Lactobacillus plantarum AR113 attenuates liver injury in D-galactose-induced aging mice via the inhibition of oxidative stress and endoplasmic reticulum stress
Yongjun Xi,Yujie Gong,Xingn Lin,Yijin Yng,Xin Song,Gungqing Wng,Zhiqing Xiong,Yngyng Qin,Zhun Lio,Hui Zhng,Linzhong Ai,
a School of Health Science and Engineering, Shanghai Engineering Research Center of Food Microbiology, University of Shanghai for Science and Technology, Shanghai 200093, China
b College of Life Sciences, Linyi University, Linyi 276000, China.
c Department of Gastroenterol., Digestive Endoscopy Center, Changhai Hospital, Shanghai 200082, China
Keywords: Lactiplantibacillus plantarun AR113 HepG2 D-galactose Oxidative stress ER stress
ABSTRACT Probiotics could effectively eliminate excess reactive oxygen species (ROS) generated during aging or lipid metabolism disorders,but their mechanism is unclear.The major purpose of this study was to investigate the mechanism of Lactiplantibacillus plantarun AR113 alleviating oxidative stress injury in the D-galactose induced aging mice.The result showed that pretreatment with L.plantarun AR113 significantly relieving H2O2 induced cytotoxicity in HepG2 cells by maintain cell membrane integrity and increasing antioxidant enzyme activities.In D-galactose induced aging mice,L.plantarun AR113 could signif icantly attenuate liver damage and inf lammatory inf iltration by promoting endogenous glutathione (GSH) synthesis and activating the Nrf2/Keap1 signaling pathway in mice,and increasing the expression of regulated phase II detoxif ication enzymes and antioxidant enzymes.Further analysis shown that gavage of L.plantarun AR113 could signif icantly reduce the expression of G protein-coupled receptor 78 (GPR78) and C/EBP homologous protein(CHOP) proteins,and promote the restoration of endoplasmic reticulum (ER) homeostasis,thereby activating cell anti-apoptotic pathways.These results were also conf irmed in H2O2-treated HepG2 experiments.It indicated that L.plantarun AR113 could inhibit D-galactose-induced liver injury through dual inhibition of ER stress and oxidative stress.L.plantarun AR113 have good application potential in anti-aging and alleviating metabolic disorders.
1.Introduction
Free radicals were generated during oxidation processes in most living organisms.However,the production-scavenging balance in cells is disturbed when free radicals were accumulated excessive,which could result in DNA damage,protein denaturation and lipid peroxidation[1-3].Moreover,oxidative stress is confirmed related to various diseases including inf lammation,cancer,non-alcoholic fatty liver disease,Crohn’s disease and Elazar Pakinson’s disease[4-7].
Many chemical oxidants,such as hydrogen peroxide,heavy metal ions,and ethanol,could cause cells to produce a large amount of reactive oxygen species (ROS),causing cells to be in a state of oxidative stress[8-9].While,the scavenging of ROS is closely related to the antioxidant capacity of cells.Nuclear factorerythroid-2-related factor 2 (Nrf2),a member of the NF-E2 family of basic leucine zipper transcription factors,regulates their gene expression by binding to antioxidant response elements (AREs) to activate the Nrf2-ARE signaling pathway,which is considered to play an important role in the stress defense system against oxidative stress[10-12].During cellular response to oxidative stress,the expression of antioxidant enzymes could be activated by Nrf2 gene.
Accumulating evidence demonstrated that probiotics exerted various beneficial effects including improvement in the health of intestinal tract,enhancement of immune system,reduction of intestinal disorders[13-14].
In recent years,several lactic acid bacteria (LAB) strains have reported possess the antioxidant ability such as scavenge free radicals,improve the activity of antioxidant enzymes and inhibit lipid oxidation[15-17].Several studies have shown that probiotics have good application potential in alleviating oxidative stress[18-19].The antioxidant capacity of LAB is strain-specific,which is related to the antioxidant mechanisms of the strains.Shen et al.showed that the culture supernatant ofBifidobacterium animalis01 had better free radical scavenging ability than intact cells and intracellular cellfree extracts[20].While,in another study,the results shown that free radical scavenging ofBifidobacterium longumATCC 15708 andLactobacillus acidophilusATCC 4356 were more efficient in intact cells[21].In addition,metabolites such as extracellular polysaccharides of LAB also have better antioxidant capacity[22-23].
Preliminary research shows that theLactiplantibacillus plantarunAR113 which could relieve oxidative damage in mice induced byD-galactose and oxidized oil[24-25].While,howL.plantarunAR113 alleviates the oxidative injury of liver remains unclear.In addition to enhancing antioxidant enzyme activity,does AR113 alleviate oxidative damage caused by ROS through other pathways? Compared with other reactive oxygen species,H2O2is relatively stable during the modeling period and could cause oxidative damage to HepG2 cells.It has been widely used as an inducer for oxidative stress modelsin vitro[26-27].Thus,the major purpose of the current study was to investigate the effect of different treatment ofL.plantarunAR113 on the H2O2induced HepG2 cells andD-galactose induced aging mice,and analyze the mechanism of AR113 alleviating oxidative stress injury.
2.Methods and materials
2.1 Bacteria strains and cell culture
L.plantarunAR113 was obtained from Chinese fermented cabbage and kept at the China General Microbiological Culture Collection Center (preservation number,CGMCC No.13909).AR113 was cultured in MRS broth at 37 °C for 16 h.After cultured,cells were collected after centrifugation at 1 180 ×gfor 10 min at 4 °C.Thereafter,these cells were washed twice with sterile phosphate-buffered saline (PBS,pH 7.2) and resuspended to a final concentration of 109CFU/mL in PBS.
2.2 HepG2 experiments
2.2.1 Establishment of H2O2-treated HepG2 cells model
The HepG2 cells (5 × 104cells/well,DEME) were seeded into a 96-well plate (Corning,USA) under conditions (5% CO2,37 °C) overnight before treatments.The DMEM medium containing 10% fetal bovine serum,100 U/mL penicillin and 100 µg/mL streptomycin.After culture,the cells were washed withD-Hank’s and treated with H2O2at different final concentrations (0,100,200,300,400,500,600,700,800,900,1 000 µmol/L) for 2 h.Then,the medium was removed and cells were incubated with 10 µL of CCK-8 working solution for 60 min at 37 °C.Cell viability was determined by a standard CCK-8 (Cell Counting Kit-8) assay.Briefly,the absorbance was measured using SpectraMax i3x (MD,USA) at 450 nm and the cell viability was determined.The OD450nmof the control cells was denoted as 100% viability.
2.2.2 Antioxidant effects of L.plantarun AR113 on H2O2-treated HepG2 cells
To determine the antioxidant effects ofL.plantarunAR113,HepG2 cells were divided into 7 groups: control,model,vitamin C(VC,1 mmol/L),live cells (1E-8,108CFU/mL),live cells (1E-9,109CFU/mL),dead cells (Heat-killed,D1E-8,108CFU/mL),dead cells (Heat-killed,D1E-9,109CFU/mL).In low dose group,L.plantarunAR113 cells were adjusted the concentration to 1 × 108CFU/mL after washed twice with sterile PBS.In high dose group,AR113 was adjusted the concentration to 1 × 109CFU/mL after washed twice with sterile PBS.In dead cells groups,AR113 cells were inactivated by boiling water broth for 20 min.
