Biochemistry and transcriptome analysis reveal condensed tannins alleviate liver injury induced by regulating cholesterol metabolism pathway
Xingxin Li,Yijing Pu,Bngdi Liu,Xioming Fng,Wenjun Peng,Weibo Jing,Wenli Tin,
a Institute of Apicultural Research, Chinese Academy of Agricultural Sciences, Beijing 100093, China
b College of Food Science and Nutritional Engineering, China Agricultural University, Beijing 100083, China
c Academy of Agricultural Planning and Engineering, Ministry of Agriculture and Rural Affairs, Beijing 100125, China
Keywords: Condensed tannins High cholesterol Liver injury Antioxidants Transcriptomic analysis
ABSTRACT Free cholesterol has been considered to be a critical risk factor of nonalcoholic fatty liver disease (NAFLD).It remains unknown whether dietary intake of condensed tannins (CTs) have distinguishable effects to alleviate liver damage caused by a high cholesterol diet.Male C57BL/6 mice were fed a high cholesterol diet for 6 weeks,and given CTs treatment at a dosage of 200 mg/(kg·day) at the same time.The results indicated that compared with mice fed a normal diet,a high cholesterol diet group resulted in significant weight loss,dysregulation of lipid metabolism in blood and liver,and oxidative stress in the liver,but CTs treatment dramatically reversed these negative effects.Hematoxylin and eosin (H&E) staining and frozen section observation manifested that CTs treatment could effectively reduce the deposition of liver cholesterol and tissue necrosis caused by high cholesterol intake.CTs alleviated liver injury mainly by regulating the expression of related genes in cholesterol metabolism pathway and AMPK phosphorylation.Our results conf irmed that CTs have remarkable cholesterol lowering and anti-liver injury effects in vivo.1 The authors contribute equally to this article.*Corresponding authors.
1.Introduction
Non-alcoholic fatty liver disease (NAFLD) is the most common chronic liver disease with a prevalence of 20%-30% in the population[1].It may cause a range of liver injuries,including simple steatohepatitis,non-alcoholic steatohepatitis (NASH),associated cirrhosis,and hepatocellular carcinoma in severe cases[2].In addition,it is a multi-system disease that increases the risk of type 2 diabetes mellitus (T2DM),cardiovascular disease (CVD),heart disease,and chronic kidney disease (CKD)[3].Current studies have shown that the excessive fatty acid intake can lead to metabolism-related fatty liver diseases.Increasing evidence suggests that accumulation of free cholesterol in liver cells induces to liver injury and the formation of non-alcoholic steatohepatitis[4].Another study have found that excessive intake of cholesterol led to a serious accumulation of cholesterol in liver cells and caused to the formation of cholesterol crystals,which further promoted pathological changes in liver cells and ultimately apoptosis[5].The prevalence of NAFLD is increasing worldwide,but specif ic treatment and effective prevention methods are still lacking at present.Although previous studies have proved that lipid-lowering drugs (such as statins) can relieve liver damage caused by excessive intake of cholesterol by reducing the accumulation of liver lipids,the side effects of statins cannot be ignored[6].Therefore,there is an urgent need for safe and effective approaches to prevent liver damage caused from a high cholesterol diet.
Natural plant functional foods are considered as a potential material to prevent chronic metabolic diseases such as hyperlipidemia and fatty liver due to their benef icial biological activities and health benef its[7].Dietary plant polyphenols are the most abundant source in natural products.Previous studies have shown that plant polyphenols can influence the absorption and metabolism of fatty acids and cholesterol by regulating fatty acid metabolism,fatty acid oxidation,sterol synthesis,sterol metabolism,adenosine 5’-monophosphate(AMP)-activated protein kinase (AMPK) and peroxisome proliferator-activated receptor (PPAR) in liver of mice fed high fat diet[8-11].Condensed tannins is a polyphenolic substance formed by condensation of flavane-3-alcohol structural units through 4-8 or 4-6 C-C bonds or C=O bonds linked to C bonds[12].CTs are believed to have many health benefits,such as antioxidant,anti-inflammatory,antibacterial,anti-allergy,heart and nerve protection,anti-obesity and other functions[13].The most interesting thing is that in our previous studies,it was found that CTs could not only bind cholesterol and bile saltsinvitro[14],but also react with cholesterol esterase[15],which indicated that CTs had potential cholesterol-lowering effects.However,there are few reports on the effect of CTs on hepatic cholesterol metabolism pathway in mice fed with high cholesterol diet.Therefore,we need to further verify the cholesterol-lowering effect and explore the regulating mechanism of CTsinvivo.
Herein,we investigated the effect of CTs on lipid level,liver function,oxidative stress level and liver tissue damage in mice with excessive intake of cholesterol,so as to determine the potential anti-liver injury effect of CTs.In addition,RNA-seq technology was utilized to analyze the differentially expressed genes (DEGs) in liver of different treatment groups.Our study explored the regulatory effect of CTs on liver cholesterol in mice with high cholesterol intake at the transcriptional level,providing theoretical basis for further exploration of CTs as natural functional active substances to reduce cholesterol.
