A novel strain of Levilactobacillus brevis PDD-5 isolated from salty vegetables has benef icial effects on hyperuricemia through anti-inf lammation and improvement of kidney damage
Jue Xu,Maolin Tu,Xiankang Fan,Yuxing Guo,Tao Zhang,Xiaoqun Zeng,,Zhendong Cai,Zhen Wu,Daodong Pan,
a State Key Laboratory for Managing Biotic and Chemical Threats to the Quality and Safety of Agro-products, Ningbo University, Ningbo 315211, China
b Key Laboratory of Animal Protein Food Deep Processing Technology of Zhejiang Province, College of Food and Pharmaceutical Sciences, Ningbo University, Ningbo 315211, China
c Department of Food Science and Technology, School of Food Science and Pharmaceutical Engineering, Nanjing Normal University, Nanjing 210023, China
Keywords: Lactic acid bacteria Purine nucleoside Hyperuricemia Uric acid nephropathy
ABSTRACT Hyperuricemia is a metabolic disorder caused by abnormal purine metabolism,resulting in abnormally high serum uric acid.In this study,a novel Levilactobacillus brevis PDD-5 isolated from salty vegetables was verif ied with the function of alleviating hyperuricemia.The relevant effects of L.brevis PDD-5 in lowering uric acid were analyzed by in vitro and in vivo experiments.The results showed that the L.brevis PDD-5 has (68.86 ± 15.46)%of inosine uptake capacity and (95.75 ± 3.30)% of guanosine uptake capacity in vitro.Oral administration of L.brevis PDD-5 to hyperuricemia rats reduced uric acid,creatinine,and urea nitrogen in serum,as well as decreased inosine and guanosine levels in the intestinal contents of rats.Analysis of relevant markers in the kidney by ELISA kits revealed that L.brevis PDD-5 alleviated oxidative stress and inf lammation.Moreover,the gene expression of uric acid transporter 1 (URAT1) and glucose transporter 9 (GLUT9) was down-regulated,and the gene expression of organic anion transporter 1 (OAT1) was up-regulated after treatment with L.brevis PDD-5.Western blot analysis showed that L.brevis PDD-5 alleviated hyperuricemia-induced kidney injury through the NLRP3 pathway.The se f indings suggest that L.brevis PDD-5 can lower uric acid,repair kidney damage,and also has the potential to prevent uric acid nephropathy.
1.Introduction
Hyperuricemia is a metabolic disorder characterized by elevated serum uric acid levels,exceeding 415 µmol/L in adults and 330 µmol/L in adolescents[1].Complications associated with hyperuricemia include gout,atherosclerosis,obesity,hypertension,metabolic syndrome,cardiovascular disease,lipid disorders,and chronic kidney disease[2].Due to the evolutionary lack of uric acid oxidase in primates,purines are degraded via xanthine oxidase catalytic degradation to produce uric acid as its final oxidation product in humans,and the increase in uric acid levels in the blood leads to hyperuricemia[3].A variety of factors contribute to hyperuricemia,including genetics,insulin resistance,hypertension,renal insufficiency,obesity,diet and alcoholic beverage consumption[4].Foods rich in purine-like substances are more potent in exacerbating hyperuricemia[5].Althoug h there are many commercially available drugs for the treatment of hyperuricemia,such as allopurinol,these drugs had significant side effects including liver and renal toxicity and systemic rash manifestations[6].Therefo re,in addition to drugs,micro-ecological treatments without side effects are crucial to treating hyperuricemia.
Microecological therapy could achieve the balance of microecology in the body by supplementing with live bacteria or flora transplants that are beneficial to the body[7].Live microorganisms which bring health benefits to the host are called probiotics when ingested in sufficient amounts[8].Lactic acid bacteria (LAB)are regarded as a major group of probiotics and a normal flora in the intestinal tract.LAB play an important role in promoting the absorption and decomposition of nutrients,maintaining microbial flora balance of the intestine,reducing intestinal pH,and improving gastrointestinal function,bacteriostasis and immune regulation.In addition,studies have shown a correlation between the reduction of serum uric acid in mice and the intake of LAB,which can efficiently absorb purines[9].For example,Yamada et al.[10]used an isotope label to confirmLactobacillus gasseriPA-3 could incorporate purines.Wang et al.[11]screened strain DM9218,which was identified asLactobacillus plantarum,had a strong ability to assimilate purine nucleosides (inosine and guanosine).At this stage,only a few LAB were screened to absorb purines.But the species of LAB that absorb purines have not been fully expanded due to the few studies.
Hyperuricemia is accompanied by persistent chronic low-grade inflammation,which plays an important role in the early stage of hyperuricemia development and the complications caused in the late stage of hyperuricemia.LAB are equally useful in removing the effects of inflammation,and many reports illustrate that LAB can alleviate inflammation.Vieira et al.[12]discovered that oral administration ofBifidobacterium longum51Aimproved gout caused by urate crystals by inhibiting IL-1β and recombinant human C-X-C motif chemokine 1 (CXCL1).Leblanc et al.[13]demonstrated the immunomodulatory effect of Kefir against breast tumor disease,which was based on LAB intervention to reduce IL-6 by modulating the immune and endocrine systems.LAB that exhibited hypoglycaemic effects played a beneficial role in reducing insulin resistance by alleviation of inflammation via reducing tumor necrosis factor-α(TNF-α) and interleukin-6 (IL-6) levels[14].
However,studies on the interventional effects of uric acidlowering lactic acid bacteria on hyperuricemia uric acid nephropathy are relatively scarce.It is necessary to screen for LAB with the ability to absorb purines.This study aimed to screen the LAB capable of degrading purines (guanosine and adenosine)invitroand evaluate their uric acid-lowering effects and alleviating mechanisms through a hyperuricemia rat model.The study could lay a theoretical foundation for the treatment of hyperuricemia and uric acid nephropathy and provide a basis for the development of probiotics to alleviate hyperuricemia.
2.Materials and methods
2.1 Materials
Salty vegetables and fish were purchased from Nanjing Ninghai Road vegetable market (China).Milk lumps were collected from Kanxiang.Qapqal County (Xinjiang,China).Tripotassium phosphate(K3PO4) was obtained from Lingfeng Chemical Reagent Co.,Ltd.(Shanghai,China).Inosine,guanosine,and allopurinol were obtained from Sigma (Merck,Darmstadt,Germany).The rats were purchased from Sipford Biotechnology Co.,Ltd.(Beijing,China).High-purine rat chow and ordinary rat chow were obtained from Syony Pharmaceutical Bioengineering Co.,Ltd.(Jiangsu,China).Oxygen oxazine acid potassium (OXO) was obtained from Maclin Biochemical Technology Co.,Ltd.(Shanghai,China).Sodium carboxymethylcellulose (CMC-Na),malondialdehyde (MDA),superoxide dismutase (SOD),and glutathione peroxidase (GSH-Px)kits were obtained from Solarbio (Beijing,China).Enzyme-linked immunosorbent assay (ELISA) kits,XOD kits and antibodies for Western blotting were purchased from the Nanjing Jiancheng Bioengineering Institute (Nanjing,China).