The HepG2 cells (5 × 104cells/well,DEME) were seeded into 96-well plates (Corning,USA) under conditions (5% CO2,37 °C)overnight before treatments.The DMEM medium containing 10%fetal bovine serum,100 U/mL penicillin and 100 µg/mL streptomycin.After culture,the cells were washed withD-Hank’s and treated with different methods.Pre-incubation with AR113 in culture medium(without antibiotic) for 2-8 h and washes away AR113 withD-Hank’s.Then,the complete medium containing H2O2was added to HepG2 cells for 2 h.Cell viability was determined by a standard CCK-8 assay.The OD of the control cells was denoted as 100% viability.
2.2.3 Effect of inhibitor ML385 on antioxidant capacity of HepG2 cells
ML385 was used to inhibit the Nrf2 in HepG2 cells[28].The HepG2 cells (5 × 104cells/well,DEME) were seeded into 96-well plates (Corning,USA) under conditions (5% CO2,37 °C) overnight before treatments.After culture,the cells were washed withD-Hank’s and 100 µL DEME medium (without antibiotic and containing 5 µmol/L ML385) was added for incubation 1 h.Then,the experiments of different treatments (Con,Mod,IE-9,DIE-9) were carried out and the effect on HepG2 cells was analyzed.
2.3 Animals and experimental protocols
Male C57BL/6J mice (5-6 weeks old,20-22 g) were purchased from Shanghai BK Laboratory Animal Co.Ltd.(Shanghai,China).The mice were housed in IVC system (BCR-MI03,Shinva Medical Instrument Co.Ltd.,China) and maintained on a 12-h light/dark cycle at (22 ± 2) °C with (50 ± 5)% humidity.The animal experiments were carried out according to Lin et al.[18].As shown in Table 1,after the adaptation period (1 week),the mice were randomly divided into 4 groups (n=8) as follow: (a) control group;(b)D-galactose induced aging model group;(c) AR113 treatment group;(d) VC treatment group.All experiments were approved and performed following the guidelines of the IACUC (Institutional Animal Care and Use Committee) of Shanghai Jiao Tong University in accordance with the animal protocol (202201229).
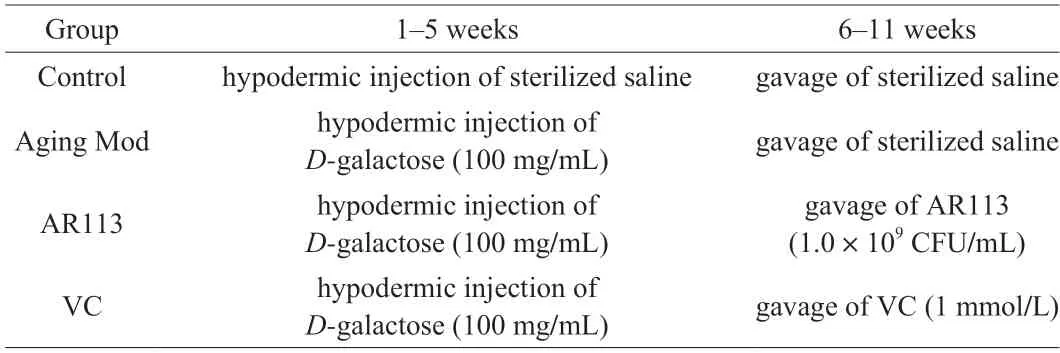
Table 1 The groups of animal experiment.
2.4 DAPI staining of nuclei in HepG2 cells
The nuclei of HepG2 cells were stained with the chromatin dye,4’-6-diamidino-2-phenylindole (DAPI) according to Se-Jin et al.[29]with little modification.After culture,the cells were washed three times withD-Hank’s and fixed with 4% paraformaldehyde for 10 min at room temperature.Then,the fixed cells were washed twice withD-Hank’s and incubated with DAPI (1 µg/mL) at room temperature for 5 min.Finally,the DAPI was removed and cells were washed 3 times with PBS,and then the cells were observed with a fluorescence microscope.
2.5 Lactate dehydrogenase assay in HepG2 cells
The supernatant of HepG2 cells were collected from different treatments.The lactate dehydrogenase (LDH) released into the supernatant were measured using anin vitrolactate dehydrogenase release assay kit (Beyotime Biotechnology,China).The cytotoxicity was calculated as follows:
2.6 Detection of intracellular ROS levels in HepG2 cells
The effects ofL.plantarunAR113 on intracellular ROS levels in HepG2 cells were determined using Reactive Oxygen Species Assay Kit (Beyotime Biotechnology,China).According to instruction of manufacturer,HepG2 cells were cultured overnight and then treated with H2O2for 2 h with or without pretreatment with VC or different treatment of AR113.Cells were harvested using trypsin-EDTA into 1.5 mL tubes and washed usingD-Hank’s.Then,the cells were treated with 10 µmol/L DCFH-DA and further incubated for 20 min.DCFH-DA-treated cells were then performed using a SpectraMax M3 (MD,USA) at excitation and emission wavelengths of 488 and 525 nm,respectively.
2.7 Detection of mitochondrial depolarization in HepG2 cells
JC-1 probe was employed to measure mitochondrial depolarization in HepG2 with Mitochondrial Membrane Potential Assay Kit (Beyotime Biotechnology,China).According to instruction of manufacturer,HepG2 cells were stained with JC-1 (10 µmol/mL)for 20 min at 37 °C after harvested by trypsin-EDTA and washed twice byD-Hank’s.The monomeric form of JC-1 in the cytosol after mitochondrial membrane depolarization and the potential-dependent aggregation of JC-1 in the mitochondria were detected by SpectraMax M3 (MD,USA),respectively.
2.8 Preparation of liver and serum in C57/BL6 mice
After 12 h of the last gavage,the eyeballs were removed to collect blood samples.Serum samples were obtained after centrifugation at 664 ×gfor 10 min at 4 °C and stored at -80 °C for later analysis.Liver samples were quickly rinsed with pre-cooled normal saline,weighed,and then homogenized by adding pre-cooled normal saline.The liver homogenate was centrifuged at 664 ×gfor 10 min at 4 °C to determine the protein content and other biochemical indicators.
2.9 Determination of biochemical indexes
The biochemical indexes (molondialdehyde (MDA),glutamic oxalacetic transaminase (AST),glutamic-pyruvic transaminase (ALT),superoxide dismutase (SOD),glutathione peroxidase (GSH-Px)and total antioxidant capacity (T-AOC)) in HepG2 cells and liver tissues were analyzed following the manufacturer’s instructions of commercial kits (Nanjing Jiancheng Bioengineering Institute,China).
2.10 RNA extraction and quantitative real-time PCR

Table 2 Primers of qPCR in HepG2.
Extraction of total RNA by using 1 mL RNAiso Plus (TaKaRa Bio,Otsu,Japan) according to the manufacturer’s instructions.The concentration and quality of RNA were evaluated by using NanoDrop ND2000C spectrophotometer (Thermo Scientific,DE) at anA260nm/A280nmratio.The cDNA was prepared according to the instructions of HiScript III RT SuperMix for qPCR reagent (Vazyme,Nanjing,China) and amplified by real-time PCR using Hieff qPCR SYBR Green Master Mix reagent (Yeasen Biotech,Shanghai,China).All primer sequences were synthesized by Sangon Biological Engineering,Shanghai,China and as presented in Tables 2 and 3.GAPDHandβ-actinas housekeeping gene was used to normalize target gene transcript levels.The thermocycle protocol includes 30 s at 95 °C followed by 40 cycles of 5 s denaturation at 95 °C,30 s annealing/extension at 60 °C,and then a final melting curve analysis to monitor purity of the PCR product.The 2-ΔΔCtmethod was used to estimate relative gene expression.