2.Materials and methods
2.1 Materials and reagent
Condensed tannins (CTs) were extracted from mature-green bananas (Musaspp.,cv.“Brazil”) bought at a fruit market in Beijing,China.CTs were obtained according to our laboratory methods[16].It was a trimer consisted of one epigallocatechin (EGC) and two epicatechins (EC),with a molecular weight of 877.06 Da.Its proposed structure as shown in Fig.S1.Cholesterol and cholic acid were purchased from Sinopharm Chemical Reagent Co.,Ltd.(China).Total cholesterol (TC) kits,triglyceride (TG) kits,high density lipoprotein cholesterol (HDL-C) kits,low density lipoprotein cholesterol (LDL-C)kits,total bile acid (TBA) kits,total bilirubin (TBil) kits,alanine aminotransferase (ALT) kits,aspartate aminotransferase (AST) kits,alkaline phosphatase (ALP) kits,glutathione S transferase (GST) kits,total antioxidant capacity (T-AOC) kits,total superoxide dismutase(T-SOD) kits,glutathione (GSH) kits,glutathione peroxidase (GSHPx) kits,catalase (CAT) kits,malondialdehyde (MDA) kits were obtained from Nanjing Jiancheng Biotechnology Company (China).Hematoxylin and eosin (H&E) staining kit was acquired from Beijing Solarbio Science and Technology Co.,Ltd.(China).Other reagents and solvents were all of the analytical grades.
2.2 Animals and experimental treatments
All experiments were carried out in accordance with the guidelines of the Ethics Committee for Experimental Animal Welfare of the Chinese Society for Experimental Animals and approved by the Ethics Committee for Experimental Animals.Six-week-old C57BL/6 male mice (18-20 g,SPF) were selected and purchased from Beijing Vital River Laboratory Animal Technology Co.,Ltd.(China).The relative humidity was (60 ± 5)%,the temperature was (23 ± 2) °C,and the light and darkness were controlled for 12 h.The mice were free to drink and eat during the experiment.After 1 week of adaptive culture,mice were randomly divided into 3 groups(n=12): (1) normal group: normal water and normal feed (NG);(2) model group: normal water and high cholesterol diet (HCG);(3) treatment group: normal water,high cholesterol diet and 200 mg/(kg·day) CTs by intragastric administration during the experiment(HCG+CTs).According to the method of Matsuzawa et al.[17],the high-cholesterol feed was mainly composed of 90.75% normal feed,7.5% oil,1.25% cholesterol and 0.5% cholic acid.The mice were weighed and recorded weekly for 6 weeks.At the end of 6 weeks,all mice were fasted overnight and anesthetized with ether,and sacrificed according to animal ethics.Blood and feces samples were collected for biochemical analysis.The liver tissue samples were separated and carefully washed with ultrapure water that removed RNase,and the excess water was sucked out with filter paper.The liver was weighed and the liver index was calculated.Liver index was calculated as liver index (%)=liver weight/body weight × 100.Liver tissue was either fixed in 4% paraformaldehyde or immediately frozen in liquid nitrogen and stored at -80 °C until analysis.
2.3 Lipid analysis of blood,liver and feces
Blood,liver and feces samples were collected and processed.The blood samples were centrifuged at 1 500 ×gfor 15 min,and the supernatant was taken and placed in a refrigerator at 4 °C for subsequent analysis.The liver tissue (about 0.5 g) was homogenized with frozen normal saline at a volume ratio of 1:9 in a mortar,and then centrifuged at 3 000 ×gfor 10 min to obtain the supernatant for analysis.The feces samples were homogenized at 0.5 g/mL and centrifuged at 3 500 ×gfor 10 min to obtain the supernatant for subsequent lipid analysis.According to the methods on the kits,the UV-160A spectrophotometer (Shimadzu,Kyoto,Japan) was utilized to analyze the levels of lipid-related indicators in serum,liver and feces,including TC,TG,HDL-C,LDL-C,TBA,TBil.
2.4 Analysis of liver function and oxidative stress level
The liver samples were treated according to section 2.3.The levels of ALT,AST,ALP,GST,T-AOC,T-SOD,GSH,GSH-PX,CAT and MDA in liver samples were analyzed by UV-160A spectrophotometer(Shimadzu,Kyoto,Japan) according to the corresponding kits methods.
2.5 Histological analysis
Partial tissue was taken from the left lobe of mouse liver and fixed in 4% paraformaldehyde for histopathological analysis.The fixed tissue was dehydrated by a series of graded ethanol and embedded in paraffin.The paraffin was thinly sliced (6 μm)and stained with H&E.Olympus light microscope was used for observation and photography[18].Liver tissue was fixed in a 10%optimal cutting temperature (OCT) compound solution and placed in a -80 °C refrigerator for forming for subsequent frozen sections.The crystal structure of cholesterol was observed using a polarizing microscope[19].