2.2 Screening of LAB that degrades purine nucleosides
Fifty strains were isolated from Chinese salty vegetables,gastrointestinal tract contents from healthy fish (Ctenopharyngodon idellus),and milk lumps (Xinjiang,China).The LAB strains were incubated in de Man,Rogosa and Sharpe (MRS) broth for 24 h at 37 °C.In order to identify LAB with the ability to efficiently absorb inosine and guanosine,strains were grown in a standard assay mixture consisting of 12.6 mmol/L inosine,12.6 mmol/L guanosine,and 0.1 mol/L sodium phosphate buffer (pH 7.0).Then the supernatants were centrifuged (10 000 ×g,2 min),followed by filtration through a 0.22 µm filter.The content of inosine and guanosine in the supernatants was determined using high-performance liquid chromatography (HPLC).
Quantitative analysis of inosine and guanosine was performed by HPLC as described by Yamada et al.[10]with slight modifications.Reductions in substrate (inosine or guanosine) concentrations were analyzed by an Agilent HPLC (1260 series,Agilent Technologies,Palo Alto,CA) and Agilent ZORBAX SB-Aq column (250 mm ×4.6 mm i.d.;5 μm;Agilent,Palo Alto,CA).The mobile phase consisted of solvent A (Milli-Q water) and solvent B (methyl alcohol).Elution was started isocratically with a constant flow of 2% B,5 min;followed by 2%-12% B,5-10 min;12%-15% B,10-12 min;and 15% B,12-30 min,with the eluent monitored at 254 nm.
2.3 Physiological characteristics and probiotic properties of LAB
A slight modification of the method created by Conway,Gorbach,and Goldin[15],MRS were prepared and inoculated with 1.0% bacterial suspensions of all collected LAB strains for 12 h at 37 °C.The bacterium was collected by centrifuging 1 mL of bacteria solution,and inoculated in artificial gastric fluid for 2 h and artificial intestinal fluid for 4 h,respectively[16].The survival rate was calculated based on the value of the viable cells.
In order to determine the antibacterial activity of strains,the AGAR punching method was used as described by Schillinger et al.[17]with slight modifications.The pathogen was inoculated on the surface of a solid medium and perforated,and 100 μL LAB was inoculated into the hole for culture.The inhibitory effect of the same volume of sodium chloride was tested as a negative control on each plate.Inhibition zones (mm) were measured.
Bacteria adhesion to the solvent (BATS) was measured according to the method of Rosenberg et al.[18]with some modifications.Bacteria were harvested by centrifugation at 4 000 ×gfor 10 min,washed twice with saline,and resuspended in 0.1 mol/L KNO3(pH 6.2) to approximately 108CFU/mL.The absorbance of the cell suspension was measured at 600 nm (A0).One milliliter of solvent was added to 3 mL of cell suspension.The two-phase system was mixed by vortexing for 2 min.The aqueous phase was removed after 20 min of incubation at room temperature,and its absorbance at 600 nm (A1) was measured.Three different solvents were tested in this study: xylene,which is an apolar solvent;trichloromethane,a monopolar and acidic solvent;and ethyl acetate,a monopolar and basic solvent.The cellular surface hydrophobicity percentage was calculated as follows:
2.4 Identification of target LAB
LAB with better probiotic characteristics and absorption of purine nucleosides were further identified by 16S rRNA analysis.The 16S rRNA sequence analysis procedure was as follows: total DNA of a strain was extracted using a bacterial genomic DNA extraction kit.The general primers 1492R and 27F were used for amplification.The PCR fragments (1 500 bp) were purified using a quick PCR purification kit and sequenced by Sangon Biotech (Shanghai,China).The 16S rRNA gene sequence was submitted to the National Center of Biotechnology Information with accession number OP692768.Then,a phylogenetic tree was constructed by generating a complete alignment of the 16S rRNA gene of the selected members in GenBank by using MEGA software (https://www.megasoftware.net/) bootstrap values.Finally,the identified strain was deposited in the China General Microorganisms Collection (CGMCC,Beijing,China) with ID number 20573.
2.5 Establishment of hyperuricemia model and animal study
The experimental protocol was performed in accordance with the guidelines for the ethical treatment of experimental animals and approved by the Animal Ethics Committee of the Nanjing Normal University,China (SYXK (Su) 2015-0028).Forty-eight SPF adult male SD rats (200-220 g) were kept in the animal room at (22 ± 1) °C under standard housing conditions of a 12:12 h light/dark cycle for 7 days before the experiment.Since rats have the enzyme allantoin,which can metabolize uric acid into allantoin for excretion,this experiment adopted the method of treating while modeling.High uric acid was induced by intraperitoneal injection of potassium oxonate(0.35 mg/100 g body weight per day)-carboxymethylcellulose sodium(CMC-Na) solution (3 g/L) in rats.The intraperitoneal injection solution was an aqueous solution of potassium oxonate dissolved in 0.5% sodium carboxymethylcellulose (CMC-Na).
After acclimatization feeding,48 rats were randomly divided into 6 groups (n=8): control (Con) group (normal diets,intraperitoneal injection of CMC-Na,0.9% NaCl);model (Mod) group (high purine diets,intraperitoneal injection of potassium oxyzincate-CMC-Na,0.9% NaCl);L-Lab group (high purine diets,intraperitoneal injection of potassium oxyzincate-CMC-Na,1 × 108CFU/mLLevilactobacillus brevisPDD-5);M-Lab group (high purine diets,intraperitoneal injection of potassium oxyzincate-CMC-Na,1 × 109CFU/mLL.brevisPDD-5);H-Lab group (high purine diets,intraperitoneal injection of potassium oxyzincate-CMC-Na,1 × 1010CFU/mLL.brevisPDD-5);positive control (Pos) group (high purine diets,intraperitoneal injection of potassium oxyzincate-CMC-Na,42 mg/100 kg body weight of allopurinol).Each group of rats was gavaged with a daily dose of 2 mL.All rats were weighed and blood was collected at 0,7th,and 14thday by orbital blood sampling[19].On the 14thday,after fasting,all rats were anesthetized with 10%chloral hydrate intraperitoneally and then executed,and liver,kidney,blood,and intestinal contents were collected.
2.6 Determination of biochemical indicators
Blood samples were taken from the abdominal aorta of rats and the supernatant was obtained by centrifugation at 3 500 ×gfor 15 min at 4 °C.The creatinine (CRE),urea nitrogen (BUN),and uric acid (UA) levels from serum were determined using Cobas®8000 Automatic Biochemical analyzer (Roche Diagnostics,Swiss).