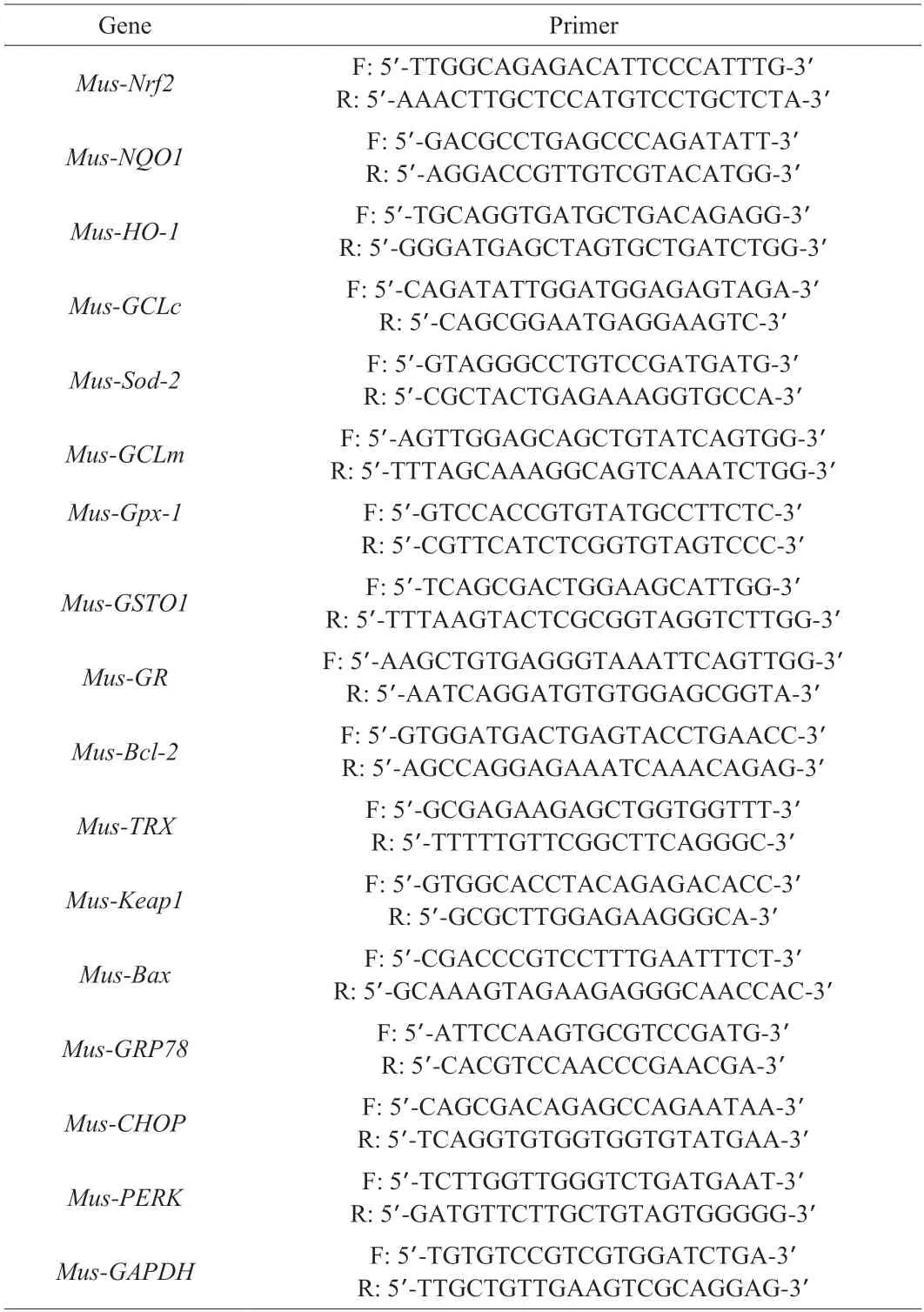
Table 3 Primers of qPCR in mouse.
2.11 Western blot in liver
Liver samples were weight and homogenized in RIPA lysis buffer (Beyotime Biotechnology,Shanghai,China).The supernatants were collected by centrifugation at 12 000 ×gfor 30 min at 4 °C.The total protein content was analyzed using a BCA Protein Assay Reagent Kit (biyotime,china) according to the instruction.The protein samples were separated by 12.5% SDS-polyacrylamide gels and transferred to polyvinylidene fluoride (PVDF) membranes.And then,the membranes were blocked with western sealing fluid(Beyotime,P0252) and incubated with rabbit anti-GPR78 (abcam,ab198787),rabbit anti-PERK (abcam,ab229912),rabbit anti-CHOP(abcam,ab11419),rabbit anti-Bcl-2 (abcam,ab196495),rabbit anti-BAX (abcam,ab3191) and mouse anti-β-actin primary antibody at 4 °C overnight.After incubation,the proteins were detected by the ECL chemiluminescence kit (Beyotime,P0018) using a horseradish peroxidase-conjugated anti-rabbit secondary antibody.Images were acquired using a ChemiDoc MP imaging system (Bio-Rad,Hercules,CA).
2.12 Histology analysis
The liver morphology was assessed using haematoxylin and eosin (H&E) staining and Oil Red O staining.The liver tissue was fixed in 4% paraformaldehyde for 18 h,dehydrated and embedded in paraffin wax for H&E stain.The lipid distribution of liver tissue was assessed by Oil Red O staining.The liver tissue was fixed in 4%paraformaldehyde for 8 h and treatment with sucrose solution,then sliced into 10 μm thick slices for Oil Red O stain.The stained slices of liver tissue (n=6 biologically independent samples for each group)were scanned with a Pannoramic MIDI digital section scanner and photographed.The slices were observed at 200 × magnification.
2.13 Statistics
Each experiment was performed three times,and all values were represented as means ± standard deviation (SD) of triplicates.A one-way analysis of variance (ANOVA) procedure of SPSS by the Turkey test was used to analyze the statistical significance of the results.Differences were considered statistically significant at aP< 0.01 or 0.05.
3.Results
3.1 Effect of L.plantarun AR113 on H2O2 induced cytotoxicity in HepG2 cells
As shown in Fig.1A,cell viability of HepG2 was decreased with the increasing concentrations of H2O2.The cell viability was(56.75 ± 8.19)% after treated by H2O2at 900 µmol/L.It was close to the lethal concentration of 50%[30].Thus,900 µmol/L H2O2was used to treat HepG2 for 2 h to induce the oxidant stress for the following experiments.
As shown in Figs.1B-E,the pre-incubation ofL.plantarunAR113 with HepG2 cells had an important effect on the cell viability.Compared with the control group,the cell viability of the H2O2-treated group was significantly reduced,only 54.07%.While,the cell viability of HepG2 cells was ranging from 81.99% to 121.63%by pre-incubation with different AR113-treatments for 2 h,and the live cell of AR113 at 1 × 109CFU/mL shown the best protective effect compared to other groups (Fig.1B).Although the protective effect of the inactivated AR113 was lower than that of the live AR113 group,it was also significantly higher than that of the Mod group,and the effect of high concentration of inactivated AR113 was better (100.18%).The viability of HepG2 cells decreased gradually with the extension of pre-culture time (Figs.1C-E).This may be due to the continuous acid production of AR113 inhibiting cell proliferation of HepG2.