2.6 Transcriptome analysis
Total liver RNA was extracted using TRIzol reagent (Invitrogen,Carlsbad,CA) according to manufacturer’s instructions.RNA concentration and OD260nm/OD280nmvalue were measured by Nanodrop 2000C microspectrophotometer.RNA integrity was determined using BioAnalyzer (Agilent Technologies,Inc.,Santa Clara,CA).The total RNA 28S/18S of the samples was greater than 0.9,and the mean RNA integrity number (RIN) was greater than or equal to 6.5.CDNA library was constructed using TruSeq Stranded Total RNA Sample Prep Kit (Illumina,Inc.,San Diego,CA).The library was sequenced using Illumina platform.Raw data in FASTQ format was processed using STAR (V2.5.2 B)[20]and HTseq[21].HTSeq software was utilized to analyze the gene expression level of the samples,and the model was union.DESeq (V1.10.1)[22]was used for DEGs analysis.Finally,GOseq (V1.22) software was used for Gene Ontology (GO)enrichment analysis and Kyoto Encyclopedia of Genes and Genomes(KEGG) enrichment analysis (http://www.kegg.jp)[23].
2.7 Statistical analysis
Three replicates were set for all experiments,and the final results were expressed as mean ± SD.The statistical analyses were performed using IBM SPSS statistical software Version 19 (SPSS Inc.,Chicago,IL,USA).Significance analyses were conducted by one-way variance(ANOVA) and the Duncan test at a 95% (P< 0.05) significance level.
3.Results
3.1 Effects of CTs on body weight and liver index of mice with high cholesterol diet
The experimental design was shown in Fig.1A.C57BL/6 male mice were randomly divided into 3 groups after 1 week of adaptation: NG,HCG,and HCG+CTs.The CTs dose was 200 mg/(kg·day).Blood,liver and feces samples were collected for analysis after 6 weeks of feeding.The effects of CTs on body weight and liver index in mice with liver injury induced by a high cholesterol diet were shown in Fig.1.Our results suggested that the body weight of mice with high cholesterol intake was significantly lower than that of mice with normal diet (Fig.1B),which was consistent with the experimental results of Duan et al.[24],indicating that excessive cholesterol intake would damage the liver of mice and weaken its ability to absorb and metabolize nutrients (Fig.1C).Meanwhile,CTs treatment mitigated the effects of high cholesterol intake on body weight in mice (Fig.1D).Liver index can indirectly reflect the health status of liver and the growth of mice.As could be seen in Fig.1E,the liver index of mice in the HCG was markedly higher than that in the NG and the HCG+CTs (P< 0.05).The results manifested that high cholesterol intake would lead to the increase of lipid components in the liver of mice,which might cause liver damage.The mice with CTs treatment observably reduced the increase of liver index caused by a high cholesterol diet.
3.2 Effects of CTs on lipid content in blood,liver and feces of mice with high cholesterol diet
Dyslipidemia is a critical risk factor for NAFLD,mainly associated with lipid overconsumption[25].The effects of CTs on lipid in blood,liver and feces of mice fed with high cholesterol diet were shown in Table 1.The content of total cholesterol and triglyceride can reflect the level of lipid metabolism in animals.Our results displayed that CTs treatment dramatically reduced the elevation of TC and TG in blood and liver of mice induced by high cholesterol intake.HDL and LDL are related to cholesterol synthesis and degradation in the body.Previous studies have reported that the content of HDL in blood was negatively correlated with chronic diseases caused by lipid metabolism disorders,while LDL was positively[26].We observed that the high cholesterol diet resulted in a significant decrease in HDL-C content in serum and liver of mice,and a significant increase in LDL-C content.CTs treatment effectively alleviated this trend.Blood bile acid and bilirubin levels can be used to determine liver damage.When the liver is damaged,blood levels of bile acids and bilirubin rise.The results indicated that CTs treatment memorably lowered the levels of TBA and TBil in serum of mice caused by excessive cholesterol intake.

Fig.1 (A) Experiment design and (B-E) effects of condensed tannins on body weight and liver index of liver injury mice induced by high cholesterol diet.Values with different letters are significantly different (P < 0.05) by Duncan’s test.