The kidney tissue samples of each group of rats were taken and the levels of xanthine oxidase (XOD),MDA,SOD,and GSH-Px were measured according to the manufacturer’s instructions of the kits.
2.7 Measurement of inosine and guanosine content in rat intestinal contents
Intestinal contents of rats were collected and diluted with 80 °C distilled water at 1:50 (m/V),followed by shaking for 5 min and centrifugation (6 000 ×g,10 min).The supernatant was taken and centrifuged repeatedly 3 times to remove impurities and the filter membrane was sampled at 0.22 μm.The levels of inosine and guanosine in rat intestinal contents were determined by HPLC the elution procedure is consistent with the method of screening strains with a high ability to degrade purine nucleoside.
2.8 Determination of renal histopathology
The complete right kidney of rats was collected and immediately fixed with formalin and embedded in paraffin.Hematoxylin and eosin were used to stain the kidney sections,which were cut in 5 μm-thick sections.The sections were then imaged under light microscopy at 200×magnification and the kidney injury score was calculated according to the EGTI tissue scoring system optimized by Chavez et al.[20].
2.9 Determination of proinflammatory cytokine concentrations
Rat kidney tissues (about 100 mg) were homogenized on ice and centrifuged at 5 000 ×gfor 10 min,and the supernatant was collected.The concentration of TNF-α,IL-1β,and IL-6 in renal tissue homogenate supernatant were determined using commercial ELISA assay kits (ZCIBIO,Shanghai,China).
2.10 Real-time quantitative PCR
TRIzol reagent was employed to extract 1 000 ng of RNA.Total RNA was converted into cDNA using PrimeScript™ RT master mix kit.The expressions of the mRNAs of uric acid transporter 1(URAT1),glucose transporter 9 (GLUT9),and organic anion transporter 1 (OAT1) (GAPDHgene used as an endogenous control for the assay) were measured by a StepOne plus real-time PCR system (Applied Biosystems,USA) according to ChamQ SYBR qPCR master mix kit instructions.The forward and reverse primers used in this procedure are provided in Table S1.
2.11 Western blotting analysis
Lysates of kidney tissues were collected and protein concentration was determined using a bicinchoninic acid (BCA) kit (Beyotime Biotechnolgy,Shanghai,China).Proteins were resolved by SDS-PAGE and transferred to nitro cellulose membranes.Membranes were then blocked in 5% nonfat dry milk for 2 h followed by overnight incubation at 4 °C with antibodies against NLRP3 (nucleotide-binding oligomerization domain-like receptor family containing pyrin domain 3)receptor,IL-1β,and apoptosis-associated spotted proteins (ASC).Horseradish peroxidase-conjugated goat anti-rabbit IgG (H+L) was used as the secondary antibody.After washing in PBS,the membranes were incubated with secondary antibodies (1:5 000 dilution,Cell Signaling Technology,Danvers,MA),followed by washings 3 times with Tris-buffered saline-Tween.Immunoreactivity was detected with an enhanced chemiluminescence detection system and visualized in an imaging system.β-Tubulin (1:1 000;Cell Signaling Technology)was used as control.The images were quantified using Image-Pro Plus software (Media Cybernetics,Rockville,MD).
2.12 Statistical analysis
All experiments were repeated 3 times.Experimental data were analyzed using GraphPad Prism 7.0 (GraphPad Inc.,San Diego,CA),and analysis of significant differences was performed by Oneway ANOVA (P< 0.05).Data are expressed as the mean ± standard deviation (SD).
3.Results
3.1 Isolation of inosine and guanosine-degrading LAB
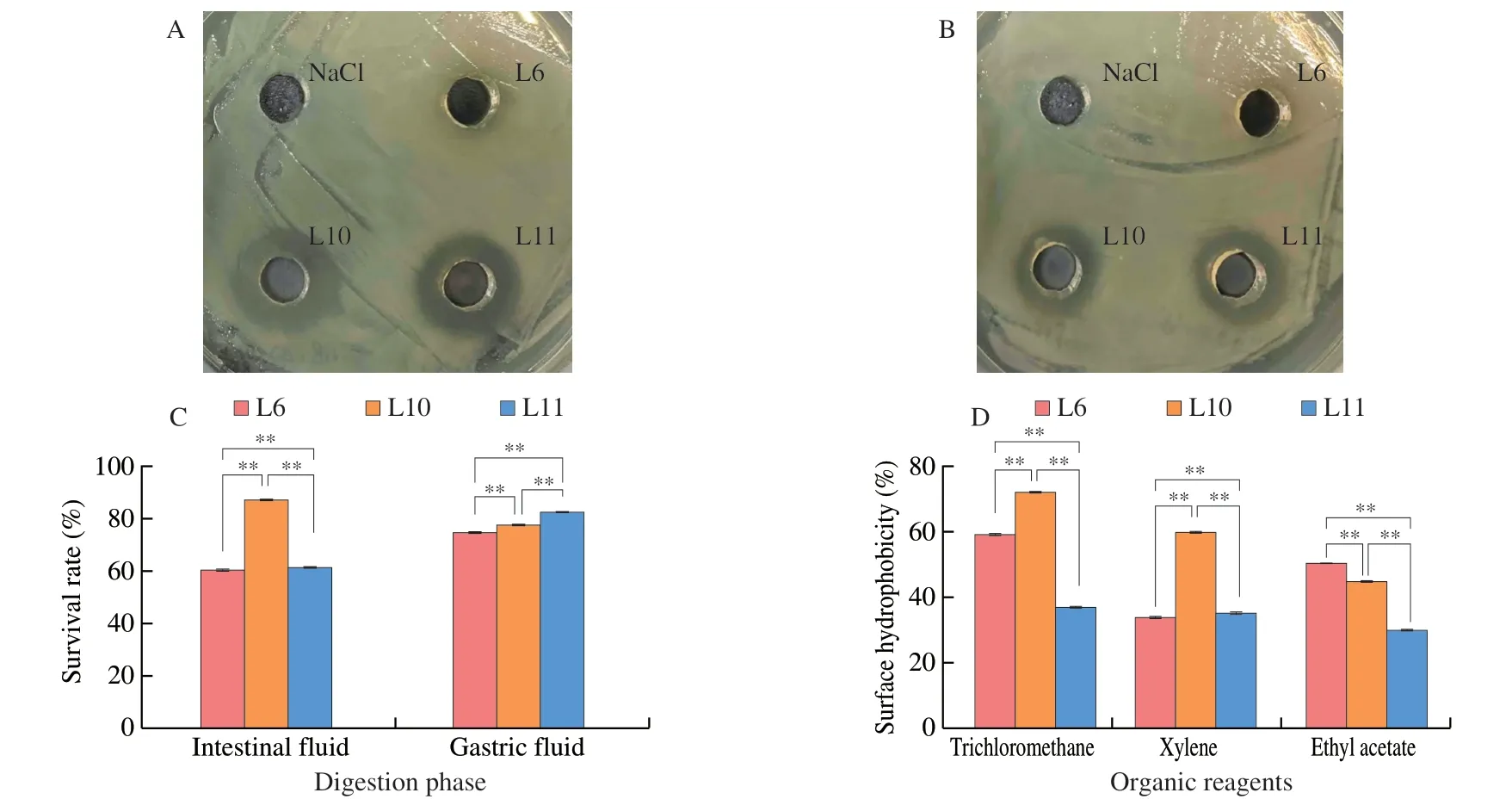
Fig.1 Probiotic properties of isolated strains.(A) The inhibitory ability of the isolates against E.coli.(B) The inhibitory ability of the isolates against S.aureus.(C) The effect of simulated gastrointestinal environment on the isolated strains in vitro.(D) Adhesion of isolated strains to three organic reagents.*P < 0.05,**P < 0.01.