The protective effects of AR113 against H2O2induced cytotoxicity were assessed by determination of LDH release rate of HepG2 cells(Fig.1F).Treatment with H2O2significantly increased the LDH leakage from HepG2 cells (53.14%),reaching 2.63 times that of the control group.Compared with the Mod group,the pre-incubation with different treatments of AR113 significantly decreased the LDH leakage.The maximum protection was observed with live AR113 at 1 × 109CFU/mL (31.40%).The inactivated AR113 also had a positive effect on reducing LDH leakage,and there was no significant difference with the live AR113 group.It indicates that AR113 could significantly reduce cell damage induced by H2O2,which is consistent with the experiments of cell viability.
The effects ofL.plantarunAR113 on ROS and mitochondrial membrane potential in H2O2-induced HepG2 cells were determined.As shown in Fig.1G,treatment with H2O2markedly increased ROS concentrations in HepG2 cells.However,pre-incubation with AR113(live and inactivated) at 1 × 109CFU/mL significantly reduced intracellular ROS levels by 38.84% and 31.83%,respectively,compared to the Mod group.JC-1 (5,5’,6,6’-tetrachloro-1,1’,3,3’-tetraethyl-imidacarbocyanine iodide) is a fluorescent probe that can be used to detect mitochondrial membrane potential.
The monomer-to-aggregates ratio of JC-1 can reflect changes in mitochondrial membrane potential of cells.After H2O2treatment,the monomer-to-aggregates ratio of JC-1 was significantly increased compared with the control group (Fig.1H).However,the ratio of JC-1 monomer-to-aggregates decreased significantly after preincubation of AR113 with HepG2 cells,and the effect of AR113 live cells was better than inactivated cells.As shown in Fig.1I,the ROS content in HepG2 cells was significantly increased after H2O2treatment.Pre-incubation with AR113 (live and inactivated) could inhibit the generation of ROS significantly at 1 × 109CFU/mL.DAPI staining showed that after H2O2treatment,the dye accumulated in the nucleus of HepG2 cells,which enhanced the fluorescence of cells(Fig.1J).The fluorescence of HepG2 cells was significantly reduced after AR113 treatment,and the effect of high dose AR113 was better.It indicates thatL.plantarunAR113 could effectively inhibit H2O2-induced apoptosis of HepG2 cells by scavenging intracellular ROS.
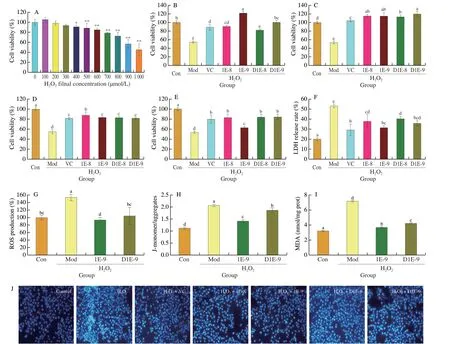
Fig.1 Effect of L.plantarun AR113 on H2O2 induced cytotoxicity in HepG2 cells.(A) Effect of H2O2 concentration on cell viability;(B-E) Effect of AR113 pre-incubation time (2,4,6,8 h) on cell viability;(F) LDH release rate;(G) Intracellular ROS;(H) Mitochondrial membrane potential;(I) MDA content;(J) DAPI staining,scale bar represented 10 μm.Results of experiments are express as the mean ± SEM for each experimental group (n =6).The significance of differences between the data was assessed using One-way ANOVA by Dunnett’s analysis,significant differences were compared with each two groups (*P < 0.05,**P < 0.01).
3.2 L.plantarun AR113 promotes endogenous glutathione synthesis in H2O2-treated HepG2 cells
The main non-enzymatic antioxidant in cells is glutathione,which maintains the redox balance in cells through the mutual conversion of reduced GSH and oxidized oxidized glutathione (GSSG)[31].As shown in Fig.2,after H2O2treatment,the content of glutathione in HepG2 cells was significantly decreased (P< 0.001),resulting in a significant decrease in GSH-Px enzyme activity (P< 0.05).Further analysis showed that the expression of genes related to GSH synthesis and transformation in HepG2 cells was weakened under H2O2induction conditions.However,L.plantarunAR113 (live and inactivated) could significantly up-regulate the expression of genes related to glutathione synthesis and transformation,and increase the GSH content in cells,and the effect of live AR113 was better than that of inactivated AR113 (P< 0.05).
3.3 Effect of L.plantarun AR113 on Nrf2/Keap1 signal pathways in HepG2 cells
To investigate the effect ofL.plantarunAR113 on antioxidant enzymes in H2O2-treated HepG2 cells,the gene expression levels of antioxidant enzymes such as catalase (CAT),SODand NAD(P)H:quinoneoxidoreductase 1 (NQO1) were measured.As shown in Fig.3A,pre-incubation with AR113 (live and inactivated)significantly increased the mRNA expression ofNrf2in the HepG2 cells(P< 0.001).After treatment with AR113,the SOD activity of HepG2 cells was significantly elevated compared with Mod group (Fig.3B).Then,the mRNA expression levels of phase II detoxification enzymes and antioxidant enzymes against oxidative stress defense systems were subsequently determined.The results shown that AR113 could significantly up-regulated mRNA levels ofCAT,NQO1,heme oxygenase-1 (HO-1) andSOD(Figs.3C-F).The expressions of those genes were consistent with the change ofNrf2levels,which indicating that AR113 may activate the Nrf2/Keap1 signaling pathway.
ML385 (C29H25N3O4S,CAS No.846557-71-9) is a specificNrf2inhibitor that could inhibit the expression of downstream genes ofNrf2.To verify the effect ofL.plantarunAR113 onNrf2gene expression,5 µmol/L ML385 was added to H2O2-treated HepG2 cells.As shown in Figs.3G-I,the protective effect of AR113 on H2O2-treated HepG2 cells was significantly reduced when ML385 was added.There was no significant difference in cell viability,LDH release rate and intracellular ROS content between AR113 treatment group and Mod group.In addition,the mRNA expression ofNrf2and its downstream genes significantly decreased after ML385 was added(Figs.3J-L).It suggests that AR113 exerts anti-oxidative damage function by activating the Nrf2 signaling pathway.
3.4 Effect of L.plantarun AR113 on endoplasmic reticulum stress in H2O2-treated HepG2 cells
To detect the endoplasmic reticulum (ER) stress level in H2O2-treated HepG2 cells,the mRNA expression levels of ER stress proteins were examined.As shown in Fig.4,the mRNA expression of ER stress-related proteins was significantly increased after H2O2treatment,resulting in a significant increase in the expression of the pro-apoptotic protein (Bax),while the expression of the antiapoptotic proteins (Bcl-xl,Bcl-2) was significantly down-regulated.Pre-incubation with AR113 could relieve ER stress by decrease the expression of GPR78 (G protein-coupled receptor 78),PERK(proteinkinase R-like ER kinase) and CHOP (C/EBP homologous protein) (Figs.4A-C).Further analysis showed that AR113 could inhibit the expression of Bax and enhance the expression of Bcl-xl and Bcl-2 (Figs.4D-F).The results were consistent with the cell viability experiments.More important,AR113 live cells were more effective in relieving HepG2 cell apoptosis induced by endoplasmic reticulum stress.