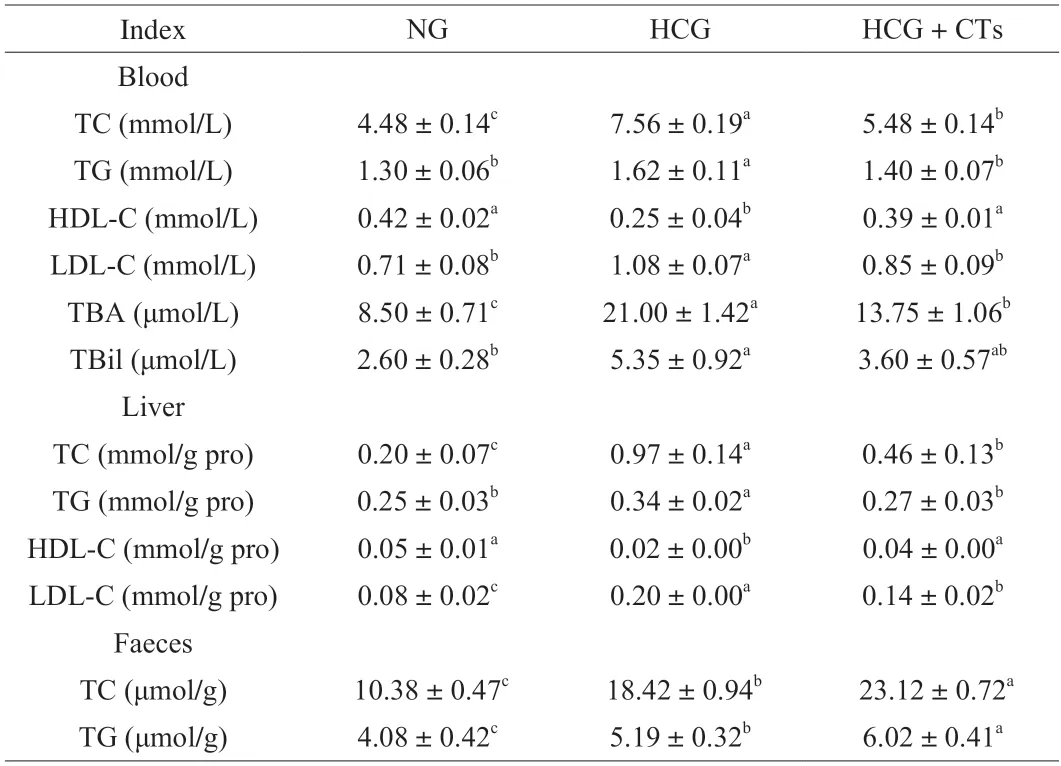
Table 1 Effect of condensed tannins on lipid levels in blood,liver and feces of mice.
The excretion of total cholesterol and triglyceride in feces of mice was analyzed,and the results exhibited that the contents of total cholesterol and triglyceride in feces of mice in normal group were the lowest,because excessive cholesterol was not added in normal diet.Compared with the HCG,the contents of total cholesterol and triglyceride in the feces of mice treated with CTs were signally increased by 25.51% and 15.99%,respectively,indicating that the intake of CTs could increase the excretion of cholesterol and triglyceride in mice.These results manifested that the intake of CTs could alleviate the lipid metabolism disorder induced by high cholesterol in mice.
3.3 Effects of CTs on liver function and histopathology in mice with high cholesterol diet
The effects of CTs on liver function of mice with liver injury induced by high cholesterol were shown in Figs.2A-D.The results demonstrated that the contents of ALT,AST,ALP and GST in plasma of mice in HCG were notably higher than those in NG,while the contents of ALT,AST,ALP and GST in HCG+CTs were remarkably decreased (P< 0.05),suggesting that CTs treatment could alleviate liver injury caused by high cholesterol diet in mice.To further investigate the effects of CTs on liver injury induced by excessive cholesterol intake,liver sections were observed by H&E staining,as shown in Fig.2E.No pathological abnormalities were found in liver sections of mice in the NG.Liver cells were radially arranged around the central vein,and liver cells were plump and neatly arranged,and hepatic sinuses were clearly visible.In addition,clear nuclei could be seen with relatively complete boundaries,and the size of nuclei was basically uniform and evenly distributed in the liver cells (Fig.2E1).Partial liver nuclei were irregularly shaped and densely distributed around the central vein.The liver cell volume was significantly enlarged and loosely arranged with more vacuoles,and a large number of cell necrosis could be observed in the HCG (Fig.2E2).After CTs treatment,the vacuoles of liver slices were strikingly reduced,and the structure of liver cells was roughly similar to that of the NG (Fig.2E3).Frozen sections of liver were observed with polarizing microscope,and it was found that densely distributed cholesterol crystals could be observed in liver sections of mice in HCG,while cholesterol content was significantly reduced after CTs treatment (Fig.2F).The above indicated that the intake of high cholesterol would lead to the destruction of the structural integrity of the liver tissue in mice,resulting in the deposition of cholesterol in liver cells and the necrosis of liver cells.CTs treatment could effectively reduce the deposition of liver cholesterol and tissue necrosis caused by high cholesterol intake.