In this study,the inosine and guanosine decreasing capacity via LAB isolated from pickled vegetables,milk lumps,and fish intestines were monitored by HPLC (Fig.S1) and the results were summarized in Table S2.The results showed that L6,L10,and L11 exhibited relatively higher capacity of decreasing the guanosine among all strains,of which their value reached to (83.57 ± 3.92)%,(95.75 ± 3.30)%,and (93.58 ± 1.75)%,respectively.Consequently,these three strains of LAB were selected for further studies.
3.2 Physiological characteristics of isolated strains and identification of target strains
One of the significant features of probiotic bacteria is the ability to inhibit the growth of pathogenic bacteria.The results demonstrated thatEscherichia coliandStaphylococcus aureuswere significantly inhibited by L10 and L11 (Figs.1A-B).However,no difference of the inhibition zone was found in the strain of L6 compared with control(NaCl).Under the simulated stomach fluid,a higher survival rate of strain L11 (82.20%) was observed compared with that of L6 and L10,whose values both reached 70%.The survival of L6 (60.26%),L10 (86.84%),and L11 (61.22%) in the intestinal fluid showed a progressive increase (Fig.1C),which would be necessary for their colonization in the intestinal tract.
The BATS method was used to evaluate the cell surface hydrophobicity properties of selected strains.The hydrophobicity capacity of bacteria is one of the important factors affecting its adhesion to host tissues[21],high cell surface hydrophobicity contributes to bacterial colonization of the intestinal mucosal surface and enhances bacterial adhesion to the intestinal epithelial cells[22].Fig.1D showed the surface hydrophobicity of L6,L10,and L11 in trichloromethane,xylenes,and ethyl acetate.Overall,L10 exhibited the highest surface hydrophobicity (> 50%) regardless of environment polarity,followed by strains of L6 and L11.The high surface hydrophobicity of L10,which could contribute to bacterial colonization of the intestinal mucosal surface epithelial cells,was in line with the result of Fig.1C.According to the results aforementioned,the strain of L10,characterized with good probiotics and high efficiency in purine nucleoside degradation,was selected for further study of 16S rRNA gene analysis (Fig.2).This strain was identified asL.brevisand was namedL.brevisPDD-5.
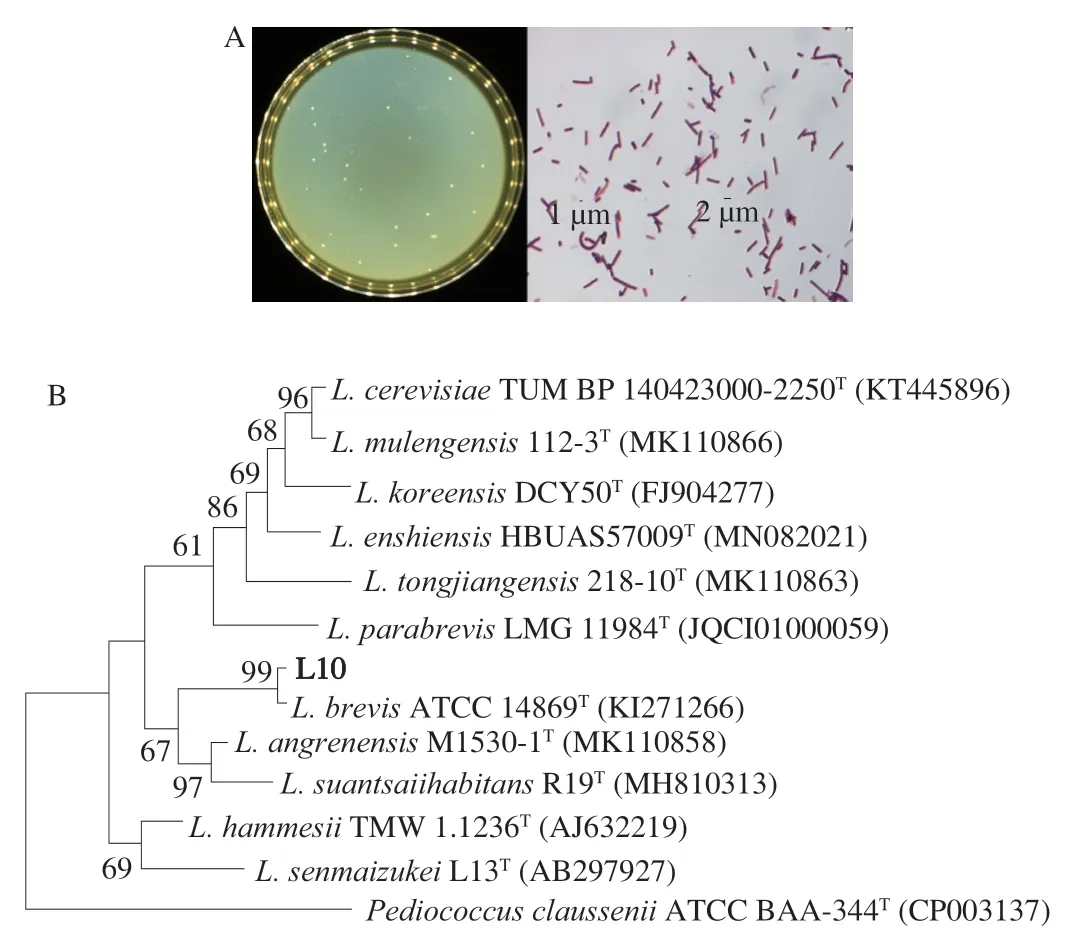
Fig.2 Identification of the strain L10.(A) Morphology of the screened strain L10 on MRS plate and under the microscope.(B) Phylogenetic tree based on 16S rRNA gene sequences of the strain L10.The evolutionary history was inferred using the Neighbor-Joining method,scale bar represents 0.01 nucleotide substitution per position.