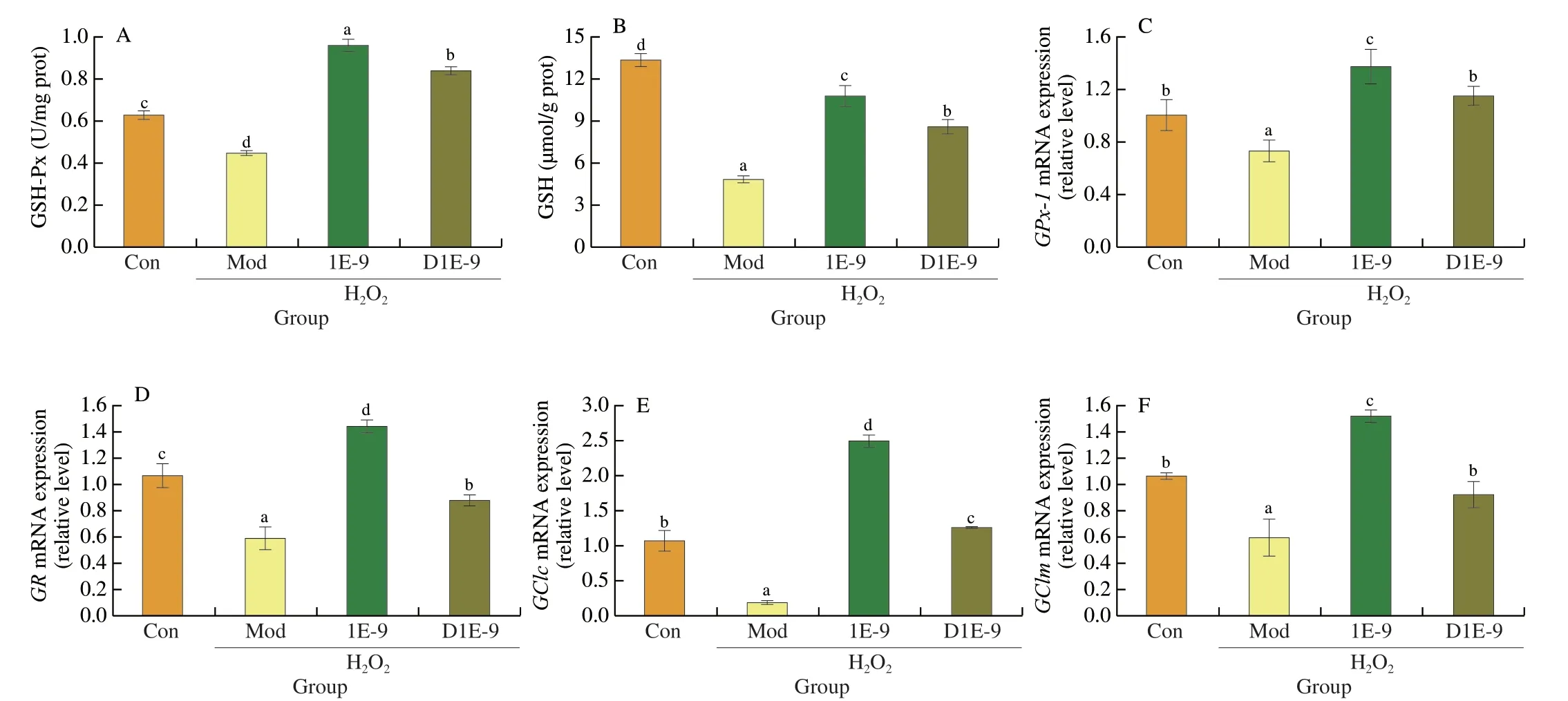
Fig.2 Effect of L.plantarun AR113 on endogenous GSH synthesis in H2O2-treated HepG2 cells.(A) GSH-Px enzyme activity;(B) GSH content;(C) The mRNA expression of GPx-1;(D) The mRNA expression of GR;(E) The mRNA expression of GClc;(F) The mRNA expression of GClm.Results of experiments are express as the mean ± SEM for each experimental group (n=6).The significance of differences between the data was assessed using One-way ANOVA by Dunnett’s analysis,with the level of significance set at P < 0.05.

Fig.3 Effect of L.plantarun AR113 on Nrf2/Keap1 signal pathways in HepG2 cells.(A) The mRNA expression of Nrf2;(B) SOD enzyme activity;(C) The mRNA expression of SOD;(D) The mRNA expression of HO-1;(E) The mRNA expression of CAT;(F) The mRNA expression of NQO1;(G) Effect of ML385 on cell viability;(H) Effect of ML385 on LDH release rate;(I) Effect of ML385 on ROS production;(J) Effect of ML385 on the mRNA expression of Nrf2;(K) Effect of ML385 on the mRNA expression of NQO1;(L) Effect of ML385 on the mRNA expression of HO-1.Results of experiments are express as the mean ± SEM for each experimental group (n=6).The significance of differences between the data was assessed using One-way ANOVA by Dunnett’s analysis,with the level of significance set at P < 0.05.
3.5 Anti-aging effect of L.plantarun AR113 on D-galactoseinduced mouse model
Previous experiments showed that AR113 could attenuate H2O2-induced oxidative damage in HepG2 cells.In order to further verify the mechanism of AR113 alleviating oxidative stress injury,theD-galactose model mice were used to conduct experiments.The experimental procedure was shown in Fig.5A.As shown in Fig.5,long-term injection ofD-galactose resulted in severe impairment of liver function in mice,including elevated AST and ALT enzyme activities,elevated MDA content,and significantly reduced antioxidant capacity (SOD,GSH-Px and T-AOC).Compared with the Mod group,after gavage ofL.plantarunAR113,the activity of ALT and AST decreased significantly by administration with AR113,especially for AST (decreased by 2.46 times) (Figs.5B,C).The content of lipid peroxidation product MDA also decreased by 2.81 times and returned to the level of the Con group (Fig.5D).L.plantarunAR113 could increases the antioxidant capacity of the liver significantly.Compared with the Mod group,AR113 increased SOD,GSH-Px and T-AOC in liver by 95.74%,195.62%and 856.18%,respectively (Figs.5E-G).And what’s more,the activities of SOD,GSH-Px and T-AOC also significantly higher than Con group.The results were consistent with HepG2 cells experiments.The effect of AR113 on the pathological observation in liver tissues was observed by H&E staining and Oil Red O staining.Oil red O staining showed thatD-galactose led to obvious fatty infiltration in the liver,and the color of liver sections were red.However,after administration with AR113,the structure of hepatocytes was clear and intact,and the number of lipid droplets was significantly reduced (Fig.5H).As shown in Fig.5I,in Mod group,exposure of mice toD-galactose resulted in disordered hepatocyte cords,irregular shape and arrangement of hepatocytes,and pyknotic nuclei.However,administration with AR113 significantly reduced liver damage and inflammatory infiltration.It indicated thatL.plantarunAR113 could significantly attenuateD-galactose-induced hepatic oxidative stress injury.