3.4 Effects of CTs on antioxidant capacity of mice with high cholesterol diet
To evaluate the effects of CTs on oxidative stress induced by high cholesterol intake,total antioxidant capacity and the activities of several common antioxidant enzymes,including T-SOD,GSH,GSH-Px and CAT,were analyzed.The specific results were shown in Fig.3.Significantly decreased levels of T-AOS,GSH,GSH-Px and CAT were observed in HCG,while the level of T-SOD decreased but not remarkably (Figs.3A-E).Compared with HCG,the levels of T-AOS,GSH and GSH-Px in liver of mice were observably increased by 16.78%,51.90% and 11.75% in HCG+CTs.Meanwhile,the levels of CAT and T-SOD increased by 13.86% and 17.95%,respectively.The change of MDA content can reflect the degree of lipid peroxidation and body damage.High cholesterol intake led to a distinct increase in concentration of MDA in liver (Fig.3F),while CTs treatment obviously reduced MDA content,which was reduced by 10.25%compared with the HCG.These results revealed that CTs treatment could restore the level of antioxidant enzymes in the liver of mice,enhance the ability of scavenging free radicals,and protect cells from free radical damage such as O2-,thus alleviating oxidative stress and liver damage caused by cholesterol.
3.5 Effects of CTs on DEGs in liver of mice with high cholesterol diet
Transcriptome sequencing was performed on mouse livers using lllumina high-throughput sequencing platform.After removing the joint and low-quality reads,more than 6.6 Gb of data were obtained for each sample,and the average sequencing error rate of clean reads was controlled below 0.02%.The Q20 and Q30 values were both higher than 90%,and the GC content of each sample was about 48%.Based on the comparison results of C57BL/6 mouse genomes and the prediction of new transcripts,a total of 55 950 genes were detected(Table S1).Correlation analysis of FPKM values between biological duplications of each sample showed that the correlation coefficient(R2) of gene expression levels between biological duplications of NG,HCG and HCG+CTs were all greater than 0.98 (Fig.S2),indicating that the repeatability between biological samples was good and could be further analyzed.
Transcriptomic results exhibited that many genes were memorably changed after high cholesterol intake compared with NG (adjustedP-value < 0.05).The total number of DEGs was 1 967,of which 1 073 genes were up-regulated and 894 genes were down-regulated.Compared with the HCG,there were a total of 2 178 DEGs after CTs treatment,including 977 up-regulated genes and 1 201 down-regulated genes (Figs.4A-D).GO and KEGG analyses were performed for the total DEGs in each group.GO is a comprehensive database describing gene functions,which can be divided into three parts,namely biological process,cellular components and molecular functions[27].Functional classification and functional significance enrichment analysis were carried out by GO database,and the results represented that the number and the GO category of DEGs in liver of each group were obviously different.The 30 GO terms with the most significant enrichment were selected and shown in Fig.4.The results demonstrated that compared with NG,the gene expressions in biological processes,cell components and molecular functions in the liver of mice in the HCG were mainly up-regulated above,and the most significant differences in the up-regulation genes were found in metabolic process,intracellular process and protein binding process,respectively (Fig.4E).On the contrary,compared with the HCG,the gene expressions in biological processes,cell components and molecular functions in the liver of mice in the HCG+CTs were mainly down-regulated,and the most significant differences in the down-regulated genes occurred in metabolic processes,intracellular processes and protein binding processes,respectively (Fig.4F).
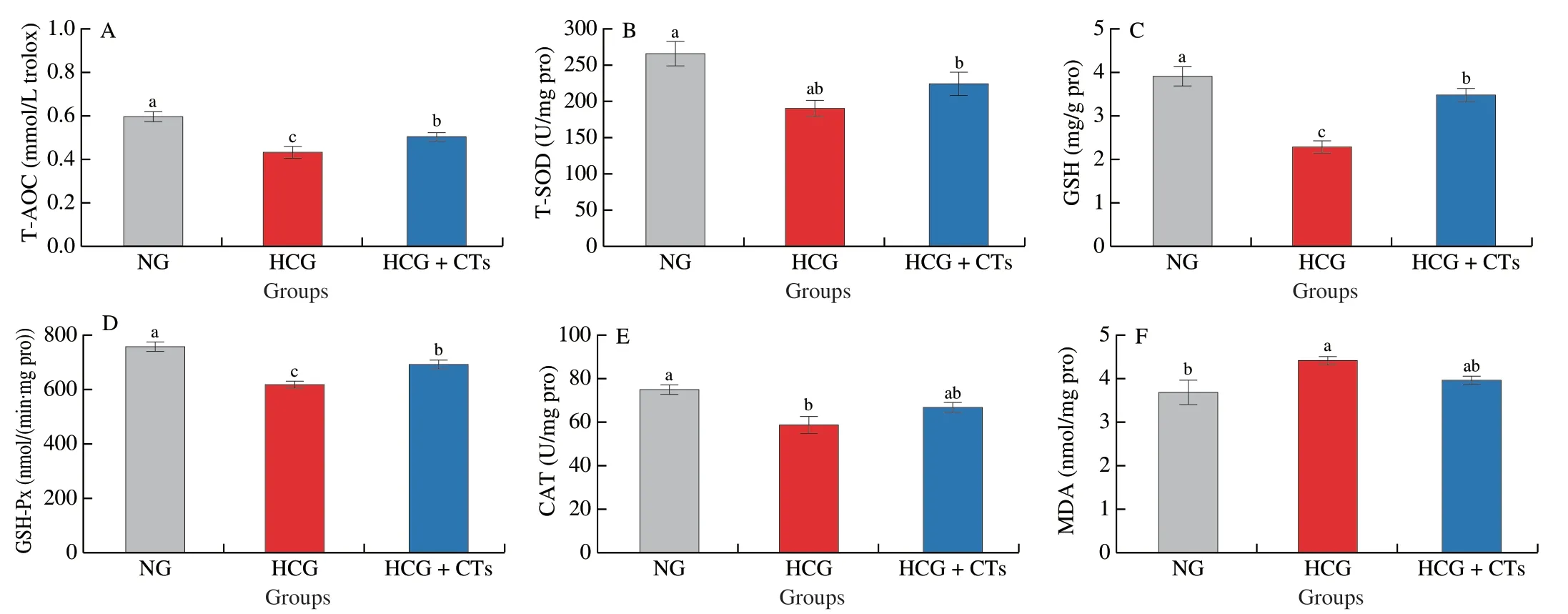
Fig.3 Effects of condensed tannins on the oxidative stress index of mice liver induced by high cholesterol diet.Values with different letters are significantly different (P < 0.05) by Duncan’s test.