3.3 Effect of L.brevis PDD-5 on body weight, organ index,XOD, and serum biochemical indicators
As showed in Fig.3A,there was a gradual increase of body weight of rat in control group and a significant decrease was observed in model rat within 2 weeks.The intervention ofL.brevisPDD-5 showed a dosing dependent alleviation effect of body weight reduction,among which the body weight value of H-Lab was almost identical to that of allopurinol.Similarly,the decrease of organ index,at the intervention ofL.brevisPDD-5,also followed a dosing dependent trend,where in the H-Lab group the index was almost the same to that of in Pos group (Fig.3B).In addition,as shown in Fig.3C,XOD activity was significantly increased in the liver of hyperuricemia rats (model group) compared to control mice (P< 0.05),whereas,as expected,the levels were significantly reduced after treatment with M-Lab,H-Lab (P< 0.05) and allopurinol (positive control group) (P< 0.05).The serum levels of UA,CREA,and BUN in the rat model group were substantially different from the blank control group (P< 0.05),as shown in Figs.3D-F,demonstrating successful modeling.In the H-Lab group,all three serum markers dramatically dropped,which was consistent with the allopurin effect.
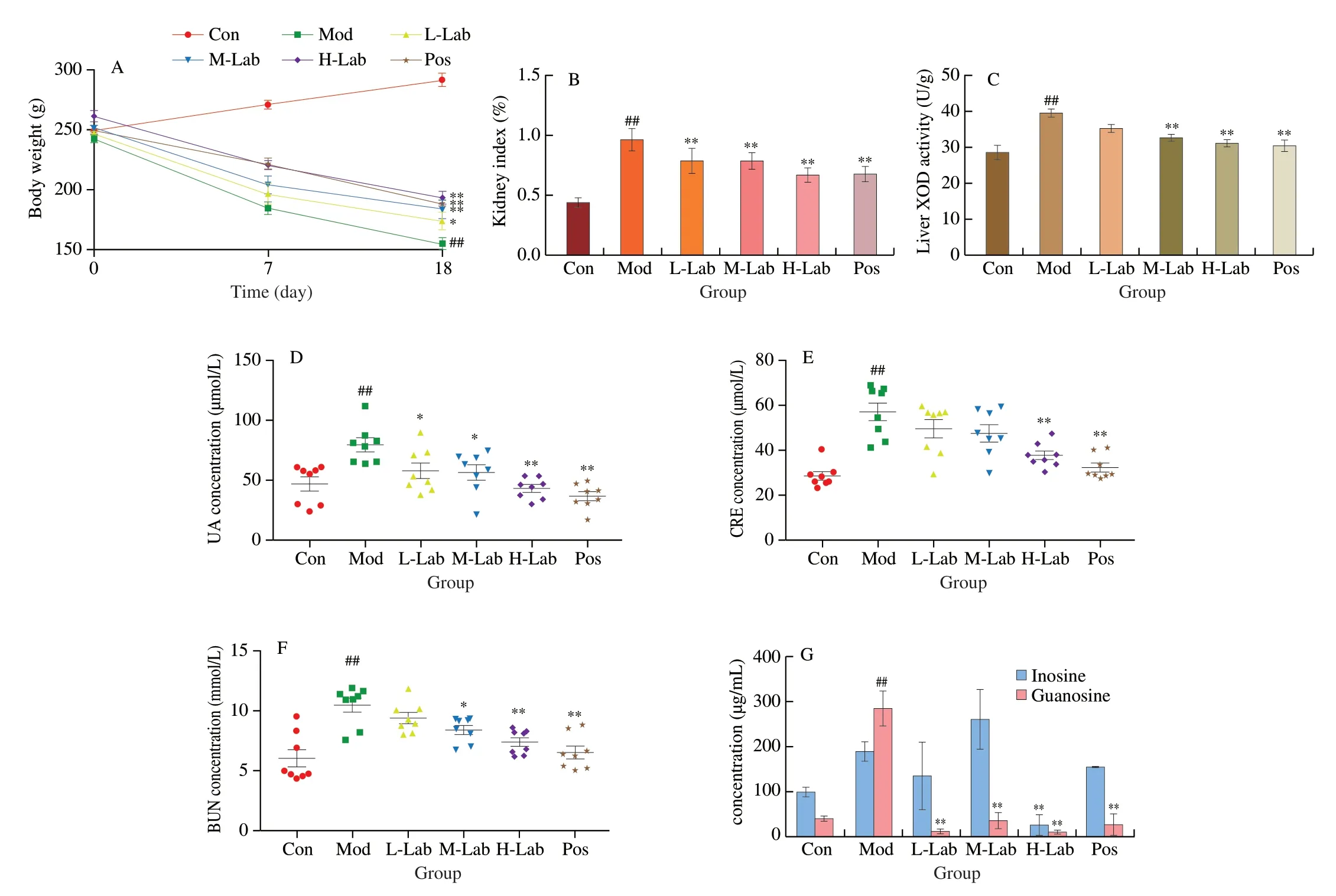
Fig.3 (A) Body weights of rats with different treatments.(B) Rat renal organ index.(C) Liver XOD activity of rats in different treatment groups.(D-F) Indicators of serum UA,CRE,and BUN in each group of rats on day 14.(G) The content of inosine and guanosine in the intestinal contents.The data are shown as the mean ± SD,n=8 per group.*P < 0.05,**P < 0.01 vs.model group.##P < 0.01 vs.control group.
3.4 Concentration of inosine and guanosine in rat intestinal contents
The efficiency ofL.brevisPDD-5 in absorbing and degrading inosine and guanosine in the intestinal of the rats was also investigated.As shown in Fig.3G,the overall decrease of inosine and guanosine were also observed in rats at the presence of low and high dose ofL.brevisPDD-5,whereas a higher level of inosine than that of Mod and Pos was observed in M-Lab ofL.brevisPDD-5.The above results indicated that a higher level ofL.brevisPDD-5(> 10 (lg CFU/mL)) had a better effect on the absorption of inosine/guanosine.
3.5 Effect of L.brevis PDD-5 on the kidney of hyperuricemia rats
MDA is the product of an oxygen radical chain reaction,which indirectly responds to the degree of the cell damage caused by oxygen radicals.SOD and GSH-Px are important antioxidant enzymes in the body,which can block oxygen radicals from causing damage to cells and protect the body from oxidative stress damage.After H-Lab intervention,renal SOD levels were significantly increased and MDA levels were significantly decreased,while GSH-Px showed no significant difference (Figs.4A-C).MDA was positively correlated with inflammatory factors TNF-α,IL-1β and IL-6,and SOD was negatively correlated with inflammatory factors TNF-α,IL-1β and IL-6 (Figs.4D-F).