3.6 Effects of AR113 on Nrf2/Keap1 signaling pathway in D-galactose-induced aging mice
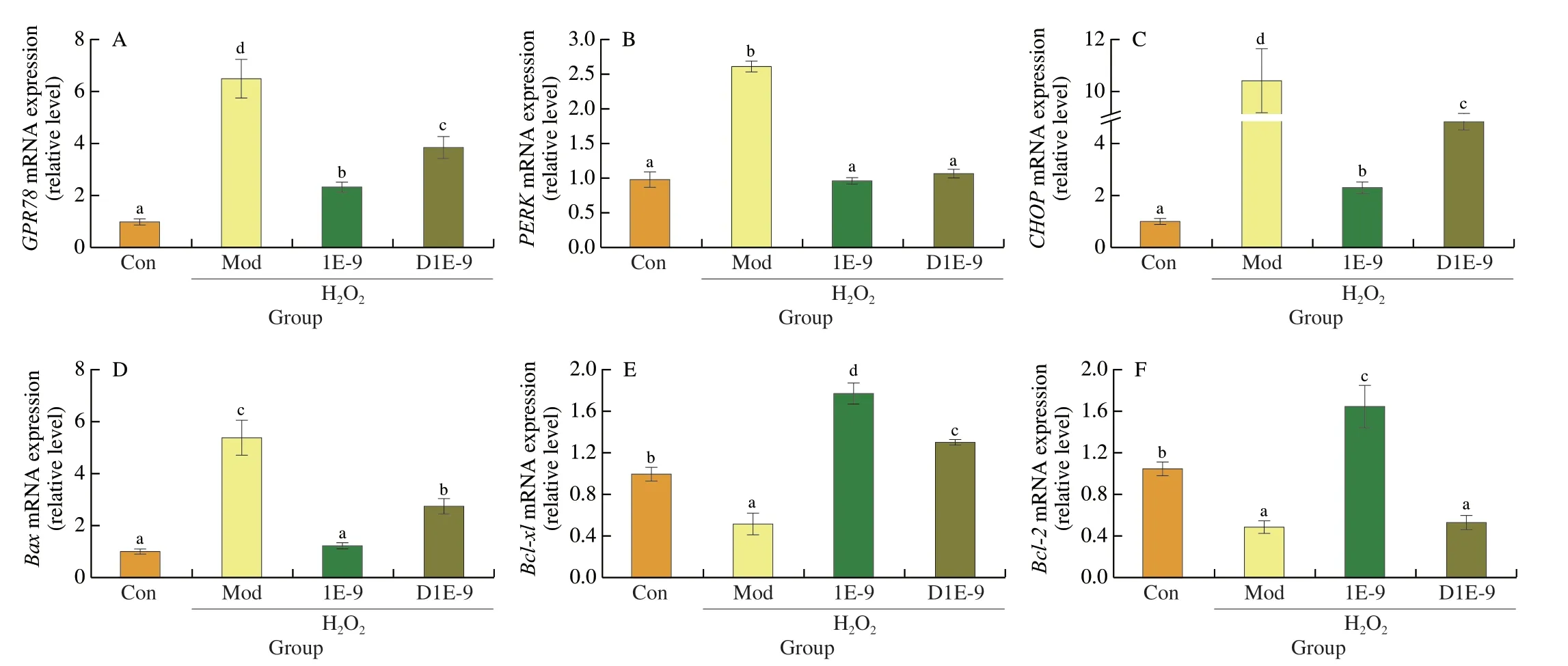
Fig.4 Effect of L.plantarun AR113 on ER stress in H2O2-treated HepG2 cells.(A) The mRNA expression of GPR78;(B) The mRNA expression of PERK;(C) The mRNA expression of CHOP;(D) The mRNA expression of Bax;(E) The mRNA expression of Bcl-x1;(F) The mRNA expression of Bcl-2.Results of experiments are express as the mean ± SEM for each experimental group (n=6).The significance of differences between the data was assessed using One-way ANOVA by Dunnett’s analysis,with the level of significance set at P < 0.05.
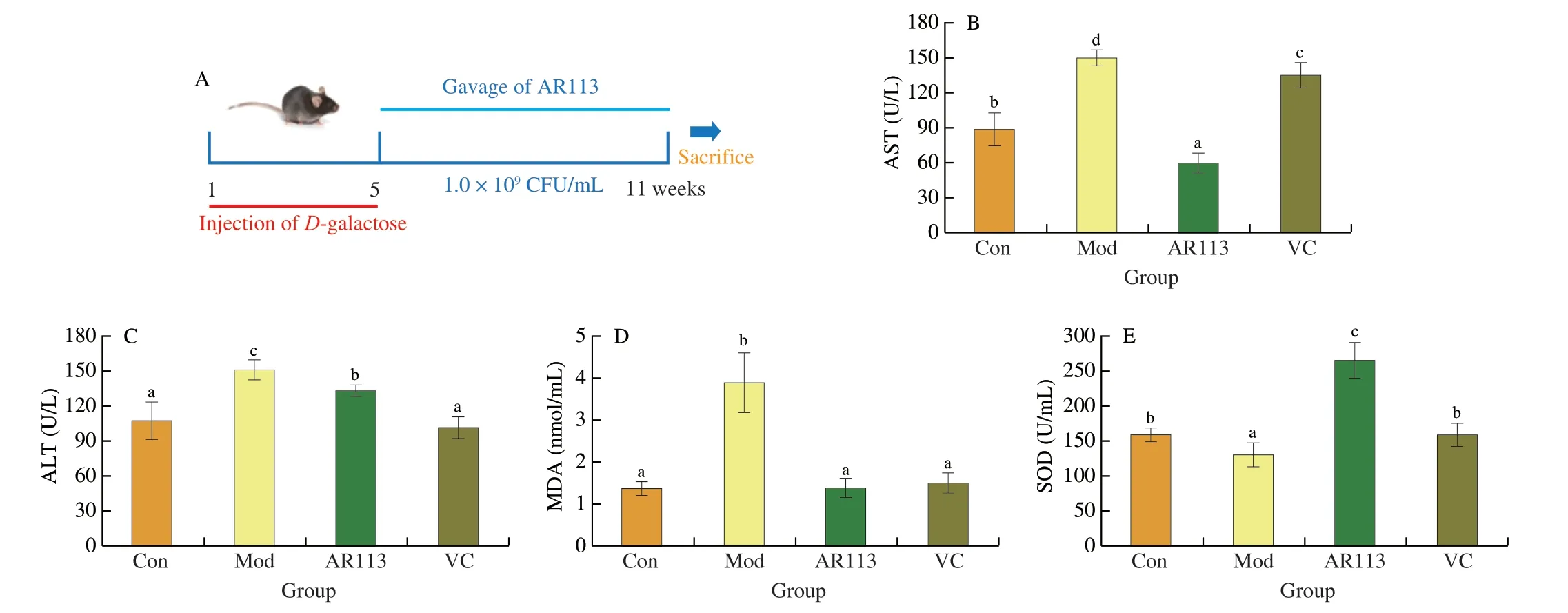
Fig.5 Anti-aging effect of L.plantarun AR113 on D-galactose-induced mouse model.(A) The experimental procedure;(B) MDA content;(C) AST enzyme activity;(D) ALT enzyme activity;(E) SOD enzyme activity;(F) GSH-Px enzyme activity;(G) T-AOC;(H) Oil Red O staining of liver tissue;(I) H&E staining of liver tissue.Original magnification 200×.Results of experiments are express as the mean ± SEM for each experimental group (n=8).The significance of differences between the data was assessed using One-way ANOVA by Dunnett’s analysis,with the level of significance set at P < 0.05.