To obtain more information on the prediction functions of the DEGs,KEGG pathway analysis was carried out (Table S2).The results suggested that compared with the NC,FoxO signaling pathway,renal cell carcinoma and insulin resistance were the main pathways enriched by up-regulated DEGs in HCG.The down-regulated DEGs enrichment pathways were mainly metabolic pathways and steroid biosynthesis.This indicated that high cholesterol intake could cause damage to different aspects of the mice.It was noteworthy that the pathways of up-regulated DEGs in the liver of mice in the HCG+CTs were mainly metabolic pathways,drug metabolism-other function,steroid biosynthesis and steroid hormone biosynthesis.There was no significant down-regulated DEGs enrichment pathway in HCG+CTs.The abovementioned results illustrated that CTs had a prominent intervention effect on the regulation of lipid anabolic pathway in mouse liver.
3.6 CTs alleviate liver injury in mice with high cholesterol by regulating cholesterol-related pathways
Cholesterol metabolism in animals was mainly divided into the following processes: 1) receptor mediated cholesterol transport process;2) synthesis of endogenous cholesterol;3) the conversion of cholesterol to cholate[28].The above processes were regulated by different genes at multiple levels (Fig.5).

Fig.4 DEGs (A-D) and GO analysis of DEGs (E-F) in liver of mice in different treatment groups.(A) HCG vs NG;(B) HCG+CTs vs HCG;(C) HCG+CTs vs NG.(2-4) Biological process;Cellular component;Molecular function.
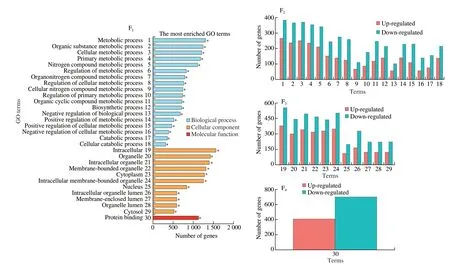
Fig.4 (Continued)
CTs inhibited the absorption of cholesterol in mice and promoted the reversal of cholesterol transport by regulating the gene expression of lecithin cholesterol acyltransferase (Lcat),low-density lipoprotein receptor (Ldlr),ATP binding cassette transporter G5 (Abcg5),ATP binding cassette transporter G8 (Abcg8),and ATP binding cassette transporter A1(Abca1) (Figs.5A,S3).Meanwhile,CTs reduced cholesterol biosynthesis by lowering the gene expression level of 3-hydroxy-3-methylglutaryl-CoA reductase (Hmgcr) (Figs.5B,S3).In addition,CTs promoted cholesterol conversion to cholate by upregulating gene expression of cholesterol 7α-hydroxylase (Cyp7a1)and sterol 12α-hydroxylase (Cyp8b1) (Figs.5C,S3),thus reducing cholesterol accumulation in the body.Moreover,CTs could advance AMPK phosphorylation by up-regulating the gene expression levels ofStrada,calcium/calmodulin-dependent protein kinase kinase 2(Camkk2),adrenoceptor alpha 1A (Adra1a),and adiponectin receptor 2 (Adipor2),while down-regulating the gene expression levels ofPpp2r5bandPpp2r5e(Fig.5D),thus promoting catabolism in cells and regulating cholesterol synthesis in the body.
4.Discussion
Existing studies have found that the main cause of liver cell damage in NAFLD model is lipid toxicity[29].NAFLD is marked by the accumulation of lipid droplets containing triglycerides in liver cells.However,the accumulation of triglycerides in liver cells alone does not induce inflammation,but certain types of lipids can damage liver cells,such as free fatty acid palmitic acid,cholesterol,lysophosphatidylcholine and ceramide[30].Cholesterol,as a common lipid toxic substance,has attracted more attention in recent years.Experimental studies and clinical evidence highlight the role of cholesterol,especially free cholesterol,in the pathogenesis of NASH[31],and demonstrate that dysregulation of cholesterol metabolism promotes the accumulation of free cholesterol,thereby affecting all liver cell populations and causing liver injury.Although lipid-lowering drugs (such as statins) can relieve liver damage caused by excessive intake of cholesterol by reducing the accumulation of liver lipids,the side effects of statins cannot be ignored[6].Therefore,there is an urgent need for safe and effective plant ingredients to prevent liver damage caused from a high cholesterol diet.