In addition,the right kidney of rats was shown in Fig.5A,a slightly lighter color combined with uneven texture patches or dense white granular spots were observed in the model group,which was different from that of the control.Relative to the model group,a gradual smooth and even morphology of kidney tissue was exhibited in the increasing dose ofL.brevisPDD-5,thus showing a better morphology than that of the allopurinol group.For further observation of the internal organization of the kidney,the histopathology of kidney was photographed after hematoxylin-eosin sectioning(Fig.5B).Severe atrophy and fibrosis were observed in Model group in comparison with control.The intervention ofL.brevisPDD-5 effectively improved the histopathology of the kidneys.The least amount of dilated tubules and urate crystals were observed in H-Lab which was almost equivalent to that of purinergic groups,suggesting that a dose-response relationship was also found between kidney histopathology andL.brevisPDD-5.In addition,analysis of renal tissue scores showed that the high-dose group ofL.brevisPDD-5 had the highest mitigating effect on kidney injury among all groups ofL.brevisPDD-5 (P< 0.05,Fig.5C),which was in line with the result of TNF-α,IL-1β and IL-6 (P< 0.01,Figs.4D-F).
3.6 Effects of L.brevis PDD-5 on the expression of uraterelated transporter proteins in the kidney of hyperuricemia rats
The uric acid transport proteins URAT1,GLUT9,and OAT1 were associated with uric acid excretion in the kidney.URAT1andGLUT9mRNA levels were significantly reduced andOAT1mRNA levels were significantly increased after medium to M-Lab and H-Lab intervention (P< 0.05,Figs.5D-F).
3.7 Protein expression of NLRP3 inflammatory pathway in rat kidney
As shown in Fig.6,the Western blot results showed that the protein expression of NLRP3,ASC,and Caspase-l was significantly increased in the kidney tissues of the model rats compared with the control group (P< 0.01).These indicated that urate nephropathy correlated with increased expression of related proteins in NLRP3 inflammatory body signaling.There was a significant effect of either low,medium,or high dose of lactic acid bacteria intervention compared to the model group (P< 0.01) and a gradual decrease level was monitored with the increasing dose ofL.brevisPDD-5.These results indicated thatL.brevisPDD-5 intake reduced the stimulation of the NLRP3 signaling pathway and decreased the expression of downstream IL-1β.

Fig.4 Effect of L.brevis PDD-5 on inflammation and oxidative stress induced by high uric acid.The levels of (A) MDA,(B) GSH-Px,and (C) SOD in rat kidney tissues.The concentrations of (D) TNF-α,(E) IL-1β,and (F) IL-6 in different treated groups.*P < 0.05,**P < 0.01 vs.model group.#P < 0.05,##P < 0.01 vs.control group.

Fig.5 Effect of L.brevis PDD-5 on renal tissue in hyperuricemia rats.(A) Intact right kidney of rats with different treatments.(B) Histological morphology of rat kidneys stained using H&E from all groups.(C) Histopathological scores of the kidneys in each group.Effect of L.brevis PDD-5 on mRNA expression of(D) URAT1,(E) GLUT9,and (F) OAT1 in renal tissues of hyperuricemia rats.*P < 0.05,**P < 0.01 vs.model group.##P < 0.01 vs.control group.
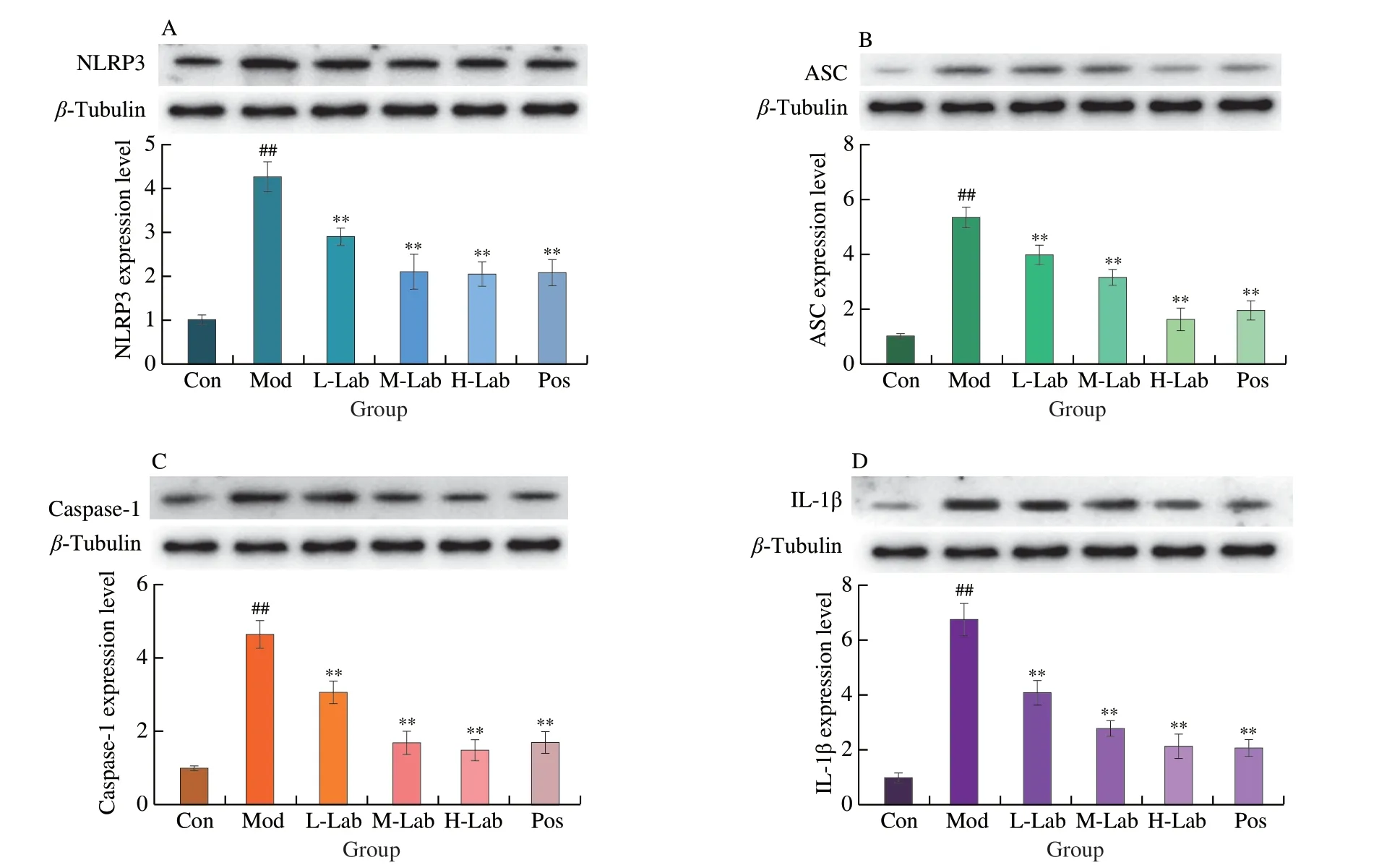
Fig.6 Effect of L.brevis PDD-5 intake on the levels of NLRP3 pathway-related protein in renal tissues.Western blot analysis for (A) NLRP3,(B) ASC,(C) caspase-1,and (D) IL-1β.Data are expressed as means ± SD (n=8).**P < 0.01 vs.model group.##P < 0.01 vs.control group.