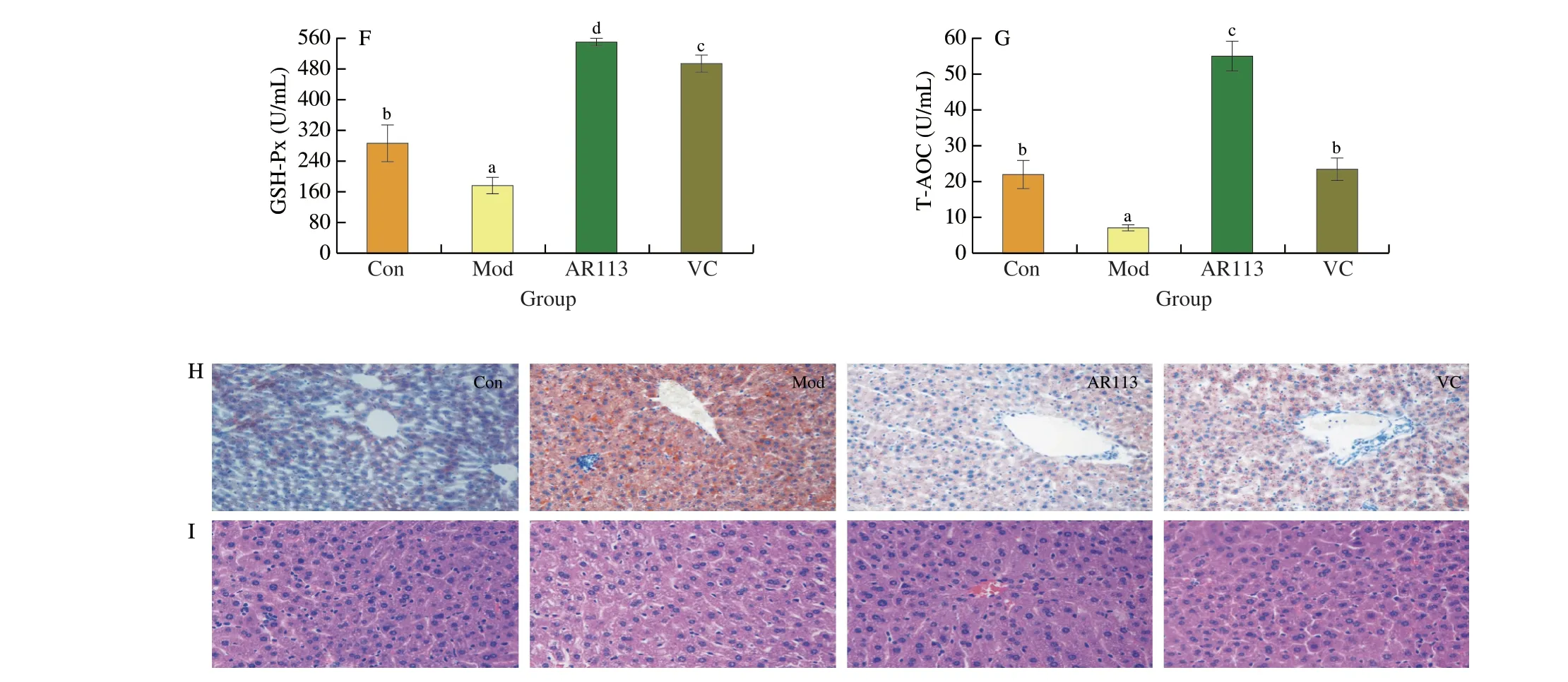
Fig.5 (Continued)

Fig.6 Effects of L.plantarun AR113 on Nrf2/Keap1 signaling pathway and GSH synthesis in D-galactose-induced aging mice.The mRNA expression of (A) Nrf2;(B) Keap1;(C) HO-1;(D) Sod2;(E) NQO1;(F) TRX;(G) GClm;(H) GClc;(I) GR;(J) GPx-1;(K) G6PDH;(L) GSTO1.Results of experiments are express as the mean ± SEM for each experimental group(n=8).The significance of differences between the data was assessed using One-way ANOVA by Dunnett’s analysis,with the level of significance set at P < 0.05.
The Nrf2/Keap1 signaling pathway plays an important role in alleviating oxidative damage in the body[32].As shown in Figs.6A and B,after long-term injection ofD-galactose (Mod group),the expression ofKeap1in the liver was significantly up-regulated,indicating thatNrf2was dissociated fromKeap1.However,the expression ofNrf2did not increase,but was lower than that in the Con group,and the gene expression levels of its regulated antioxidant enzymes and phase II detoxification enzymes were significantly lower than those in the Con group.
Gavage ofL.plantarunAR113 could significantly increase the expression ofNrf2(P< 0.001),which was 2.57 times that of the Mod group;and the expression ofKeap1was decreased,which was 0.22 times that of the Mod group.In addition toGRandGCLM,administration with AR113 could significantly increase the gene expression of antioxidant enzymes and phase II detoxification enzymes (Figs.6C-L).Among them,the expression ofSod2recovered to no significant difference with the Con group;while the expression ofGpx-1andG6PDHwas higher than that of the Con group,3.53 times and 13.04 times that of the Mod group,respectively.It indicated thatL.plantarunAR113 could also activate the Nrf2/Keap1 signaling pathway in mice,promote the expression of regulated phase II detoxification enzymes and antioxidant enzyme genes,thereby alleviatingD-galactose-induced liver oxidative damage,which is consistent with cell experiments.
3.7 Effects of AR113 on endoplasmic reticulum stress in D-galactose-induced aging mice
ER stress is an adaptive response of the body to stimuli such as oxidative stress,which can restore the homeostasis of proteins in the ER through various pathways[33].As shown in Fig.7,D-galactose disrupted the protein homeostasis in the mouse liver,resulting in a sharp increase in the mRNA expressions ofGPR78andPERK,which were 28.76 and 3.17 times that of the Con group,respectively(Fig.7A,7B).Elevated expression ofGPR78could be used as a marker of ER stress.Sustained ER stress activated and significantly increasedCHOPexpression and led to apoptosis (Fig.7C).The expression of anti-apoptotic gene Bcl-2 was significantly decreased,which was 0.20 times that of the Con group,and the expression of the pro-apoptotic geneBaxwas significantly increased,which was 44.00 times that of the Con group (Figs.7D,7E).However,L.plantarunAR113 intervention could significantly inhibitD-galactose-induced ER stress.The mRNA expression levels of the marker proteinsGPR78andCHOPwere reduced to 0.04 and 0.26 times that of the Mod group,respectively,while the expression ofPERKincreased to 2.09 times that of the Mod group.AR113 also improves the expression of apoptosis-related proteins.The expression level ofBcl-2was significantly increased,which was 12.24 times that of the Mod group,while the expression level ofBaxwas reduced to 0.01 times.As shown in Fig.7F,the results of Western blot were consistent with the qPCR results.AR113 could significantly reduce GPR78 and CHOP protein level in liver tissue and restore the protein homeostasis of ER.Further analysis shown that the ER stress induced apoptotic was relieved and AR113 could significantly reduce liver tissue damage under the condition ofD-galactose induced aging.
4.Discussion
As a safe dietary supplement,probiotics could improve health problems such as metabolic disorders in the body through antioxidant,anti-inflammatory and other functional properties.Previous studies have shown thatL.plantarunAR113 has good antioxidant activity and could colonize the intestinal environment[34].However,the specific mechanism is still unclear.Therefore,this paper analyzes the mechanism of AR113 alleviatingD-galactose-induced oxidative stress injury through cell and animal experiments.
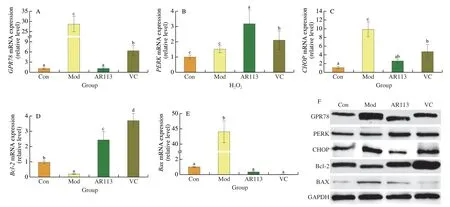
Fig.7 Effects of L.plantarun AR113 on endoplasmic reticulum stress in D-galactose-induced aging mice.The mRNA expression of (A) GPR78;(B) PERK;(C) CHOP;(D) Bcl-2;(E) Bax;(F) Western blot.Results of experiments are express as the mean ± SEM for each experimental group (n=8).The significance of differences between the data was assessed using One-way ANOVA by Dunnett’s analysis,with the level of significance set at P < 0.05.