There are a lot of phenolic compounds in banana flesh and peel,such as gallic acid,catechin,epicatechin,tannins and anthocyanins,and these compounds have convergence in immature banana[32].Flavanols are the most abundant phenols in immature bananas,and they exist in various forms such as monomer,dimer and polymer.The polymer of flavan-3-ols is also named condensed tannin.Each 1 kg of banana fruit contains 3.952 g catechin equivalent condensed tannin,and the content of epigallocatechin is the highest in the monomer composition,which can reach 158 mg/100 g dry weight[33].CTs used in our study were mainly extracted from unripe bananas by ethanol,separated and purified by AB-8 macroporous resin,and characterized as trimer tannins[16].Some published studies have reported that tannins can promote the excretion of cholesterol in the small intestine,which may have beneficial effects on chronic diseases caused by excessive cholesterol intake[34].Our previous studies have shown that CTs bind to cholate and cholesterolinvitro,thereby reducing their absorption and promoting their excretion[14-15].The combination of CTs with these components may be one of the main mechanisms of cholesterol-lowering effect of polyphenols.
Thus,we investigated the effect of CTs on liver injury in mice fed with high cholesterol.The intake of CTs in bananas restored the lipid metabolism disorders in serum and liver caused by a high cholesterol diet to some extent.Meanwhile,CTs treatment reduced the levels of ALT and AST,and alleviated tissue damage and accumulation of cholesterol in liver,indicating that CTs had conspicuous anti-liver injury effect.
In addition,we found that CTs treatment significantly increased the reduction in GSH levels associated with a high cholesterol diet.In addition,cholesterol can also activate sterol regulatory element binding protein element 2 (SREBP-2) to upregulate LDL-R,thereby reducing the bioconversion of cholesterol to bile acids,which ultimately leads to apoptosis,inflammation and liver fibrosis[35].Notably,the underlying pro-inflammatory mechanism of cholesterol involves the accumulation of free cholesterol crystals in liver cells.Dietary excess cholesterol leads to a buildup of cholesterol in the liver,which forms cholesterol crystals that serve as damage associated molecular patterns (DAMPs) and promote the activation of the NOD-like receptor thermal protein domain associated protein 3(NLRP3) and other pro-inflammatory pathways[36].Some researchers also speculated that another reason for the damage of cholesterol crystals to liver cells might be the physical damage of the crystals themselves[5].Our observation of frozen section confirmed that CTs reduced the accumulation of cholesterol crystals in the liver to a certain extent,thus protecting the liver from damage.

Fig.6 Possible mechanism by which condensed tannins alleviate liver injury induced by high cholesterol diet.(1) Cholesterol synthesis pathway.BCTs inhibit the synthesis of cholesterol in liver by inhibiting the expression of HMGCR gene.(2) Bile acid metabolism pathway.BCTs promote cholesterol metabolism to bile acids by up-regulating the gene expression of CYP7A1 and CYP8B1,two key enzymes in the classical pathway of bile acid metabolism.(3) Reverse cholesterol transport pathway.BCTs promote the reversal process of cholesterol by up-regulating ABCA1,LCAT,CETP and LDL-R,and promote the excretion of free cholesterol from liver by up-regulating ABCG5/G8.(4) AMPK pathway.BCTs increase AMPK phosphorylation by up-regulating the expressions of AMPK upstream regulators (Strada,Camkk2,Adra1a and Adipor2),and reduce AMPK dephosphorylation by down-regulating the expressions of Ppp2r5b and Ppp2r5e,thereby activating AMPK pathway.It provides energy for the whole cholesterol synthesis and metabolism pathway.BCTs: Banana condensed tannins;HMGCR: 3-hydroxy-3-methylglutaryl-CoA Reductase;AMPK: adenosine 5’-monophosphate (AMP)-activated protein kinase;LDL-R: low-density lipoprotein receptor;LDL: low density lipoprotein;HDL: high density lipoprotein;HDL-C: high density lipoprotein-cholesterol;CETP: cholesterol ester transfer protein;LCAT: lecithin cholesterol acyltransferase;ABCA1: ATP binding cassette transporter A1;ABCG5: ATP binding cassette transporter G5;ABCG8: ATP binding cassette transporter G8.