4.Discussion
Increasing evidence suggests that hyperuricemia is a major risk factor for many diseases.Increased serum uric acid levels will trigger hyperuricemia,which is associated with increased uric acid synthesis and impaired uric acid excretion[23].The precursor substance of uric acid is purine,which exists in the human body mainly in the form of purine nucleosides.Purine nucleosides are readily absorbed by the intestine leading to elevated uric acid[24].Therefore,reduction of purine food intake is the source of managing uric acid and improvement of renal excretion is the treatment for controlling uric acid.However,there are several negative effects associated with the medications used to treat hyperuricemia at the moment.Therefore,it is necessary to find secure means of treatment.LAB,which are used to treat diabetes and hypercholesterolemia,have been shown in studies to colonize the human gastrointestinal and reproductive systems as well as lower cholesterol,lower glucose,and improve host microecological balance[25].Finding a microecological therapy that can treat hyperuricemia and shield the liver and kidneys from harm has thus become a hot research subject[26].The purine nucleosidedegradingL.brevisPDD-5 strain was examined for its probiotic qualities,which may be related to itsinvivofunction,as well as itsinvitrotherapeutic potential.Additionally,we investigated the mechanisms through whichL.brevisPDD-5 ameliorates hyperuricemia and uric acid nephropathy,as well as the antihyperuricemic action ofL.brevisPDD-5invivoand its adjuvant effect on hyperuricemia-induced uric acid nephropathy in a model of hyperuricemia.
Since adenine and guanine are the major nucleic acids found in food such as vegetables,which are little responsible for hyperuricemia.In contrast,the nucleic acid of meat products,fish,and shrimp is mainly composed of inosine,and the nucleic acid of beer is mainly composed of guanosine[10,27].Moreover,purines are not as easily absorbed by the gut as nucleosides.Thus,inosine and guanosine were selected as absorption targets of LAB in this study.Surprisingly,L.brevisPDD-5 screened from salty vegetables,in which inosine absorption capacity of (68.86 ± 15.46)% and guanosine absorption capacity of (95.75 ± 3.30)% within 1 h,exhibited a survivability against the gastric and intestinal fluid.L.brevisPDD-5 showed superior uptake in terms of the rate of purine nucleosides compared to other probiotics.2 h treatment was required for purine nucleoside degradation byLacticaseibacillusparacaseifound by Lee et al.[28].In anticipation of the positive physiological function of probiotics,Nguyen et al.[29]used protectant-embeddedL.plantarumto increase the survival ofLactobacillusto 28.7% and 14.0% byinvitrosimulated gastrointestinal fluid experiments,compared to 86.84% and 77.45% survival of our screenedL.brevisPDD-5 in artificial intestinal and gastric fluids.In addition,the hydrophobicity ofL.brevisPDD-5 was greater than 50%,which is similar to the hydrophobicity ofL.plantarumscreened as a fermentative agent by Jung et al.[30].Hydrophobicity contributes to the adhesion of LAB to the intestinal mucosa[31].
Based on this,we validated the remission treatment in rats with hyperuricemia using this strain.Since rats possess uric acid oxidase,we chose to intraperitoneally inject potassium oxazinate to inhibit uric acid oxidase and feed them a high purine diet.Successful establishment of the hyperuricemia model was indicated when the blood uric acid level in the model group was significantly increased compared with the control group.We should know that majority of purines in food were oxidized by XOD to uric acid,which is rarely used by the body.Uric acid is partially excreted by urine and partially absorbed by the body,Unhealthy people are unable to balance the production and excretion of uric acid,so uric acid accumulates and leads to hyperuricemia[32].When rats were treated withL.brevisPDD-5,serum levels of UA,CREA,and BUN were significantly reduced and XOD levels were effectively inhibited.When treated withL.brevisPDD-5,serum levels of UA,CREA,and BUN were significantly reduced and XOD levels were effectively inhibited in rats,which is consistent with the findings of Cao et al.[33].The levels of inosine and guanosine in the intestinal contents after the removal of the organisms were statistically significant in the high-dose group,which was consistent with the results of serum uric acid levels.Certainly,as we expected,there was a decrease in intestinal inosine and guanosine levels with LAB intervention,which may be due to the role of LAB absorbed purine analogs in the intestine.In the intestine,whereL.brevisPDD-5 is present,on the one hand,it probably absorbs degraded purine nucleosides through its cell surface proteins and polysaccharides adhering to the intestinal membrane[34].On the other hand,L.brevisPDD-5 did not colonize the intestine directly,but competed with intestinal epithelial cells in the intestine to absorb purine analogs leading to lower uric acid levels.Whereas the mechanism of uric acid reduction by allopurinol is to inhibit the production of XOD,thereby reducing the degradation of purines to uric acid and preserving purine-like substances[35].Theoretically,the positive control model group should have higher inosine and guanosine contents than the blank control group,but the results of this paper showed the opposite (Fig.3G).Therefore,we hypothesize that the treatment with allopurinol leaves the animal in a relatively healthy state,where the intestinal barrier is not damaged,inflammation and oxidative stress in the organism are eliminated,and purines enter metabolism and are reused,leaving the purines in the body in a balanced state.
On the other hand,uric acid excretion is dependent on uric acid transporter-related proteins in the parietal or basolateral membrane of the proximal tubular epithelium of the kidney,whose function is to regulate uric acid secretion in the blood and filtrate.The uric acid reabsorption transport proteins URAT1 and GLUT9 are responsible for regulating the reabsorption of uric acid and thus controlling the amount of uric acid returned to the blood.The uric acid excretion transporter OAT1 regulates uric acid excretion by secreting uric acid into the urethra.The balance of uric acid secretion and reabsorption is a key factor in the regulation of serum uric acid concentration.Although there are natural extracts such as resveratrol[36]and green tea polyphenols[37]that are effective in modulating uric acid-related transporters to inhibit uric acid reabsorption,there are few studies on the effect of probiotics on uric acid transporter proteins.The results of this study showed that the use of LAB intervention downregulated the expression of the reabsorption proteins GLUT9 and URAT1 and also upregulated the uric acid excretion protein OAT1 (Figs.5D-F).