The intake of dietary antioxidants could significantly reduce the risk of colorectal cancer,Parkinson’s disease and other diseases,and help maintain bone mass,which is related to the overall antioxidant level of the diet[35-36].However,studies have shown that long-term intake of antioxidants (N-acetylcysteine and vitamin E) may have negative health effects,especially in healthy people,excess dietary antioxidants could disrupt the homeostasis of ROS signaling,thereby accelerated aging[37-38].ROS mainly generated from mitochondrial respiration as byproducts,and they are tightly controlled by multiple anti-oxidant mechanisms[39].ROS are integral in host defense and redox signaling,but are also involved in inflammatory or degenerative diseases[40].Oxidative stress occurs from the imbalance between production and elimination of ROS which are believed to cause of cellular damage and underlie a variety of metabolic and neurodegenerative diseases[41-42].In this study,L.plantarunAR113 could significantly improve the survival rate of H2O2-treated HepG2 cells,effectively eliminate intracellular ROS to normal levels,and reduce cell damage (LDH and JC-1) (Fig.1).AR113 could upregulate the expression of endogenous antioxidant GSH synthesis genes(G6PDH,GClcandGClm),increase the intracellular GSH content and the activities of related antioxidant enzymes,thereby effectively eliminating the cell damage caused by continuous oxidative stress(Fig.2 and Fig.6).More importantly,AR113 could restore the antioxidant capacity of mice,and plays an important role in regulating liver lipid metabolism disorders and fat droplet deposition[9,43].
In general,probiotics are able to achieve antioxidant capacity by scavenging free radicals,sequestering pro-oxidative metal ions,and modulating related enzymes and gut microbiota.However,the antioxidant properties of probiotics are strain-specific,and different strains exert different mechanisms of antioxidant capacity.Previous studies have shown thatL.plantarunAR113 could tolerate 3.5 mmol/L H2O2
[44].When subjected to H2O2-induced oxidative stress,AR113 could highly express GSH-related enzymes and antioxidant enzymes(npx,nox,cat) for scavenging excess ROS.This is consistent with the study ofL.fermentumME-3 andBifidobacterium animalissubsp.lactis01[45-46].However,the genes associated with antioxidant by probiotics are different.Zhang et al.[47]showed thatL.plantarunZLP001 has no genes encoding SOD enzymes.AR113 could encode genes of various antioxidant enzymes,so it could quickly respond to oxidative stress and exert antioxidant function in the presence of a large amount of ROS.Interestingly,the inactivatedL.plantarunAR113 could alleviating oxidative stress injury in H2O2-induced HepG2 cells.Studies have shown that different probiotics have different material bases for their antioxidant function,and metabolites such as extracellular polysaccharides and surface proteins make probiotics still have good antioxidant activity after heat inactivation.Mws et al.shown that both living and heat-killedL.brevisB13-2 cells showed antioxidant activity and immunomodulating activity[48].Heat-killedL.plantarun(L-137) could still increase the activity of antioxidative enzyme (SOD and CAT) significantly in genetically improved farmed tilapia[49].
In addition to their own antioxidant functions,probiotics could also affect the host’s antioxidant capacity.In theD-galactose mouse model,AR113 could activate the Nrf2 pathway in the liver,thereby increasing the expression of various antioxidant enzymes such as HO-1,NQO1,TRX,and SOD2.In the meantime,the experiment of adding inhibitor ML385 into HepG2 cells confirmed that AR113 could promote the expression of intracellular phase II detoxification enzymes by activating the Nrf2/Keap1 signaling pathway,and effectively eliminate ROS generated by excessive oxidative stress (Fig.3).Studies have shown that the activation of Nrf2 signaling pathway by probiotics is one of the most important mechanisms for exerting antioxidant functions[50-51].In animal models that could induce a large amount of ROS production,such as aging and high-fat diet,probiotics could upregulate the expression of SOD,CAT and other antioxidant enzymes by activating the Nrf2 signaling pathway,and ultimately enhance antioxidant defense capabilities[52-54].Researches shown that Nrf2 could involves in ER stress.Nrf2 could ameliorate neurological deficit by ER stress and ER stress-induced apoptosis in Nrf2 knockout mice[55].Additionally,Nrf2 signaling pathway could reduce ER stress in mouse hepatocytes[56].
ER stress is an adaptive response to stimuli such as oxidative stress.It could maintain environmental homeostasis in cells by regulating various mechanisms.However,persistent ER stress induces inflammatory responses and cell damage,and is associated with various diseases caused by oxidative stress,such as lipid metabolism disorders[57-58].In this study,both hydrogen peroxide treatment and long-term injection ofD-galactose triggered persistent ER stress,which resulted in the accumulation of many misfolded proteins and disrupted endoplasmic reticulum function,which was manifested in a significant increase in the expression of GPR78 protein,and finally activate apoptosis pathway.Oral administration of AR113 could alleviate ERS,significantly reduce the expression of GPR78 and CHOP proteins,and promote the restoration of ER homeostasis,thereby activating cell anti-apoptotic pathways and reducing cell damage.More importantly,AR113 could activate endogenous GSH synthesis and Nrf2/Keap1 signaling pathway in cells,so its alleviation effect on ERS is better than that of single antioxidant VC.
Researches showed that inhibition of ERS and oxidative stress is important for balance of metabolism and energy[59-60].Additional,Jiang et al.[61]showed that dual inhibition of ERS and oxidative stress reprogrammed M2 macrophages to improve the efficacy of PD-1 antibodies.There is a close relationship between oxidative stress and ER stress.Continuous exposure to ROS will aggravate the degree of oxidative stress,promote lipid peroxidation and produce a series of harmful compounds,resulting in alterations in the structure,function and activity of cellular proteins[62].Protein folding is the critical function of ER,and sustained oxidative stress will lead to many unfolded protein responses,resulting in ER stress[63].At the same time,the sustained unfolded protein responses would further promote the generation of ROS,leading to further aggravation of oxidative stress[64].L.plantarunAR113 has a good inhibitory effect on oxidative stress and ERS,indicating that probiotics with antioxidant function have good application potential in anti-aging and alleviating metabolic disorders.
5.Conclusion
In this paper,it was confirmed in H2O2-treated HepG2 cells andD-galactose mouse model thatL.plantarunAR113 could enhance endogenous GSH synthesis and activate Nrf2 signaling pathway,promoting the expression of SOD and other phase II detoxification enzymes,thereby reducing liver damage.At the same time,AR113 could inhibit the ER stress of liver cells and alleviate the apoptosis caused by ROS.It indicated thatL.plantarunAR113 could inhibitD-galactose-induced liver injury through multiple mechanisms.In the future,further research is needed to investigate whether AR113 is effective in preventing aging-related diseases.
Declaration of conflicting interest
The authors declared that they have no conflicting interest.
Acknowledgements
This work was supported by the National Science Fund for Distinguished Young Scholars (32025029),the Shanghai Education Committee Scientific Research Innovation Projects(2101070007800120),the Yili Health Science Foundation of Chinese Institute of Food Science and Technology (2021-Y06),the Shanghai Engineering Research Center of food microbiology program(19DZ2281100).
Data availability statement
The original contributions presented in the study are included in the article,further inquiries can be directed to the corresponding author.
- 食品科学与人类健康(英文)的其它文章
- Protective effects of oleic acid and polyphenols in extra virgin olive oil on cardiovascular diseases
- Diet and physical activity inf luence the composition of gut microbiota,benef it on Alzheimer’s disease
- Inf luence of nitrogen status on fermentation performances ofnon-Saccharomyces yeasts: a review
- Ganoderma lucidum: a comprehensive review of phytochemistry,eff icacy,safety and clinical study
- Resveratrol combats chronic diseases through enhancing mitochondrial quality
- Demonstration of safety characteristics and effects on gut microbiota of Lactobacillus gasseri HMV18