In addition,our study confirmed that CTs have strong antioxidant capacity and can alleviate oxidative stress in the liver of mice caused by a high cholesterol diet.Oxidative stress and lipid peroxidation are considered to be the main factors causing the “second blow” of NAFLD[37].Excessive intake of oxidative components or imbalance of intracellular reactive oxygen species (ROS) will lead to excessive accumulation of ROS in cells and tissues,resulting in oxidative stress and cytotoxicity,and ultimately cells and tissues damage.ROS in the body mainly includes peroxide,superoxide,hydroxyl radical,singlet oxygen,α-oxygen and other oxygen-containing chemical reactive components[38].MDA is a vital marker of oxidative stress state and can reflect the degree of lipid peroxidation and body damage[39].After the intake of high cholesterol diet,MDA level in liver of mice increased visibly,resulting in oxidative stress,while CTs effectually inhibited oxidative stress.In addition,changes in the level of antioxidant enzymes are also important indicators in the process of oxidative stress.Common antioxidant enzymes include SOD,CAT and GSH-Px.SOD is the primary component of scavenging free radicals in the body,so that cells can avoid the damage of free radicals such as O2-.CAT,as a kind of oxidoreductase,can eliminate the toxicity of H2O2and phenolic amines.The physiological effects of GSH-Px can decompose the components of reduced GSH and hydrogen peroxide in the body,thus reducing the formation of lipid peroxides[40].Our study also discovered that CTs treatment could restore the level of antioxidant enzymes and play a crucial role in alleviating oxidative stress.
To determine which pathways were involved in the anti-liver injury effect of CTs,liver transcriptome analysis was performed.The results manifested that CTs exert their effects mainly by affecting the gene expression of the key pathway of cholesterol anabolism in mouse liver.LCAT is a key enzyme in the process of cholesterol reversal.It can regulate the synthesis of cholesterol esters and HDL in blood,bind free cholesterol to HDL,and promote the conversion of HDL into HDL-C,which is transported to liver cells for catabolism,thus reducing the level of free cholesterol in blood[41].LDL-R removes LDL-C from the blood by transporting it to the liver for metabolism[42].The intake of CTs dramatically increased gene expression ofLCATandLDL-R,thereby facilitating the transfer of free cholesterol from the blood to the liver and reducing cholesterol accumulation in the blood.ABCG5 and ABCG8 play an important role in cholesterol transport by transferring cholesterol from the liver into the bile duct and excreting bile acids into feces[43].The intake of CTs significantly improved the gene expression ofAbcg5andAbcg8,promoting the transfer of cholesterol from liver to gallbladder and eventual excretion of bile acids,and alleviating the liver cholesterol metabolism disorder caused by excessive cholesterol intake.CYP7A1 and CYP8B1 are the main enzymes in bile acid metabolism pathway.CYP7A1 is the most critical rate-limiting enzyme in cholesterol excretion pathway,and plays an important role in maintaining cholesterol and cholate homeostasis[44].The intake of CTs upregulated the gene expression ofCyp7a1andCyp8b1,thus maintaining the bile acid balance.However,CTs had little effect on the non-classical metabolic pathway of cholesterol,because CTs had no evident effect on the gene expression levels of sterolCyp27a1and oxysterolCyp7b1in liver.In addition,we focused on the AMPK pathway.AMPK is a considerable kinase that regulates energy homeostasis.When activated,AMPK is phosphorylated,which promotes catabolism in cells and regulates certain metabolic processes in the body,such as lipid biosynthesis,glucose metabolism and other metabolic diseases[45].CTs treatment upregulated the expression of upstream regulators of AMPK,such asStrada,Camkk2,AdralaandAdipor2,thus increasing phosphorylation of AMPK.It also down-regulated the expression ofPpp2r5bandPpp2r5e,reducing the dephosphorylation of AMPK.Furthermore,CTs treatment increased the expression of protein kinase AMP-activated non-catalytic subunit gamma 2 (PRKAG2) and promoted the phosphorylation of AMPK,and then inhibited the expression of HMGCR,which was a key enzyme gene of cholesterol biosynthesis,thereby reducing the biosynthesis of cholesterol and inhibiting the excessive accumulation of cholesterol in the liver.
In summary,our study confirmed that CTs have noteworthy anti-liver injury and cholesterol-lowering effects in mice fed with a high cholesterol diet,as well as inhibiting oxidative stress.These beneficial effects of CTs may be attributed to the regulation the related pathways of cholesterol metabolism by CTs.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Basic Research Program of China (2013CB127106).
Appendix A.Supplementary data
Supplementary data associated with this article can be found,in the online version,at http://doi.org/10.26599/FSHW.2022.9250078.
- 食品科学与人类健康(英文)的其它文章
- Protective effects of oleic acid and polyphenols in extra virgin olive oil on cardiovascular diseases
- Diet and physical activity inf luence the composition of gut microbiota,benef it on Alzheimer’s disease
- Inf luence of nitrogen status on fermentation performances ofnon-Saccharomyces yeasts: a review
- Ganoderma lucidum: a comprehensive review of phytochemistry,eff icacy,safety and clinical study
- Resveratrol combats chronic diseases through enhancing mitochondrial quality
- Demonstration of safety characteristics and effects on gut microbiota of Lactobacillus gasseri HMV18

