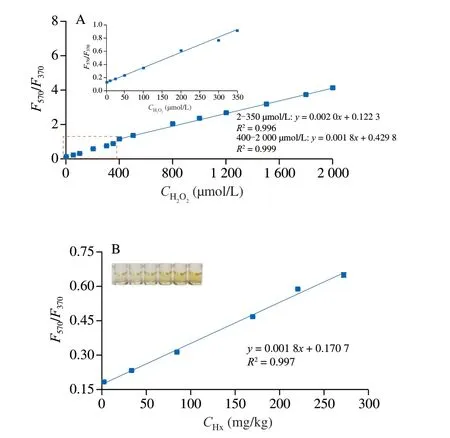
Fig.4 Working curves for H2O2 (A) and Hx (B).100 mmol/L acetate buffer(pH 5.0),0.8 mg/mL Co doped g-C3N4,0.5 mmol/L OPD,60 °C of reaction temperature and 15 min of reaction time.The excitation wavelength was 310 nm.Enzymatic conditions: 10 mmol/L PBS,5 µL of 5 U/mL XOD,and 10 min of incubation time.
For investigating the selection of the probe,ions (Na+,K+,Mg2+,and Zn2+),Glu,sucrose,ATP,AA,and UA were used as the potential interferents.Fig.5A shows that theI(F570/F370) value of Hx is significantly higher than those of interferents,the deep yellow solution of Hx (inset) further visually confirms the high selectivity of the method.Fig.5B shows the interference,in which theI(F570/F370) values of Hx is not significantly different (P> 0.05)from those of the mixtures (Hx+interferents),indicating that these coexisting substances do not interfere with the Hx detection.The results show that the ratiometric fluorescent probe has good selectivity for Hx detection.
3.4 Real sample detection
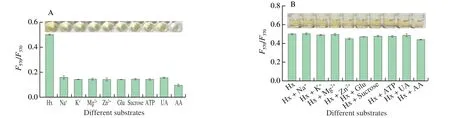
Fig.5 Selectivity (A) and interference tests (B) of Hx detection.Hx and each of the concentrations of interferences were set as 0.5 mmol/L.
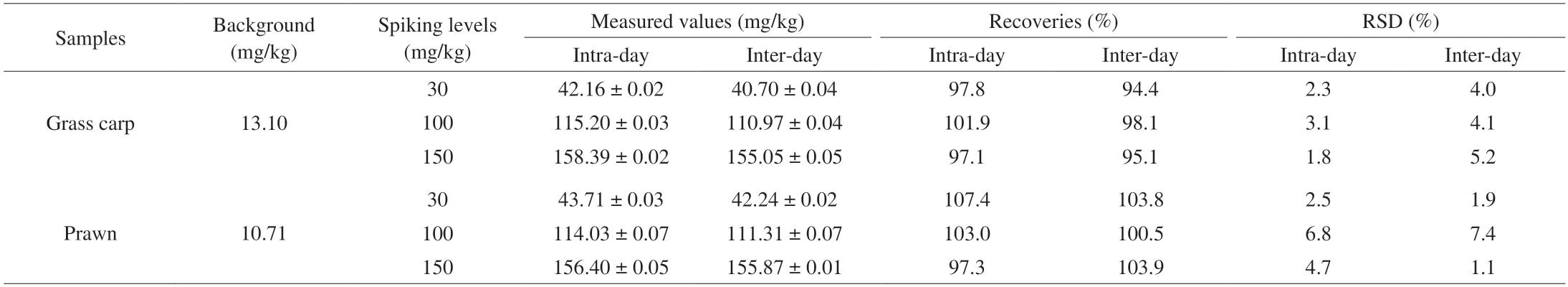
Table 1 Recovery tests of Hx in aquatic products by spiking standards.
Hx concentrations in fresh prawn,sea bass,and grass carp were detected at the intervals of 6 h during storage at room temperature,and the results were verified by HPLC method.As shown in Fig.S5,Hx levels increase with the storage time and reach the maximum at 12 h,and then decrease gradually.The data were mostly consistent with those of HPLC method.The slight differences between the two methods were due to the sampling time in which the data of the early sampling test were lower than those of the later sampling whichever method was used.
Spiked recovery tests were used to further verify the accuracy and reliability of the ratiometric fluorescent probe.Results in Table 1 show the recoveries obtained by intra-day and inter-day through two samples of grass carp and prawn.The recoveries ranging from 97.1%to 107.4% with relative standard deviation (RSD) of 1.8%-6.8% were obtained for intra-day measurement,while recoveries of 94.4%-103.9%(RSD 1.9%-7.4%) were achieved for inter-day.The results indicate that the ratiometric fluorescent probe is accurate and reliable,and can be used for Hx detection in aquatic products.
4.Conclusion
A ratiometric fluorescent probe was constructed based on the excellent mimic enzyme and fluorescence characteristics of Co doped g-C3N4.By means of xanthine oxidase,Hx could be detected by the measurement of fluorescent ratios ofI(F570/F370) with a linear range of 1.7-272.2 mg/kg.The LOD of the method was 1.5 mg/kg (3σ/K,n=9),and the recovery tested by standard addition ranged from 94.4% to 107.4% with RSD less than 10%.The ratiometric fluorescent probe was successfully applied to the detection of Hx in aquatic products.The results proved that the ratiometric fluorescent probe was sensitive,accurate and reliable.
Conflict of interest statement
We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (21804050),the National Key R and D Program of China (2018YFD0901003),the Science and Technology Planning Project of Xiamen,China (3502Z20183031),the Fujian Provincial Fund Project (2018J01432),and the Xiamen Science and Technology Planning Project,China (3502Z20183031).
Appendix A.Supplementary data
Supplementary data associated with this article can be found,in the online version,at http://doi.org/10.26599/FSHW.2022.9250075.
- 食品科学与人类健康(英文)的其它文章
- Protective effects of oleic acid and polyphenols in extra virgin olive oil on cardiovascular diseases
- Diet and physical activity inf luence the composition of gut microbiota,benef it on Alzheimer’s disease
- Inf luence of nitrogen status on fermentation performances ofnon-Saccharomyces yeasts: a review
- Ganoderma lucidum: a comprehensive review of phytochemistry,eff icacy,safety and clinical study
- Resveratrol combats chronic diseases through enhancing mitochondrial quality
- Demonstration of safety characteristics and effects on gut microbiota of Lactobacillus gasseri HMV18