In our study,the organ index was significantly elevated in the model group rats,suggesting that the kidneys of the rats may be edematous or hyperplastic and may even cause complications.In addition,brownish-black monosodium urate (MSU) was clearly observable in the kidney tissue sections.This was due to inflammation caused by sodium urate deposition in the kidney tissue as a result of hyperuricemia.In the early stages of the inflammatory response,several inflammatory factors,TNF-α,IL-1β,and IL-6,are secreted[38].In our study,L.brevisPDD-5 could modulate the inflammatory response by decreasing the levels of pro-inflammatory factors(TNF-α,IL-1β,and IL-6) consistent with the literature reporting that Lactobacillus can reduce the secretion of inflammatory cytokines[39].Wu et al.[40]found thatLimosilactobacillus fermentumJL-3 inhibited UA-induced changes in the intestinal flora and reduced the ratio of Firmicutes to Bacteroidetes (F/B ratio) and decreased UA levels.Cao et al.[33]found thatLactobacillus paracaseiX11 restored intestinal floraα-diversity and reduced hyperuricemia.Some studies have reported a direct correlation between the expression of inflammatory factors and changes in the intestinal flora[41].Therefore,the present study hypothesized thatL.brevisPDD-5 may reduce the level of pro-inflammatory factors by affecting the intestinal flora of rats.In many patients,purines are oxidized to UA in the liver by XOD with the production of superoxide anions,and the excessive production of superoxide anions can lead to oxidative stress and increased disease risk of hyperuricemia[42].Uric acid salts exacerbate oxidative stress by releasing free radicals and inactivating innate antioxidant enzymes,causing an inflammatory response.This suggests a key role of antioxidant and anti-inflammatory effects in the regulation of hyperuricemia[43].Fig.3C showed that M-Lab and H-Lab could effectively reduce hepatic XOD activity.MDA,GSH-Px,and SOD can be used as oxidative stress markers.MDA indirectly reacts to the damage of oxygen free radicals on cells,and SOD is an important antioxidant substance in the body,which can improve the activity of antioxidant enzymes in the body,scavenging the oxygen free radicals generated by metabolism in the body and protect the body from oxidative stress damage[44].GSH-Px is a peroxidolytic enzyme that reacts mainly to the body’s selenium levels and is able to reduce toxic peroxides to non-toxic hydroxyl compounds[45].Figs.4A-C demonstrated that H-Lab can reduced MDA levels and increased SOD levels,but there was no significant difference on GSH-Px(P> 0.05).This may be because SOD is the first line of defense against free radicals.GSH-Px can only inhibit the production of free radicals and cannot scavenge them directly.Fig.4 indicated that H-Lab can effectively alleviate the inflammatory response induced by oxidative stress.
What’s more,hyperuricemia led to the deposition of urate in the kidney tissues,which caused kidney tissue lesions.Since the NLRP3 inflammatory signaling pathway was associated with hyperuricemia complications,we explored the anti-inflammatory effects ofL.brevisPDD-5 using an MSU-induced rat model of uric acid nephropathy.MSU promoted the activation of the NLRP3 inflammasome,subsequently leading to the release of IL-1β[46].In our study,the expression of pro-inflammatory proteins (NLRP3,caspase-1,IL-1β,and IL-6)invivowas consistent with that reported in the literature[47].We found thatL.brevisPDD-5 induced the downregulation of the expression of NLRP3 inflammasome-related proteins as well as those of the downstream factor caspase-1 and the inflammatory factor IL-1β and IL-6invivo(Fig.4).Notably,L.brevisPDD-5 downregulated the expression levels of ASC,which is NLRP3 inflammasomerelated proteins.These observations suggested that the regulatory effects ofL.brevisPDD-5 on hyperuricemia are related to its ability to inhibit the NLRP3 signaling pathway.As expected,analysis of tissue morphology further showed thatL.brevisPDD-5 can protect against renal damage,at least partially (Fig.5B).Combined,these results demonstrated that the attenuating effects ofL.brevisPDD-5 on MSU-induced renal injury are likely mediated through inhibiting the NLRP3 pathway.
Of note,the effect of high-doseL.brevisPDD-5 intervention was comparable to the therapeutic effect of allopurinol.This result may be due to the fact allopurinol has been linked to a very distinctive form of acute liver injury[48-49],whereas high doses ofL.brevisPDD-5 had no adverse drug reactions.This study predicts thatL.brevisPDD-5 colonizes the intestinal and competes with related protein receptors in the intestine for the uptake of purine nucleosides,achieving uptake and degradation of purine nucleosides in the hepaticintestinal circulation,thereby reducing blood uric acid levels in rats,regulating uric acid transport proteins in the kidney,improving renal excretion and alleviating hyperuricemia.In addition,the production of oxygen free radicals and the release of inflammatory factors,such as TNF-α,IL-1β,and IL-6,can lead to cellular damage.It has been suggested that inhibition of oxidative stress-induced inflammation can effectively improve hyperuricemia[43].In this study,it was shown thatL.brevisPDD-5 intake effectively alleviated inflammation and oxidative stress induced by uric acid,thereby reducing MSU-stimulated NLRP3-ASC interactions,and decreasing caspase-1 protein expression.In addition,L.brevisPDD-5 can reduce the level of IL-1β expression and alleviate hyperuricemia-induced uric acid nephropathy by inhibiting NLRP3 inflammation.These provide new means and new ideas of adjuvant therapy for the microecological treatment of hyperuricemia and gout diseases.
5.Conclusion
Collectively,our findings identified a novel strain ofL.brevisPDD-5 from salty vegetables that not only absorbed and degraded inosine and guanosineinvitrobut also alleviated hyperuricemiain vivo.This strain attenuated oxidative stress and anti-inflammatory propertiesinvivoand ameliorated hyperuricemia and uric acid nephropathy in a dose-dependent manner.Furthermore,we hypothesized thatL.brevisPDD-5 improves renal uric acid metabolism and reduces hyperuricemia-induced renal injury via the NLRP3 pathway.These findings highlight thatL.brevisPDD-5 could be used as a potential probiotic strain to improve hyperuricemia.
Conflict of interest
There are no conflict of interest among all authors.
Acknowledgements
The authors thank the National Natural Science Foundation of China (31 972048;32272339) and the National Key R &D Program of China (2021YFD2100104) for financial support.
Appendix A.Supplementary data
Supplementary data associated with this article can be found,in the online version,at http://doi.org/10.26599/FSHW.2022.9250077.
- 食品科学与人类健康(英文)的其它文章
- Protective effects of oleic acid and polyphenols in extra virgin olive oil on cardiovascular diseases
- Diet and physical activity inf luence the composition of gut microbiota,benef it on Alzheimer’s disease
- Inf luence of nitrogen status on fermentation performances ofnon-Saccharomyces yeasts: a review
- Ganoderma lucidum: a comprehensive review of phytochemistry,eff icacy,safety and clinical study
- Resveratrol combats chronic diseases through enhancing mitochondrial quality
- Demonstration of safety characteristics and effects on gut microbiota of Lactobacillus gasseri HMV18

